Synopsis
Magnetic resonance imaging (MRI) has rapidly become a leading research tool in the study of multiple sclerosis (MS). Conventional imaging is useful in diagnosis and management of the inflammatory stages of MS, but has limitations in describing the degree of tissue injury as well as the cause of progressive disability seen in the later stages of disease. Advanced MRI techniques hold promise to fill this void. Magnetization transfer imaging is a widely available technique that can characterize demyelination and may be useful in measuring putative remyelinating therapies. Diffusion tensor imaging describes the three-dimensional diffusion of water and holds promise in characterizing neurodegeneration and putative neuroprotective therapies. Spectroscopy measures the imbalance of cellular metabolites and could help unravel the pathogenesis of neurodegeneration in MS. Functional (f) MRI can be used to understand the functional consequences of MS injury, including the impact on cortical function and compensatory mechanisms. These imaging tools hold great promise to increase our understanding of MS pathogenesis and provide greater insight into the efficacy of new MS therapies.
Keywords: MRI, imaging, magnetization transfer imaging, spectroscopy, functional MRI, diffusion tensor imaging
Introduction
Magnetic resonance imaging (MRI) is a non-invasive imaging technique, providing excellent contrast between intact and demyelinated white matter. MRI lesions typically persist for decades, providing a long term record of MS injury within the brain and spinal cord. MRI was formally integrated into the MS diagnostic criteria in 2000 and can be used to demonstrate both of the classic demyelinating hallmarks of MS – dissemination in space and dissemination in time [PG1][rf2].105 MRI also has been an important tool in the study of new MS agents, where reduction in new lesions is usually the primary outcome of phase II trials of anti-inflammatory therapies.
Conventional MRI modalities include T2-weighted, T1-weighted, and post-gadolinium T1-weighted images. Although useful in the diagnosis and management of MS, conventional imaging has several limitations. Lesions are non-specific, indicating areas of inflammation, demyelination, ischemia, edema, cell loss, gliosis. Conventional imaging is unable to differentiate between these different pathologies. Conventional imaging also poorly characterizes the degree of injury in demyelinated lesions. In addition, conventional imaging does not identify all of the pathology in MS: there are widespread abnormalities in the white matter which appears normal on T2- and T1-weighted images. Cortical demyelination is common in MS patients, but is rarely seen on conventional imaging. Gradually progressive disability is common on the later stages of MS, even though conventional imaging usually shows no changes. To address these short-comings, advanced imaging modalities have been developed and applied to MS. These advanced imaging methods provide a more sensitive and specific assessment of MS tissue injury. MRI is also useful in studying the pathophysiology of MS, with different imaging modalities providing pathologic insights into the MS injury and later recovery. This review will describe some of the ways MRI is used to study MS.
Brain Atrophy
Inflammatory injury in MS causes both demyelination and axonal loss.157 The end result of this injury can be loss of tissue, and this loss of tissue can be measured by brain atrophy.15 Brain atrophy begins early in the disease course and progresses throughout the different stages of MS. Although atrophy correlates only modestly with existing clinical disability, progression of atrophy more strongly predicts later disability progression.61
MS therapies might also impact the progression of brain atrophy, although the relationship is not always straightforward.81,107,135 The anti-inflammatory effect of MS therapies can reduce brain volume, called a “pseudo-atrophy” effect.167 Even patient hydration status can also affect atrophy measurements.48 Brain atrophy is an attractive outcome metric for progressive MS trials using putative neuroprotective therapies, where conventional lesion measures do not characterize the underlying neurodegeneration.1
Quantitative Analysis of Conventional Imaging
Since new and enlarging lesions define active inflammation, sensitive and accurate quantitative measures of these lesions are a valuable research tool. Image analysis software has been helpful in measuring lesions accurately and reproducibly. Application of quantitative lesion measures to longitudinal studies can characterize changes in lesions over time, including new lesions and changes in overall lesion burden. Quantitative measures of conventional imaging are now standard outcomes metrics in MS clinical trials. New gadolinium-enhancing or T2 lesions are typical primary outcome measures in Phase II anti-inflammatory MS trials, while these lesion measures are relegated to secondary outcomes in phase III trials.
Although useful in many ways, quantitative measures have several limitations. The measures are relatively dependent upon pulse sequence and other scanner settings. Changes in scanner settings, including scanner and pulse sequence upgrades, can significantly impact quantitative measures. Different software programs work differently, yielding different measures of lesion burden and atrophy.60 Artifacts such as patient motion can also impact quantitative measures. Because imaging abnormalities are not specific for MS, not all “lesions” seen on imaging represent actual MS lesions. Nonetheless, quantitative image analysis software provides powerful tools to assess the inflammatory components of MS injury.
Magnetization transfer imaging
In vivo markers of myelin are essential for quantifying demyelination and remyelination in the brains of MS patients. Unfortunately, the protons associated with myelin have T2 relaxation times that are too short (<1 ms) to be directly detected by conventional brain MRI. Instead, the protons associated with myelin can be indirectly detected by harnessing a physical phenomenon called magnetization transfer (MT). MT ratio (MTR) imaging is one of the most promising MRI modalities with sensitivity and specificity to myelin and widespread availability.
MT is a phenomenon in which protons of two or more environments (pools) with distinctly different magnetic resonance (MR) properties exchange magnetization. In a simple model of the brain, two pools of protons with distinctly different MR properties and biological properties are liquid (or mobile) protons and macromolecular (or bound) protons. The protons associated with water (both intracellular and extracellular) contribute to the liquid pool of protons. The protons associated with myelin, cell membranes and proteins contribute to the macromolecular pool of protons. To detect the macromolecular pool of protons, an off-resonance radio-frequency pulse (often referred to as the MT pulse) is used. This pulse preferentially excites the macromolecular pool of protons and is added immediately prior to a conventional (usually T1-weighted or proton-density-weighted (PDw)) MRI sequence. Adding this pulse induces the transfer of magnetization from the macromolecular protons to nearby liquid protons, yielding an MRI with intensities that have been modulated by the presence of myelin.
One type of MT imaging is quantitative MT (qMT) imaging. In a qMT imaging session, many different MRIs with different parameters (e.g. MRI modalities without the MT pulse, MRI with variable MT pulse duration or pulse offset frequency, etc.) are acquired. After the acquisition, these MRIs are analyzed to completely characterize the two- (or more) pooled model of protons in the brain. One of the quantities that can be extracted from this analysis is a 3-dimensional (3D) image of the fraction of macromolecular protons (fB). The sensitivity and specificity of fB to myelin density in lesional white-matter (WM) and normal-appearing WM (NAWM) has been demonstrated in a study that performed qMTI on unfixed brain slices, followed by histopathological analyses139. Unfortunately, qMT imaging can only be performed at specialized centers and it typically yields low-resolution images if performed on the whole brain31,156.
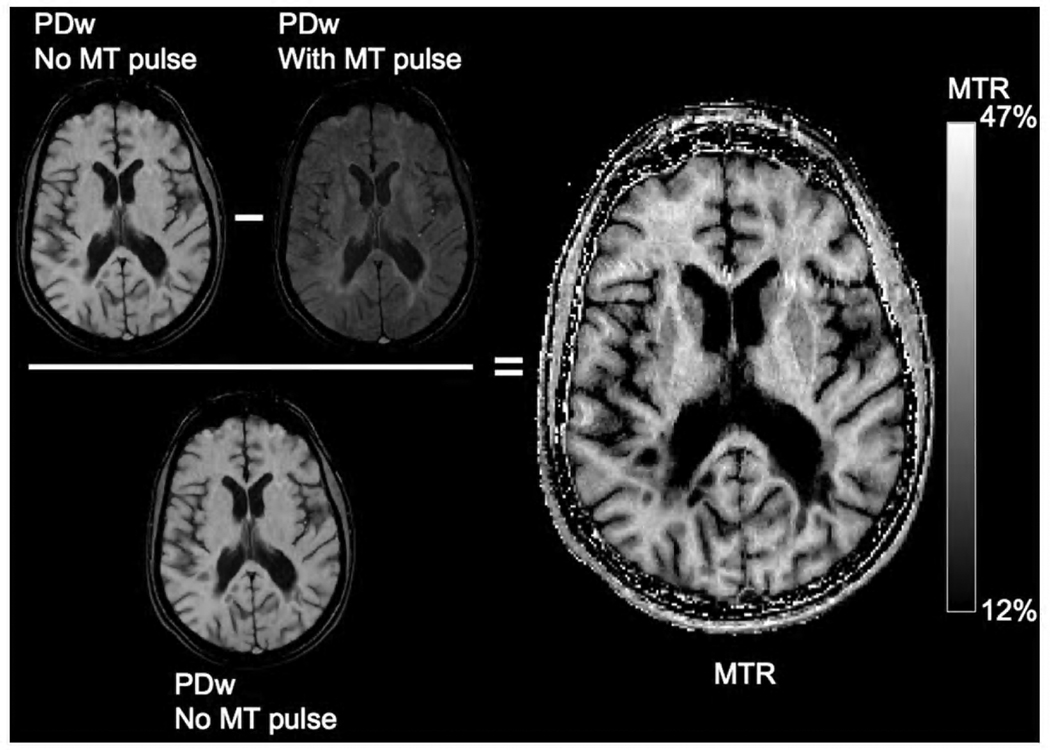
Another type of MT imaging is MTR imaging (Figure 1). To obtain an MTR image, two different MRIs are acquired in a single session: 1) an MToff image, which is a conventional MRI (T1w or PDw); and 2) an MTon image, which is the same conventional MRI acquired with the additional MT pulse. After acquisition, a 3D MTR image is calculated by measuring the percentage difference in the MTon image relative to the MToff image (MTR = 100*[MToff − MTon]/MToff). The sensitivity of MTR to myelin density has been demonstrated by post mortem imaging and histopathological analyses in MS brains that revealed strong associations between myelin content and MTR in WM lesions and NAWM11,138. In addition, in vivo imaging and post mortem histopathology in MS brain are validated MTR metrics for remyelination and demyelination within an initially enhancing WM lesion.34
Figure 1.
