Abstract
Please cite this paper as: Neumann G, Kawaoka Y. (2011) The first influenza pandemic of the new millennium. Influenza and Other Respiratory Viruses DOI: 10.1111/j.1750‐2659.2011.