Magnetization Transfer Ratio imaging in a secondary-progressive MS patient at 1.5 T: The 3D MTR image is calculated by measuring the percentage difference in the MTon image relative to the MToff image. The MT pulse was applied to a 3D Fast Low Angle Shot PDw (TR=30 ms, TE=11 ms, flip angle=15°).
The relative specificity of MTR to myelin density in brain has been demonstrated by post mortem imaging and histopathological analyses that revealed no significant associations with axonal count (after accounting for the correlation between myelin content and axonal count) or gliosis,138 and by in vivo imaging of acute MS lesions that revealed no significant effect of inflammation on the correlation of MTR with qMT-derived macromolecular content.67 Although changes in brain MTR values underestimate demyelination in acute lesions due to edema,67 several observations suggest that MTR imaging is a powerful tool for MS research: the strong correlation between MTR and qMT-derived macromolecular content over the 10-month evolution of acute lesions, the strong associations between MTR and histopathologically-derived metrics of myelin density, and the clinical feasibility of performing whole-brain MTR imaging on most modern scanners at a clinically relevant resolution.
MTR in MS
MTR has provided many insights regarding the evolution of demyelination and remyelination in acute WM lesions. Decreased in MTR prior to the appearance of a WM lesion on T2-weighted MRI or Gadolinium (Gd)-enhanced T1w MRI suggests early myelin pathology not detectable by conventional MRI.56,124,131 The evolution of MTR within new lesions varies from lesion to lesion: mean lesional MTR may recover over 1–5 months,58,91,131,142,159 suggesting remyelination; or, it may remain low58,131,159 or decrease further47,58,159, suggesting on-going demyelination. This variable MTR outcome for acute lesions is supported by post mortem observations of both remyelinated shadow plaques and demyelinated lesions in the same brain.23 The evolution of MTR in individual lesion voxels (typically 1 to 3 mm3) has also been shown to vary: within a given lesion there may be groups of voxels that remain stable with low MTR, suggesting static demyelination; or increase significantly, suggesting remyelination; or decrease significantly, suggesting on-going demyelination.33,34 This variable MTR outcome for different regions of a lesion is supported by post mortem observations of inactive demyelinated WM lesions with variable peripheral remyelination,126 and in vivo MTR with post mortem histopathology validating that the spatial variability in MTR outcome was associated with demyelinated and remyelinated lesion regions.34
MTR of non-lesional brain tissue has also been found to be abnormally low in MS patients, in both NAWM42 and normal-appearing grey-matter (NAGM).42,127 MTR of non-lesional brain tissue has been shown to be associated with concurrent disability. Mean MTR of normal-appearing brain tissue was associated with existing cognitive impairment.59 MTR in cortical regions was associated with the regionally relevant clinical disability scores.84. Mean cortical MTR was significantly lower in cognitively impaired compared to cognitively preserved benign MS patients.2
MTR of non-lesional brain tissue predicts disability progression. Mean MTR in NAWM predicted disability progression over five years.137 Peak height of the NAGM MTR histogram best predicted progression over three years.83 Despite the clinical relevance of these MTR findings in brain regions without MRI-detectable WM lesions, studies of NAWM and NAGM using both MRI (including high-resolution MTR) and histopathology (including immunohistochemical analyses to determine myelin distribution and pathology, and electron microscopy to investigate myelin and tissue ultrastructure) have not been performed to adequately understand the substrate for these MTR differences.
MTR of cervical spinal cord has also been found to be abnormally low in MS patients and associated with disability.21 Performing MTR imaging in spinal cord is challenging, in part due to the motion of the cord during each scan and between the two scans. A new approach acquiring only the MTon scan and normalizing the intensities using the cerebrospinal fluid (MTCSF) has demonstrated associations between column-specific MTCSF values and the relevant measures of sensorimotor impairment.165
Future Challenges to MTR Imaging
An important goal for MS therapies could be to enhance remyelination, yet there is no well-accepted MRI metric of remyelination. The sensitivity and specificity of MTR to myelin suggests that image processing of MTR may yield a metric of in vivo remyelination. As described earlier, there is preliminary validation of MTR as useful metrics for remyelination and demyelination of acute WM lesions.34 Another method of detecting significant changes in MTR within lesional and non-lesional brain voxels has also been proposed,50 although further validation of all of these methods is still needed. An advantage with MTR is that most imaging systems have MTR easily available, making implementation in multi-centered clinical trials more straight-forward than other advanced imaging techniques. Additional advances in standardized image acquisition and analysis will help increase the application of MTR to assessing therapies which target remyelination. Several ongoing clinical trials are using MTR, and initial results appear promising.3
Cortical demyelination is commonly observed in MS brains through post mortem histopathology and the extent of demyelination can be quite great. Some cortical lesions can be observed by double-inversion-recovery (DIR),29,66,143 fluid-attenuated-inversion-recovery and spoiled-gradient-recalled-echo,9 DIR and phase-sensitive-inversion-recovery (PSIR),116 DIR and PSIR and magnetization-prepared-rapid-acquisition-with-gradient-echo (MPRAGE),115 MPRAGE and T2w imaging.10 However, all of these methods identify only a small proportion of the overall cortical lesions seen on histopathology. The most common cortical lesions involve the layers nearest to the pial surface, and these are typically not visualized with current techniques, including MTR. Advanced image-processing of MTR, however, may yield a metric of in vivo cortical demyelination that is not always visually apparant. As described earlier, image processing of cortical MTR has detected abnormalities, but further MRI and histopathological studies are needed to determine the relationship between cortical lesions and abnormal cortical MTR.
Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) is an advanced imaging method which describes the three-dimensional diffusion of water within tissue. In the 1960's Stejskal and Tanner found that a magnetic field gradient can be used to measure the ease by which molecules can diffuse.150 Moseley et al recognized that adding diffusion weighting gradients (DWG, or a gradient in the magnetic field across the imaging plane) into an MRI acquisition could detect edema on acute ischemia, leading to the earliest clinical application of diffusion imaging.110
DWG can be applied in only one direction per scan (or single image acquisition), while DTI takes advantage of the fact that the direction of the DWG affects the signal contrast within tissues. When a DWG is parallel to the axons and myelin sheaths (the least restricted direction of diffusion), there is relatively more signal attenuation. When a DWG is perpendicular to myelin sheaths, the most restricted direction of diffusion, the signal is brighter (Figure 2). By integrating the signal contrast when DWG are applied in different directions over multiple scans, a three-dimensional characterization of water diffusion can be derived.
Figure 2.
An illustration of the contrast induced by diffusion weighting gradients (DWG). When the DWG is aligned left-to-right (A), signal attenuation is greatest in regions with highly organized bundles of myelin aligned left right (B, arrows. When the DWG is aligned anterior-to-posterior (C), signal attenuation is greatest in regions with myelin sheaths aligned anterior posterior (D, arrow).
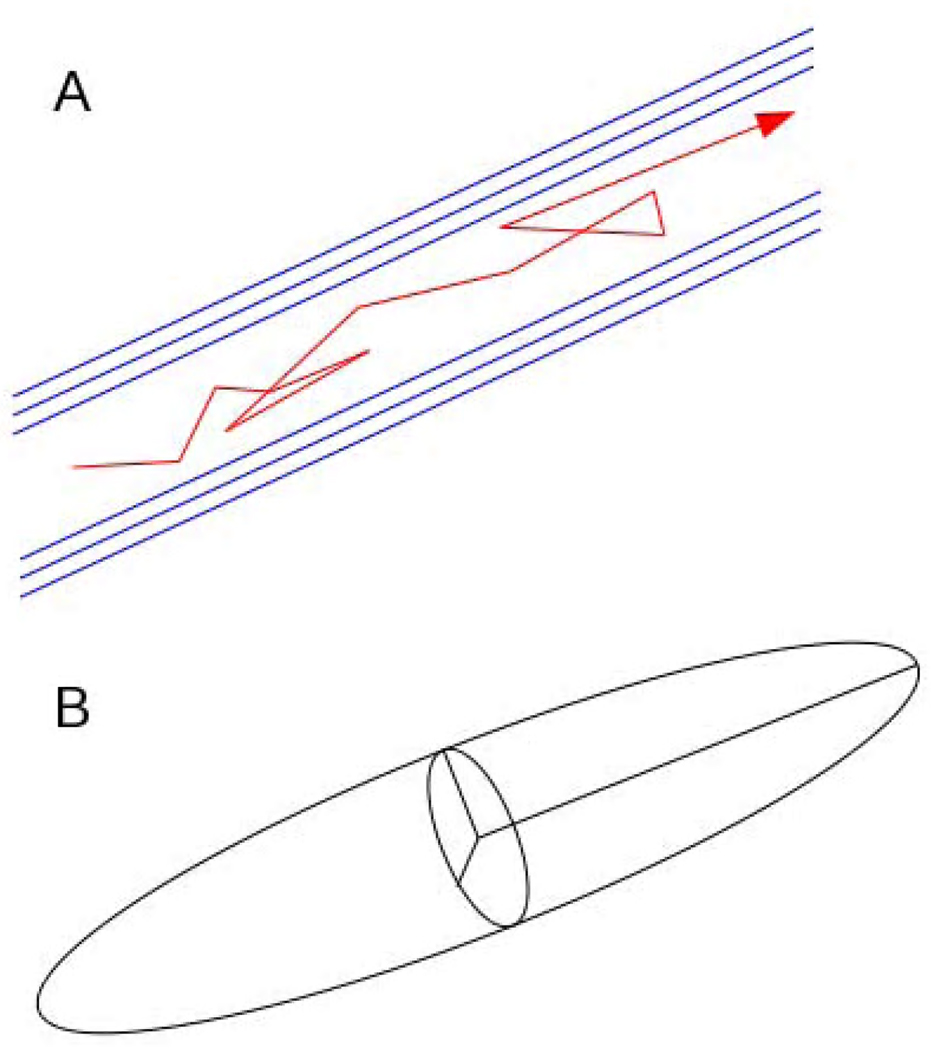
The “diffusion ellipsoid” provides an intuitive description of the diffusion tensor (Figure 3). The shape of the ellipsoid corresponds with tissue microstructure, with the long (principal) axis parallel to fiber bundles. Although only six DWG are needed to fully describe the ellipsoid, tensor estimates will vary depending upon the orientation of the ellipsoid with respect to the primary magnetic field if only six DWG’s are used. Therefore, typically 30 or more non-collinear (i.e. different directions) DWG's are utilized to reliably estimate the diffusion tensor.79
Figure 3.
(A) Cartoon of an axon with myelin sheaths (blue). A water molecule diffusing in this environment follows a path with movement preferentially aligned along the myelin sheath (red arrow). (B) Diffusion ellipsoid representation of diffusion tensor, with long axis aligned with preferred direction of water diffusion.
A number of metrics can be derived from the tensor to correlate with degree and type of tissue injury. The two most commonly reported metrics are mean diffusivity (MD), which is reflects the overall amount of diffusion, and fractional anisotropy (FA), which represents the degree of elongation of diffusion. An increase of MD can accompany loss of physiological barriers to diffusion associated with cell loss or injury and is commonly observed in MS patients. FA is large in highly organized white matter, since diffusion is relatively elongated; tissue injury (e.g. demyelination) can therefore lead to a decrease in FA.
The individual components of the diffusion ellipsoid (called eigenvalues) can be evaluated separately, too. Longitudinal diffusivity (LD) is simply the largest of the three eigenvalues and is also known as axial diffusivity. Relative decreases in LD correlates with axonal injury at the acute stage of inflammatory injury,52,86 and such abnormalities are found distal from site of injury.25,44 This observations suggests a picture in which transection of axons reduces the ease by which water moves along axon bundles.37,164 Transverse diffusivity (TD) is the mean of the smaller two eigenvalues and is also known as radial diffusivity. In highly organized fiber tracts, radial diffusivity corresponds to diffusion across fibers. Relative increase of TD correlates with demyelination and suggests a picture in which fragmented or missing myelin leads to fewer barriers for water diffusion.25,146
DTI Tractography
Tractography is a diffusion analysis technique that aims to identify specific white matter pathways and may enable association between white matter injury and functional deficit. For example, measures of TD in the motor pathway has been found to correlate with arm function (the 9 hole peg test) in MS patients. Injury has been found in pathways connected distal to MS lesions, providing an imaging correlate to Wallerian degeneration.37,144,164 These changes may also explain advanced imaging abnormalities in white matter regions that appear normal on conventional imaging.
The basic concept behind tractography entails connecting the orientation information at each voxel. In the most common implementation, the principal eigenvector, which is associated with the largest axis of the diffusion ellipsoid, is used. Streamline is constructed by connecting principal eigenvectors in each voxel, thus creating whole-brain maps of fibers.12,109 Unfortunately, such methods fail with small fibers, in regions of fiber crossings and in the presence of tissue injury such as MS lesions.14 A number of higher-order methods have been developed to model crossing fibers,118 78,158 and more complex tractography methods have been introduced to capture more of the fascicle anatomy.88,148
Analysis of tractography results typically involves either of two approaches. One can measure tract-specific values such as LD, TD, FA, and MD.98 This approach has found correlation with clinical measures of disability in MS and with functional MRI measures of transcallosal inhibition. Another approach essentially counts streamlines generated by the tractography algorithm. However, this counting approach and the related tract volume metric demonstrate relatively high variability and reduced sensitivity.72
Tract based spatial statistics (TBSS) is a tractography method which constructs a white matter skeleton from fractional anisotropy images.145 Imaging metrics associated with these skeletons can be compared between subjects and so TBSS shows promise for bridging the gap between imaging and clinical disability.46,68
DTI in MS
The sensitivity and physiological interpretation of DTI suggest a range of applications relevant to MS. DTI is sensitive to abnormalities in “normal appearing white matter,” which appears normal on conventional imaging,54,65 and can differentiate different types of lesions.112,161 DTI-related parameters have also been used to assess the impact of new drug therapies.140,141 Correlation with a number of clinical, physiological, and psychological scores suggest the potential of DTI for assessing and perhaps predicting patient-specific progression of disease and response to therapy.63,98,130
Future Challenges to DTI
Modeling water diffusion as a tensor is helpful in highly organized white matter tracts, but the tensor models are a less accurate description of water diffusion in more complex tissues, including those with crossing fiber tracts, gray matter, and tissue injury. Therefore, several methods have been proposed to model multi-component diffusivity, which may provide additional information about tissue. Examples include diffusion spectrum imaging,160 q-space imaging,6 multi-exponential modeling,111 and diffusion kurtosis imaging.77
The wide dynamic range of measurement and the relative pathologic specificity of DTI to axonal and myelin integrity suggests that it may be useful in measuring the impact of MS therapies. As therapies emerge with potential remyelination and neuroprotection effects, DTI is an attractive metric to assess efficacy.62 Methods to assure comparability of DTI measures across different magnet types and different centers are needed to effectively implement DTI in multi-center clinical trials.
Spectroscopy
Magnetic resonance spectroscopy (MRS) is an imaging tool used to study the chemical characteristics of tissues. Where conventional MRI characterizes the physical characteristics of a region of tissue relative to surrounding regions, MRS characterizes the chemical properties of a region of tissue, most commonly focusing on cellular metabolites. The sensitivity of MRS measurements is proportional to Aγ3, where A is the natural abundance of the isotope of the MR active nucleus, and γ is the gyromagnetic ratio, which is determined by the magnetic moment of the nucleus. The sensitivities of the isotopes commonly used in clinical MRS are listed in Table 1.
Table 1.
The abundance and sensitivity of isotopes commonly measured in clinical spectroscopy studies
| Isotope | γ (MHz T−1) | Abundance (%) | %Sensitivity |
|---|---|---|---|
| 1H | 42.58 | 99.98 | 1.00 |
| 31P | 17.25 | 100.0 | 6.65 × 10−2 |
| 13C | 10.71 | 1.108 | 1.76 × 10−4 |
Of the three isotopes listed in Table 1, proton (1H) spectroscopy is used most frequently in clinical practice. Table 2 lists some of the major resonances appearing in in vivo MR spectra from cerebral imaging.41,70 Imbalances in the relative amount of the metabolites measured in vivo indicate presence of diseases, and so precise identification and accurate quantification of the peaks in MR spectra are sometimes necessary for diagnostic purposes.
Table 2.
Major resonance peaks in MR spectra
| Metabolite | Description |
|---|---|
| N-Acetylaspartate (NAA) | predominantly present in cell bodies and acts as neuronal marker |
| creatine (Cr) / phosphocreatine (PCr) | PCr is converted to Cr during the conversion of ADP to the high energy compound ATP |
| choline (Cho) containing compounds | usually grouped within the B-complex vitamins and are present in the synaptic ends of cholinergic neurons and cell membranes, and are part of lipid metabolism |
| glutamate (Glu) and glutamine (Gln) | Glu is the major excitatory neurotransmitter and Gln is a regulator of Glu metabolism |
| myo-inositol (mI) | acts as glia cell markers in brain tissue |
| lactate (Lac) and glucose | Lac is the final product of the anaerobic glycolysis cycle, and is important in assessing ischemic tissue and tumors; glucose is important in energy metabolism of the brain |
| alanine (Ala) | a glucogenic amino acid and is readily converted to pyruvate, which can enter the TCA cycle |
| gamma amino butyric acid (GABA) | a major inhibitory neurotransmitter |
| macromolecules and lipids | cellular components |
MRS in MS
MRS has been used to study changes and imbalances in different cellular metabolites over the course of MS. MRS has also been explored as a diagnostic tool, although MRS is not considered a standard modality in the diagnosis of MS.136
N-Acetylaspartate (NAA)
MS lesions usually have lower NAA, which is an indicator of axonal/neuronal loss or dysfunction. Significant reduction in NA (sum of NAA and N-acetyl aspartyl glutamate (NAAG)) was found in chronic white matter lesions in relapsing remitting (RR), secondary progressive (SP) and primary progressive (PP) MS patients, while no such reduction in NA was seen in benign MS patients.39 The same study found a decrease in NA level in normal appearing white matter (NAWM) in PPMS, while no such effect was seen in benign MS. MRS has shown a decrease in NAA/Cr ratio in patients with moderate to severe chronic disease.4 In a longitudinal study, transient changes of NAA level in acute plaques were observed, indicating that reduced NAA is not necessarily associated with axonal loss.113 The average NAA level within the spectroscopic volume was found to be inversely correlated with the total lesion volume in the whole brain in the same study. Reduction in NAA level in cortical gray matter (CGM), NAWM and lesion have been reported in mild RRMS, suggesting widespread neuronal loss or dysfunction early in the course of the disease.80 Decrease in NA levels in CGM and NAWM has been reported in early RRMS.32 Significant reduction in whole brain NAA level has been reported in RRMS patients, where the observed decrease in NAA was higher in older than younger patients.69
Creatine
MRS has found mixed results for Cr levels. Cr level was similar in NAWM and CGM between RRMS and healthy controls,155 and in NAWM between PPMS and controls.93 In other studies, however, Cr level was modestly higher in MS NAWM than in controls,71,76 although Cr concentration was similar within T1 isointense lesions and NAWM in RRMS. Cr levels in CGM correlated with clinical disability as measured by the MS Functional Composite (MSFC).32
Choline (Cho)
No significant difference between Cho concentration in isointense lesions in T1 weighted MRI and NAWM in RRMS was seen in MRS study, while NAWM Cho concentration was reported to be 14% higher in MS patients compared to controls.71 The increase in Cho and Cr levels was interpreted as (i) attempted remyelination in isointense lesions and ongoing gliosis, and (ii) increased cellularity (gliosis, inflammation) along with membrane turnover. Significant increase in Cho level in MS plaques has been reported,92 and a decrease in NAA/Cho ratio observed in the same study was speculated to be related either to axonal degeneration or gliosis. In a short-term serial study,5 Cho levels in large demyelinating lesions was found to increase 3 days after the onset of symptoms, and at 8 months the level remained high at the center of the lesion. The Cho levels surrounding the lesion, however, normalized by 8 months. The abnormal Cho level was interpreted to indicate persistent demyelination. In a longer-term longitudinal study, Cho levels within NAWM that became a visible MS lesion 6–12 months later showed higher Cho compared to regions that did not become lesions.153 Similarly, lesions which increased in size after six months had higher Cho/Cr ratio at baseline than lesions which remained stable in size. Significant reduction in CGM Cho level was observed in RRMS.32
Glutamate (Glu) and Glutamine (Gln)
Elevated Glu has been reported in acute lesions and NAWM, while no significant elevation in Glu within chronic lesions was observed.147 These observations suggest an alteration of Glu metabolism in MS. A significant reduction in Glx (combined Glu and Gln) levels has been observed in CGM, and a significant correlation between CGM Glx level and disability (measured by MSFC) has also been reported in MS.32 The correlation between clinical disability and CGM Cr and Glx levels but not between disability and NAWM NA is suggestive of close correlation between reduced NAA and neuronal metabolic dysfunction rather than neuronal loss in early RRMS.32
Myo-inositol (mI)
MRS studies in MS patients have observed increased mI levels in acute MS lesions and chronic T2 lesions.43,80 mI levels are also elevated in NAWM, and these elevations are inversely correlated with disability as measured by MSFC.32 This correlation is speculated to relate to glial proliferation and function in MS patients. mI levels are also elevated in NAWM and cortical gray matter of patients with either MS or clinically isolated syndromes (CIS) suggestive of MS.53,80
Other metabolites
Elevated lactate levels have been reported in acute MS lesions.43 Elevated levels of macromolecule have been observed in acute MS lesions compared to chronic lesions in MS patients and NAWM from healthy controls indicating that macromolecule resonances may be a useful marker of acute MS lesions.101 Lipid resonances were observed to be elevated in enhancing MS lesions, which probably represented lipid products of myelin breakdown. These lipid levels remained elevated for a mean of five months.40 Elevated lipid peaks have also been observed in chronic T2 lesions, suggesting possible alternative pathophysiologic processes leading to demyelination.113 Strong lipid resonances in GM and NAWM have been reported in PPMS even in the absence of lesions, which is suggestive of regionally altered myelin macromolecular structure.114
MRS to measure the impact of MS therapies
Since MRS is thought to provide a quantitative (relative and absolute) measure of many different metabolites, it is reasonable to hypothesize that MRS may measure the efficacy of MS therapies. However, to date only a few studies have used MRS to evaluate the effect of MS therapies, with mixed results.136
Future Applications and Challenges of MRS
With the recent development of improved hardware (higher strength clinical scanners, multi-channel phased array coils etc.) and software (improved pulse sequences for scans, data analysis softwares), MRS is becoming a more useful tool than ever in the understanding of MS. These developments should allow increased understanding of MS pathophysiology and its relationship with brain function.
Spinal cord MRS
Most MRS research in MS has focused on the brain. While the spinal cord is known to be involved in the disability associated with MS,13,154 few MRS studies have focused on this region. 1H MRS could provide useful information on axonal damage in spinal cord. Spinal cord MRS, however, is technically challenging due to the small size of the cord, susceptibility artifacts at tissue-bone interfaces, artifacts from cardiac and respiratory motion and CSF pulsations, and hence only a few spinal cord MRS studies have been performed. Nonetheless, the feasibility of cervical cord MRS and metabolite quantification has recently been demonstrated using 3 tesla magnets in healthy controls.104 Significant differences in NA, Cr, Cho and mI concentrations were observed between spinal cord and brainstem. A study in MS patients found reduced level of NAA in the cervical spinal cord in MS supporting the presence of axonal loss and damage in normal-appearing spinal cords of MS patients.82 Significant correlations were also observed between Cr, Cho, and mI levels in the cervical cord and disability, as measured by EDSS and 9-hole peg test.38
Identifying cortical marker of MS
Damage to cortical GM in MS has long been acknowledged in pathological studies.22,85 MRS can be explored as a potential cortical marker of the disease. For this purpose, it would be most appropriate to study metabolites contained within neurons, which are present primarily in GM. GABA is an inhibitory neurotransmitter which is present in GM at a much higher level than in WM.35,123 While pathological study has explored the role of GABA in MS,49 MRS of cortical GABA in MS is largely not explored. In vivo cortical GABA measurement by MRS is technically challenging due to very low cerebral GABA level and presence of stronger overlapping resonances. Nonetheless, a recent preliminary study of MRS of GABA in MS is encouraging.16
Motion
A significant problem in MRS studies is subject motion. A single scanning session is 30–45 minutes or longer, and it is very difficult for a subject to stay still throughout such a long session. Patient motion can result in scanning the wrong brain region and thus can lead to inaccurate results.89 The advent of multi coil technology and parallel imaging have reduced the total scan time, but it remains [rf3] important for subjects to remain still for each 5–10 minute [PG4] and this is often difficult for MS patients. Moreover, identification of motion from final spectrum is not always possible,18 and so it is very important to have other mechanisms to identify motion during the scan. While several studies have proposed methods to address the issue of subject motion,18,51,125,166 the problem can still persist with patient population for longer scans.
Functional MRI
Functional MRI (fMRI) techniques take advantage of the relationship between brain activity and small changes in MRI signal. This effect, called the blood oxygen level-dependent (BOLD) effect, is the result of a cascade of physiological events that link neural activity to MRI signal changes.27,96 Upon initiating a task, the neurons in the brain regions involved in that task increase neuronal firing. This neural activity leads to increased metabolic demand, to which the brain responds. Through a combination of metabolic and synaptic signaling, cerebral blood flow (the hemodynamic response) is increased in the local blood capillaries within approximately one millimeter of the neural activity. This increased blood flow results in increased total blood oxygen content. In appropriately tuned MR acquisitions, the increased blood oxygen produces an increase in the MRI signal. The BOLD signal change is typically only a few percent of the baseline signal, which can make it difficult to detect from background variability, or noise.
The BOLD effect has been used to identify and study brain activity in response to various tasks or stimuli by looking at the time evolution of changes in signal across the brain.90,117 The typical MRI pulse sequence for fMRI is the echo-planar imaging (EPI) sequence. This pulse sequence allows fast (2–3 seconds per whole-brain sample) imaging of the BOLD effect across the entire brain with spatial resolution of a few tens of cubic millimeters. Neuroimaging fMRI studies typically involve the performance of some task during the scan session. The subject’s response to the task in the scanner is compared with the MR signal to obtain various parameters relating to the neural activity, such as 3-dimensional maps of BOLD activation. The task or stimuli can be designed to stimulate a particular domain, such as motor, sensory, emotional or cognitive. The overall study design typically measures activation during a task and compares that between two groups of subjects. Comparison can be between MS patients and healthy controls, or between different MS subgroups (Figure 4). Functional connectivity MRI is a newer fMRI technique in which measures low frequency oscillations in BOLD signals across the entire brain.19,99 The same type of data as fMRI is acquired in functional connectivity MRI studies, but the subject is usually resting in the scanner instead of performing a task. Functional connectivity analysis depicts the strength of networks between cortical regions.
Figure 4.
Group averaged BOLD activation to a complex finger tapping task in MS patients and controls. MS patients showed 5% larger cortical volume of activation.
fMRI in MS
Functional MRI (fMRI) of MS has produced a range of insights into the progression of the disease. Early studies of fMRI in MS began through evaluations of the visual and motor systems. The first use of fMRI in MS was a case report of a patient fully recovered from an acute episode of homonymous hemianopsia.106 During the fMRI scan, hemifield visual stimulation was presented, and the resulting activation maps were compared to those of controls. They found that the recovered visual cortex behaved similarly to controls. Several years later, motor and visual tasks were used to evaluate changes in fMRI in MS patients.163 This study found MS patients with motor weakness experienced larger motor activation than controls while patients with optic neuritis experienced smaller visual activation than controls. The reduced visual activation in optic neuritis was confirmed shortly after and shown to correlate with increased latency of visual evoked potentials (VEP) recorded in the cortex of the affected eye.64 Subsequent studies explored attention and arithmetic performance using the paced serial addition test (PASAT),7,149 working memory (Sternberg task),73 attention processes,120,122 and verbal working memory,151 among others.
Compensation and Reorganization
The majority of the fMRI studies in MS have examined compensatory processes and reorganization of functional tissue. Observations of cortical reorganization support the hypothesis that compensatory processes in brain tissue limit the correspondence between pathology and apparent disability.94,128 The most common finding is increased extent or strength of activation in MS patients compared to controls, implying compensation or reorganization of neuronal activation.129,134 Later studies investigated how the limits of the adaptive cortical changes may play a role in clinical progression.122 The basis for these limits were seen in studies that showed regions recruited by MS patients during simple motor tasks are components recruited by healthy controls in more difficult tasks.57 Reorganization of sensory circuits to sensori-motor integration circuits (e.g. putamen) was seen in a passive motor movement fMRI study.36 These studies of adaptive reorganization suggest that clinical disability is dependent upon some combination of tissue damage, repair and cortical reorganization.132 Longitudinal fMRI studies are needed to further understand this complex relationship and provide clinical benefit to MS patients.24
Rehabilitation & Longitudinal Studies
Longitudinal studies have been used to evaluate how fMRI findings relate to disease evolution and clinical recovery. Initial longitudinal studies found reduced ipsilateral motor activation correlated with disease progression.119 Later studies found adaptive plasticity in bilateral visual cortex and lateral geniculate nucleus following recovery from acute unilateral optic neuritis.87 A change in cognitive activation in the lateral prefrontal cortices in MS patients correlated with change in PASAT scores over one year.8 The use of serial administration of PASAT suffers from the potential confound of practice effects.30 These training effects were explored by pre and post-training fMRI motor task sessions, with a reduced effect of training seen in MS patients, implying reduced capacity to adapt.108 Administration of the cholinesterase inhibitor rivastigmine in MS patients causes a normalization of fMRI activation on an attention fMRI task (Stroop).28,120 Further studies are needed to clarify how fMRI may be helpful in guiding specific rehabilitation methods.121
Fatigue
Fatigue is a common but incompletely understood symptom in MS. fMRI studies have observed a relationship between cortical activation and fatigue severity.55 Fatigue Severity Scale scores correlated inversely with right hand finger flexion-extension motor activation in several motor-associated regions: greater fatigue was associated with less relative activation in these regions. Subsequent studies indicate that non-motor functions of the basal ganglia may be involved in fatigue processes, where greater activation over time in MS patients was observed over repeated sessions of a processing speed task.45 Performance of a cognitively fatiguing mental task (PASAT) between motor fMRI scans led to an increase in activation to a paced finger task in primarily non-motor areas of MS patients, but a decrease in controls. This observation implies that the presence of fatigue may suggest an increased level of neuronal reorganization required to perform a particular task.152 Furthermore, newly-recruited tissue may not habituate or respond in the same way as older ingrained circuitry in the presence of fatigue. In a similar study, performance of a physically fatiguing motor task between motor fMRI scans showed little change in activation in MS patients in the second (post-fatigue) scan but an increase in control subject activation.162 However, a group comparison showed that MS patients had significantly greater activation in the pre-fatigue scan, such that post fatigue level of activation was already at maximum for MS patients. These studies all point to fatigue in MS patients being associated with reaching the limit of neuronal compensation. Further exploration in the area of fatigue in MS is needed.
Connectivity
Imaging biomarkers of MS progression historically relied on T2 and contrast-enhanced lesions, with the assumption that structural connections between brain regions were reduced in MS. Studies correlating structural damage (T2 lesion load) and functional changes support this hypothesis.102,133 Trans-cranial magnetic stimulation (TMS) and motor fMRI showed that loss of trans-callosal inhibitory motor fibers is correlated with increased ipsilateral motor activation.95 MS patients with damage to the superior longitudinal fasciculus (SLF) had bilateral activation to a serial addition task, which results in lateralized (language-dominant hemisphere) activation in controls and MS patients without SLF damage (no difference in task performance).20 A study of functional connectivity and diffusion tensor imaging (DTI) structural connectivity between motor cortices found a direct correlation between the two modes of connectivity in MS patients and controls.97
Future Directions
Several areas of research remain for fMRI studies. More longitudinal studies are needed to evaluate adaptive plasticity and rehabilitation.24,103 Specifically, the current imaging biomarkers of disease progression have a poor prospective correlation with eventual outcomes.75 Improved understanding of the reorganization process (including natural tissue repair, natural reorganization, rehabilitation-induced reorganization and pharmacological-induced reorganization) and the limits of cortical adaptability to various cognitive demands could improve the ability to predict outcomes in MS. However, a single fMRI scan is typically specific to one or two pathways, and but MS deficits may arise from injury or dysfunction to a number of different pathways. The pathway-specific nature of fMRI may limit its application in predicting general MS outcomes. Functional connectivity and structural connectivity, however, can be determined for many different pathways in a practical scan-time, which may overcome that limitation. Longitudinal connectivity studies and other fMRI biomarker studies are needed to better understand the ability of fMRI to measure overall disease progression.
An often under-appreciated issue in BOLD fMRI is bias and improper design. fMRI and connectivity techniques have been shown to be sensitive several [PG5][rf6] potential confounds that could bias results, and many were not recognized in previous studies.74 These include the effect of motion,26 physiologic noise,17,100 vasoreactivity and blood flow,74 among others. Sensitive behavioral measures of performance also need to be recognized. For example, bilateral fiber optic gloves to monitor hand movements are preferable over visual inspection, since visual inspection often misses subtle motor activation that may impact fMRI results. MS patients differ from healthy subjects in several ways: increased motion, altered cerebral blood flow, and reduced ability to perform behavioral tasks. A failure to account for these differences in a population-based study may question the study’s conclusions, calling into question whether the results are only non-disease-related artifacts of the patient population. Even within a single MS patient population, a change in these variables over time may mask an underlying change in fMRI or masquerade as altered activation. These short-comings not-withstanding, fMRI has become a powerful tool in understanding cortical function and the connectivity between different brain regions. The data provided by fMRI complements that obtained by conventional imaging in understanding the impact of MS disease injury.
Conclusion
Magnetic resonance imaging (MRI) has rapidly become a leading research tool in the study of MS. Advances in imaging are providing a more accurate characterization of tissue injury, including demyelination, axonal injury, and its functional and metabolic consequences. These tools are not only providing greater insight into MS pathophysiology, but several may be useful in measuring the potential benefit of remyelinating and neuroprotective therapies.
Acknowledgments
This work was supported by RG RG 4091A3/1 (RJF), RG 3751B2 (EB), RG 3753A1/2 PB) from the National MS Society, and R21 EB005302-02 (PB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altmann DR, Jasperse B, Barkhof F, et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology. 2009;72:595. doi: 10.1212/01.wnl.0000335765.55346.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato MP, Portaccio E, Stromillo ML, et al. Cognitive assessment and quantitative magnetic resonance metrics can help to identify benign multiple sclerosis. Neurology. 2008;71:632. doi: 10.1212/01.wnl.0000324621.58447.00. [DOI] [PubMed] [Google Scholar]
- 3.Arnold DL, Dalton CM, Narayanan S, et al. Magnetization transfer ratio imaging is feasible in large multicenter MS trials. Neurology. 2010;74:A118. [Google Scholar]
- 4.Arnold DL, Matthews PM, Francis G, et al. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn Reson Med. 1990;14:154. doi: 10.1002/mrm.1910140115. [DOI] [PubMed] [Google Scholar]
- 5.Arnold DL, Matthews PM, Francis GS, et al. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol. 1992;31:235. doi: 10.1002/ana.410310302. [DOI] [PubMed] [Google Scholar]
- 6.Assaf Y, Ben-Bashat D, Chapman J, et al. High b-value q-space analyzed diffusion-weighted MRI: application to multiple sclerosis. Magnetic Resonance in Medicine. 2002;47:115. doi: 10.1002/mrm.10040. [DOI] [PubMed] [Google Scholar]
- 7.Audoin B, Ibarrola D, Ranjeva JP, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51. doi: 10.1002/hbm.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audoin B, Reuter F, Duong MV, et al. Efficiency of cognitive control recruitment in the very early stage of multiple sclerosis: a one-year fMRI follow-up study. Mult Scler. 2008;14:786. doi: 10.1177/1352458508089360. [DOI] [PubMed] [Google Scholar]
- 9.Bagnato F, Butman JA, Gupta S, et al. In vivo detection of cortical plaques by MR imaging in patients with multiple sclerosis. Ajnr: American Journal of Neuroradiology. 2006;27:2161. [PMC free article] [PubMed] [Google Scholar]
- 10.Bagnato F, Yao B, Cantor F, et al. Multisequence-imaging protocols to detect cortical lesions of patients with multiple sclerosis: observations from a postmortem 3 Tesla imaging study. J Neurol Sci. 2009;282:80. doi: 10.1016/j.jns.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barkhof F, Bruck W, De Groot CJ, et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Archives of neurology. 2003;60(8):1073. doi: 10.1001/archneur.60.8.1073. [DOI] [PubMed] [Google Scholar]
- 12.Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44(4):625. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Bastianello S, Paolillo A, Giugni E, et al. MRI of spinal cord in MS. J Neurovirol. 2000;6 Suppl 2:S130. [PubMed] [Google Scholar]
- 14.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurology. 2006;5:158. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya P, Phillips M, Bermel R, et al. Impaired Motor Performance in MS is Associated with Increased GABA Level in Sensorimotor Cortex. Proc. Intl. Soc. Mag. Reson. Med. 2010;18:390. [Google Scholar]
- 17.Bhattacharyya PK, Lowe MJ. Cardiac-induced physiologic noise in tissue is a direct observation of cardiac-induced fluctuations. Magn Reson Imaging. 2004;22:9. doi: 10.1016/j.mri.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya PK, Lowe MJ, Phillips MD. Spectral quality control in motion-corrupted single-voxel J-difference editing scans: an interleaved navigator approach. Magn Reson Med. 2007;58:808. doi: 10.1002/mrm.21337. [DOI] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 20.Bonzano L, Pardini M, Mancardi GL, et al. Structural connectivity influences brain activation during PVSAT in Multiple Sclerosis. Neuroimage. 2009;44:9. doi: 10.1016/j.neuroimage.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Bozzali M, Rocca MA, Iannucci G, et al. Magnetization-transfer histogram analysis of the cervical cord in patients with multiple sclerosis. AJNR Am J Neuroradiol. 1999;20:1803. [PMC free article] [PubMed] [Google Scholar]
- 22.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1962;25:315. doi: 10.1136/jnnp.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruck W, Schmied M, Suchanek G, et al. Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol. 1994;35:65. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- 24.Buckle GJ. Functional magnetic resonance imaging and multiple sclerosis: the evidence for neuronal plasticity. J Neuroimaging. 2005;15:82S. doi: 10.1177/1051228405284093. [DOI] [PubMed] [Google Scholar]
- 25.Budde MD, Kim JH, Liang HF, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnetic Resonance in Medicine. 2007;57:688. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- 26.Bullmore ET, Brammer MJ, Rabe-Hesketh S, et al. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp. 1999;7:38. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buxton RB, Uludag K, Dubowitz DJ, et al. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23 Suppl 1:S220. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Cader S, Palace J, Matthews PM. Cholinergic agonism alters cognitive processing and enhances brain functional connectivity in patients with multiple sclerosis. J Psychopharmacol. 2009;23:686. doi: 10.1177/0269881108093271. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese M, Rocca MA, Atzori M, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 67:376. doi: 10.1002/ana.21906. [DOI] [PubMed] [Google Scholar]
- 30.Cardinal KS, Wilson SM, Giesser BS, et al. A longitudinal fMRI study of the paced auditory serial addition task. Mult Scler. 2008;14:465. doi: 10.1177/1352458507084263. [DOI] [PubMed] [Google Scholar]
- 31.Cercignani M, Basile B, Spano B, et al. Investigation of quantitative magnetisation transfer parameters of lesions and normal appearing white matter in multiple sclerosis. NMR Biomed. 2009;22:646. doi: 10.1002/nbm.1379. [DOI] [PubMed] [Google Scholar]
- 32.Chard DT, Griffin CM, McLean MA, et al. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:2342. doi: 10.1093/brain/awf240. [DOI] [PubMed] [Google Scholar]
- 33.Chen JT, Collins DL, Atkins HL, et al. Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol. 2008;63:254. doi: 10.1002/ana.21302. [DOI] [PubMed] [Google Scholar]
- 34.Chen JT, Kuhlmann T, Jansen GH, et al. Voxel-based analysis of the evolution of magnetization transfer ratio to quantify remyelination and demyelination with histopathological validation in a multiple sclerosis lesion. Neuroimage. 2007;36:1152. doi: 10.1016/j.neuroimage.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 35.Choi IY, Lee SP, Merkle H, et al. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Ciccarelli O, Toosy AT, Marsden JF, et al. Functional response to active and passive ankle movements with clinical correlations in patients with primary progressive multiple sclerosis. J Neurol. 2006;253:882. doi: 10.1007/s00415-006-0125-z. [DOI] [PubMed] [Google Scholar]
- 37.Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging--evidence of Wallerian degeneration. Journal of Neurology. 2003;250:287. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 38.Ciccarelli O, Wheeler-Kingshott CA, McLean MA, et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220. doi: 10.1093/brain/awm152. [DOI] [PubMed] [Google Scholar]
- 39.Davie CA, Barker GJ, Thompson AJ, et al. 1H magnetic resonance spectroscopy of chronic cerebral white matter lesions and normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997;63:736. doi: 10.1136/jnnp.63.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davie CA, Hawkins CP, Barker GJ, et al. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117(Pt 1):49. doi: 10.1093/brain/117.1.49. [DOI] [PubMed] [Google Scholar]
- 41.de Graaf RA. In vivo NMR spectroscopy Principles and techniques. New York: John Wiley & Sons; 1998. [Google Scholar]
- 42.De Stefano N, Battaglini M, Stromillo ML, et al. Brain damage as detected by magnetization transfer imaging is less pronounced in benign than in early relapsing multiple sclerosis. Brain. 2006;129:2008. doi: 10.1093/brain/awl152. [DOI] [PubMed] [Google Scholar]
- 43.De Stefano N, Matthews PM, Antel JP, et al. Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann Neurol. 1995;38:901. doi: 10.1002/ana.410380610. [DOI] [PubMed] [Google Scholar]
- 44.DeBoy CA, Zhang J, Dike S, et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- 45.DeLuca J, Genova HM, Hillary FG, et al. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci. 2008;270:28. doi: 10.1016/j.jns.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- 47.Dousset V, Gayou A, Brochet B, et al. Early structural changes in acute MS lesions assessed by serial magnetization transfer studies. Neurology. 1998;51:1150. doi: 10.1212/wnl.51.4.1150. [DOI] [PubMed] [Google Scholar]
- 48.Duning T, Kloska S, Steinstrater O, et al. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- 49.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 50.Dwyer M, Bergsland N, Hussein S, et al. A sensitive, noise-resistant method for identifying focal demyelination and remyelination in patients with multiple sclerosis via voxel-wise changes in magnetization transfer ratio. J Neurol Sci. 2009;282:86. doi: 10.1016/j.jns.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Felblinger J, Kreis R, Boesch C. Effects of physiologic motion of the human brain upon quantitative 1H-MRS: analysis and correction by retro-gating. NMR Biomed. 1998;11:107. doi: 10.1002/(sici)1099-1492(199805)11:3<107::aid-nbm525>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 52.Feng S, Hong Y, Zhou Z, et al. Monitoring of acute axonal injury in the swine spinal cord with EAE by diffusion tensor imaging. Journal of Magnetic Resonance Imaging. 2009;30:277. doi: 10.1002/jmri.21825. [DOI] [PubMed] [Google Scholar]
- 53.Fernando KT, McLean MA, Chard DT, et al. Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain. 2004;127:1361. doi: 10.1093/brain/awh153. [DOI] [PubMed] [Google Scholar]
- 54.Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- 55.Filippi M, Rocca MA, Colombo B, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage. 2002;15:559. doi: 10.1006/nimg.2001.1011. [DOI] [PubMed] [Google Scholar]
- 56.Filippi M, Rocca MA, Comi G. Magnetization transfer ratios of multiple sclerosis lesions with variable durations of enhancement. Journal of the Neurological Sciences. 1998;159(2):162. doi: 10.1016/s0022-510x(98)00162-2. [DOI] [PubMed] [Google Scholar]
- 57.Filippi M, Rocca MA, Mezzapesa DM, et al. A functional MRI study of cortical activations associated with object manipulation in patients with MS. Neuroimage. 2004;21:1147. doi: 10.1016/j.neuroimage.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Filippi M, Rocca MA, Sormani MP, et al. Short-term evolution of individual enhancing MS lesions studied with magnetization transfer imaging. Magn Reson Imaging. 1999;17:979. doi: 10.1016/s0730-725x(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 59.Filippi M, Tortorella C, Rovaris M, et al. Changes in the normal appearing brain tissue and cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:157. doi: 10.1136/jnnp.68.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fisher E, Barkhof F, van den Elskamp I, et al. Comparion of brain atrophy measurement methods in the context of a clinical trial. Multiple Sclerosis. 2009;15:S217. [Google Scholar]
- 61.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412. doi: 10.1212/01.wnl.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 62.Fox RJ. Picturing multiple sclerosis: conventional and diffusion tensor imaging. Seminars in Neurology. 2008;28:453. doi: 10.1055/s-0028-1083689. [DOI] [PubMed] [Google Scholar]
- 63.Fox RJ, McColl RW, Lee JC, et al. A preliminary validation study of diffusion tensor imaging as a measure of functional brain injury. Archives of Neurology. 2008;65:1179. doi: 10.1001/archneur.65.9.1179. [DOI] [PubMed] [Google Scholar]
- 64.Gareau PJ, Gati JS, Menon RS, et al. Reduced visual evoked responses in multiple sclerosis patients with optic neuritis: comparison of functional magnetic resonance imaging and visual evoked potentials. Mult Scler. 1999;5:161. doi: 10.1177/135245859900500304. [DOI] [PubMed] [Google Scholar]
- 65.Ge Y, Law M, Johnson G, et al. Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. Journal of Magnetic Resonance Imaging. 2004;20:1. doi: 10.1002/jmri.20083. [DOI] [PubMed] [Google Scholar]
- 66.Geurts JJ, Pouwels PJ, Uitdehaag BM, et al. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 67.Giacomini PS, Levesque IR, Ribeiro L, et al. Measuring demyelination and remyelination in acute multiple sclerosis lesion voxels. Arch Neurol. 2009;66:375. doi: 10.1001/archneurol.2008.578. [DOI] [PubMed] [Google Scholar]
- 68.Giorgio A, Palace J, Johansen-Berg H, et al. Relationships of brain white matter microstructure with clinical and MR measures in relapsing-remitting multiple sclerosis. Journal of Magnetic Resonance Imaging. 2010;31:309. doi: 10.1002/jmri.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonen O, Catalaa I, Babb JS, et al. Total brain N-acetylaspartate: a new measure of disease load in MS. Neurology. 2000;54:15. doi: 10.1212/wnl.54.1.15. [DOI] [PubMed] [Google Scholar]
- 70.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 71.He J, Inglese M, Li BS, et al. Relapsing-remitting multiple sclerosis: metabolic abnormality in nonenhancing lesions and normal-appearing white matter at MR imaging: initial experience. Radiology. 2005;234:211. doi: 10.1148/radiol.2341031895. [DOI] [PubMed] [Google Scholar]
- 72.Heiervang E, Behrens TE, Mackay CE, et al. Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33:867. doi: 10.1016/j.neuroimage.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 73.Hillary FG, Chiaravalloti ND, Ricker JH, et al. An investigation of working memory rehearsal in multiple sclerosis using fMRI. J Clin Exp Neuropsychol. 2003;25:965. doi: 10.1076/jcen.25.7.965.16490. [DOI] [PubMed] [Google Scholar]
- 74.Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25:978. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 75.Ingle GT, Thompson AJ, Miller DH. Magnetic resonance imaging in primary progressive multiple sclerosis. J Rehabil Res Dev. 2002;39:261. [PubMed] [Google Scholar]
- 76.Inglese M, Li BS, Rusinek H, et al. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50:190. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- 77.Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;53:1432. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 78.Jian B, Vemuri BC. Multi-fiber reconstruction from diffusion MRI using mixture of Wisharts and sparse deconvolution. Information Processing in Medical Imaging. 2007;20:384. doi: 10.1007/978-3-540-73273-0_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 80.Kapeller P, McLean MA, Griffin CM, et al. Preliminary evidence for neuronal damage in cortical grey matter and normal appearing white matter in short duration relapsing-remitting multiple sclerosis: a quantitative MR spectroscopic imaging study. J Neurol. 2001;248:131. doi: 10.1007/s004150170248. [DOI] [PubMed] [Google Scholar]
- 81.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine. 2010;362:387. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 82.Kendi AT, Tan FU, Kendi M, et al. MR spectroscopy of cervical spinal cord in patients with multiple sclerosis. Neuroradiology. 2004;46:764. doi: 10.1007/s00234-004-1231-1. [DOI] [PubMed] [Google Scholar]
- 83.Khaleeli Z, Altmann DR, Cercignani M, et al. Magnetization transfer ratio in gray matter: a potential surrogate marker for progression in early primary progressive multiple sclerosis. Arch Neurol. 2008;65:1454. doi: 10.1001/archneur.65.11.1454. [DOI] [PubMed] [Google Scholar]
- 84.Khaleeli Z, Cercignani M, Audoin B, et al. Localized grey matter damage in early primary progressive multiple sclerosis contributes to disability. Neuroimage. 2007;37:253. doi: 10.1016/j.neuroimage.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 85.Kidd D, Barkhof F, McConnell R, et al. Cortical lesions in multiple sclerosis. Brain. 1999;122(Pt 1):17. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 86.Kim JH, Budde MD, Liang HF, et al. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiology of Disease. 2006;21:626. doi: 10.1016/j.nbd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 87.Korsholm K, Madsen KH, Frederiksen JL, et al. Recovery from optic neuritis: an ROI-based analysis of LGN and visual cortical areas. Brain. 2007;130:1244. doi: 10.1093/brain/awm045. [DOI] [PubMed] [Google Scholar]
- 88.Kreher BW, Mader I, Kiselev VG. Gibbs tracking: a novel approach for the reconstruction of neuronal pathways. Magnetic Resonance in Medicine. 2008;60:953. doi: 10.1002/mrm.21749. [DOI] [PubMed] [Google Scholar]
- 89.Kreis R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 2004;17:361. doi: 10.1002/nbm.891. [DOI] [PubMed] [Google Scholar]
- 90.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai HM, Davie CA, Gass A, et al. Serial magnetisation transfer ratios in gadolinium-enhancing lesions in multiple sclerosis. Journal of Neurology. 1997;244(5):308. doi: 10.1007/s004150050092. [DOI] [PubMed] [Google Scholar]
- 92.Larsson HB, Christiansen P, Jensen M, et al. Localized in vivo proton spectroscopy in the brain of patients with multiple sclerosis. Magn Reson Med. 1991;22:23. doi: 10.1002/mrm.1910220104. [DOI] [PubMed] [Google Scholar]
- 93.Leary SM, Davie CA, Parker GJ, et al. 1H magnetic resonance spectroscopy of normal appearing white matter in primary progressive multiple sclerosis. J Neurol. 1999;246:1023. doi: 10.1007/s004150050507. [DOI] [PubMed] [Google Scholar]
- 94.Lee M, Reddy H, Johansen-Berg H, et al. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol. 2000;47:606. [PubMed] [Google Scholar]
- 95.Lenzi D, Conte A, Mainero C, et al. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28:636. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 97.Lowe MJ, Beall EB, Sakaie KE, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Human Brain Mapping. 2008;29:818. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lowe MJ, Horenstein C, Hirsch JG, et al. Functional pathway-defined MRI diffusion measures reveal increased transverse diffusivity of water in multiple sclerosis. Neuroimage. 2006;32:1127. doi: 10.1016/j.neuroimage.2006.04.208. [DOI] [PubMed] [Google Scholar]
- 99.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 100.Lund TE, Madsen KH, Sidaros K, et al. Non-white noise in fMRI: does modelling have an impact? Neuroimage. 2006;29:54. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Mader I, Seeger U, Weissert R, et al. Proton MR spectroscopy with metabolite-nulling reveals elevated macromolecules in acute multiple sclerosis. Brain. 2001;124:953. doi: 10.1093/brain/124.5.953. [DOI] [PubMed] [Google Scholar]
- 102.Mainero C, Caramia F, Pozzilli C, et al. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage. 2004;21:858. doi: 10.1016/j.neuroimage.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Mainero C, Pantano P, Caramia F, et al. Brain reorganization during attention and memory tasks in multiple sclerosis: insights from functional MRI studies. J Neurol Sci. 2006;245:93. doi: 10.1016/j.jns.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 104.Marliani AF, Clementi V, Albini-Riccioli L, et al. Quantitative proton magnetic resonance spectroscopy of the human cervical spinal cord at 3 Tesla. Magn Reson Med. 2007;57:160. doi: 10.1002/mrm.21113. [DOI] [PubMed] [Google Scholar]
- 105.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology. 2001;50:121. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 106.Miki A, Nakajima T, Fujita M, et al. Functional magnetic resonance imaging of the primary visual cortex: evaluation of human afferent visual system. Jpn J Ophthalmol. 1995;39:302. [PubMed] [Google Scholar]
- 107.Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 108.Morgen K, Kadom N, Sawaki L, et al. Training-dependent plasticity in patients with multiple sclerosis. Brain. 2004;127:2506. doi: 10.1093/brain/awh266. [DOI] [PubMed] [Google Scholar]
- 109.Mori S, Crain B, Chacko V, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 110.Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magnetic Resonance in Medicine. 1990;14:330. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- 111.Mulkern RV, Gudbjartsson H, Westin CF, et al. Multi-component apparent diffusion coefficients in human brain. NMR in Biomedicine. 1999;12:51. doi: 10.1002/(sici)1099-1492(199902)12:1<51::aid-nbm546>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 112.Naismith RT, Xu J, Tutlam NT, et al. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology. 2010;74:1694. doi: 10.1212/WNL.0b013e3181e042c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Narayana PA, Doyle TJ, Lai D, et al. Serial proton magnetic resonance spectroscopic imaging, contrast-enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann Neurol. 1998;43:56. doi: 10.1002/ana.410430112. [DOI] [PubMed] [Google Scholar]
- 114.Narayana PA, Wolinsky JS, Rao SB, et al. Multicentre proton magnetic resonance spectroscopy imaging of primary progressive multiple sclerosis. Mult Scler. 2004;10 Suppl 1:S73. doi: 10.1191/1352458504ms1035oa. [DOI] [PubMed] [Google Scholar]
- 115.Nelson F, Poonawalla A, Hou P, et al. 3D MPRAGE improves classification of cortical lesions in multiple sclerosis. Mult Scler. 2008;14:1214. doi: 10.1177/1352458508094644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson F, Poonawalla AH, Hou P, et al. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol. 2007;28:1645. doi: 10.3174/ajnr.A0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ozarslan E, Shepherd TM, Vemuri BC, et al. Resolution of complex tissue microarchitecture using the diffusion orientation transform (DOT) Neuroimage. 2006;31:1086. doi: 10.1016/j.neuroimage.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 119.Pantano P, Mainero C, Lenzi D, et al. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128:2146. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- 120.Parry AM, Scott RB, Palace J, et al. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain. 2003;126:2750. doi: 10.1093/brain/awg284. [DOI] [PubMed] [Google Scholar]
- 121.Pelletier J, Audoin B, Reuter F, et al. Plasticity in MS: from Functional Imaging to Rehabilitation. Int MS J. 2009;16:26. [PubMed] [Google Scholar]
- 122.Penner IK, Rausch M, Kappos L, et al. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol. 2003;250:461. doi: 10.1007/s00415-003-1025-0. [DOI] [PubMed] [Google Scholar]
- 123.Petroff OA, Pleban LA, Spencer DD. Symbiosis between in vivo and in vitro NMR spectroscopy: the creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magn Reson Imaging. 1995;13:1197. doi: 10.1016/0730-725x(95)02033-p. [DOI] [PubMed] [Google Scholar]
- 124.Pike GB, De Stefano N, Narayanan S, et al. Multiple sclerosis: magnetization transfer MR imaging of white matter before lesion appearance on T2-weighted images. Radiology. 2000;215(3):824. doi: 10.1148/radiology.215.3.r00jn02824. [DOI] [PubMed] [Google Scholar]
- 125.Posse S, Cuenod CA, Le Bihan D. Human brain: proton diffusion MR spectroscopy. Radiology. 1993;188:719. doi: 10.1148/radiology.188.3.8351339. [DOI] [PubMed] [Google Scholar]
- 126.Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- 127.Ramio-Torrenta L, Sastre-Garriga J, Ingle GT, et al. Abnormalities in normal appearing tissues in early primary progressive multiple sclerosis and their relation to disability: a tissue specific magnetisation transfer study. J Neurol Neurosurg Psychiatry. 2006;77:40. doi: 10.1136/jnnp.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reddy H, Narayanan S, Arnoutelis R, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123(Pt 11):2314. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- 129.Reddy H, Narayanan S, Woolrich M, et al. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002;125:2646. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- 130.Reich DS, Zackowski KM, Gordon-Lipkin EM, et al. Corticospinal tract abnormalities are associated with weakness in multiple sclerosis. Ajnr: American Journal of Neuroradiology. 2008;29:333. doi: 10.3174/ajnr.A0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Richert ND, Ostuni JL, Bash CN, et al. Interferon beta-1b and intravenous methylprednisolone promote lesion recovery in multiple sclerosis. Multiple Sclerosis. 2001;7(1):49. doi: 10.1177/135245850100700109. [DOI] [PubMed] [Google Scholar]
- 132.Rocca MA, Filippi M. Functional MRI in multiple sclerosis. J Neuroimaging. 2007;17 Suppl 1:36S. doi: 10.1111/j.1552-6569.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 133.Rocca MA, Gallo A, Colombo B, et al. Pyramidal tract lesions and movement-associated cortical recruitment in patients with MS. Neuroimage. 2004;23:141. doi: 10.1016/j.neuroimage.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 134.Rocca MA, Matthews PM, Caputo D, et al. Evidence for widespread movement-associated functional MRI changes in patients with PPMS. Neurology. 2002;58:866. doi: 10.1212/wnl.58.6.866. [DOI] [PubMed] [Google Scholar]
- 135.Rudick RA, Fisher E, Lee JC, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53:1698. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 136.Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:45. doi: 10.1016/j.nic.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santos AC, Narayanan S, de Stefano N, et al. Magnetization transfer can predict clinical evolution in patients with multiple sclerosis. J Neurol. 2002;249:662. doi: 10.1007/s00415-002-0686-4. [DOI] [PubMed] [Google Scholar]
- 138.Schmierer K, Scaravilli F, Altmann DR, et al. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- 139.Schmierer K, Tozer DJ, Scaravilli F, et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging. 2007;26:41. doi: 10.1002/jmri.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shukla DK, Kaiser CC, Stebbins GT, et al. Effects of pioglitazone on diffusion tensor imaging indices in multiple sclerosis patients. Neuroscience Letters. 2010;472:153. doi: 10.1016/j.neulet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 141.Sijens PE, Mostert JP, Irwan R, et al. Impact of fluoxetine on the human brain in multiple sclerosis as quantified by proton magnetic resonance spectroscopy and diffusion tensor imaging. Psychiatry Research. 2008;164:274. doi: 10.1016/j.pscychresns.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 142.Silver N, Lai M, Symms M, et al. Serial gadolinium-enhanced and magnetization transfer imaging to investigate the relationship between the duration of blood-brain barrier disruption and extent of demyelination in new multiple sclerosis lesions. J Neurol. 1999;246:728. doi: 10.1007/s004150050442. [DOI] [PubMed] [Google Scholar]
- 143.Simon B, Schmidt S, Lukas C, et al. Improved in vivo detection of cortical lesions in multiple sclerosis using double inversion recovery MR imaging at 3 Tesla. Eur Radiol. doi: 10.1007/s00330-009-1705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simon JH, Zhang S, Laidlaw DH, et al. Identification of fibers at risk for degeneration by diffusion tractography in patients at high risk for MS after a clinically isolated syndrome. Journal of Magnetic Resonance Imaging. 2006;24:983. doi: 10.1002/jmri.20719. [DOI] [PubMed] [Google Scholar]
- 145.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 146.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 147.Srinivasan R, Sailasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- 148.Staempfli P, Jaermann T, Crelier GR, et al. Resolving fiber crossing using advanced fast marching tractography based on diffusion tensor imaging. Neuroimage. 2006;30:110. doi: 10.1016/j.neuroimage.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 149.Staffen W, Mair A, Zauner H, et al. Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain. 2002;125:1275. doi: 10.1093/brain/awf125. [DOI] [PubMed] [Google Scholar]
- 150.Stejskal E, Tanner J. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. The Journal of Chemical Physics. 1965;42:288. [Google Scholar]
- 151.Sweet LH, Rao SM, Primeau M, et al. Functional magnetic resonance imaging of working memory among multiple sclerosis patients. J Neuroimaging. 2004;14:150. [PubMed] [Google Scholar]
- 152.Tartaglia MC, Narayanan S, Arnold DL. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. Eur J Neurol. 2008;15:413. doi: 10.1111/j.1468-1331.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- 153.Tartaglia MC, Narayanan S, De Stefano N, et al. Choline is increased in prelesional normal appearing white matter in multiple sclerosis. J Neurol. 2002;249:1382. doi: 10.1007/s00415-002-0846-6. [DOI] [PubMed] [Google Scholar]
- 154.Tartaglino LM, Friedman DP, Flanders AE, et al. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology. 1995;195:725. doi: 10.1148/radiology.195.3.7754002. [DOI] [PubMed] [Google Scholar]
- 155.Tiberio M, Chard DT, Altmann DR, et al. Metabolite changes in early relapsing-remitting multiple sclerosis. A two year follow-up study. J Neurol. 2006;253:224. doi: 10.1007/s00415-005-0964-z. [DOI] [PubMed] [Google Scholar]
- 156.Tozer D, Ramani A, Barker GJ, et al. Quantitative magnetization transfer mapping of bound protons in multiple sclerosis. Magn Reson Med. 2003;50:83. doi: 10.1002/mrm.10514. [DOI] [PubMed] [Google Scholar]
- 157.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. New Eng J Med. 1998;338:278. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 158.Tuch DS. Q-ball imaging. Magnetic Resonance in Medicine. 2004;52:1358. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- 159.van Waesberghe JH, van Walderveen MA, Castelijns JA, et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am J Neuroradiol. 1998;19:675. [PMC free article] [PubMed] [Google Scholar]
- 160.Wedeen VJ, Hagmann P, Tseng WY, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;54:1377. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 161.Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52(8):1626. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 162.White AT, Lee JN, Light AR, et al. Brain activation in multiple sclerosis: a BOLD fMRI study of the effects of fatiguing hand exercise. Mult Scler. 2009;15:580. doi: 10.1177/1352458508100034. [DOI] [PubMed] [Google Scholar]
- 163.Yousry TA, Berry I, Filippi M. Functional magnetic resonance imaging in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64 Suppl 1:S85. [PubMed] [Google Scholar]
- 164.Yu CS, Lin FC, Li KC, et al. Diffusion tensor imaging in the assessment of normal-appearing brain tissue damage in relapsing neuromyelitis optica. Ajnr: American Journal of Neuroradiology. 2006;27:1009. [PMC free article] [PubMed] [Google Scholar]
- 165.Zackowski KM, Smith SA, Reich DS, et al. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transfer-imaging abnormalities in the spinal cord. Brain. 2009;132:1200. doi: 10.1093/brain/awp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ziegler A, Decorp M. Signal-to-noise improvement in in-vivo spin-echo spectroscopy in the presence of motion. J Magn Reson. 1993;102:26. [Google Scholar]
- 167.Zivadinov R, Reder AT, Filippi M, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71:136. doi: 10.1212/01.wnl.0000316810.01120.05. [DOI] [PubMed] [Google Scholar]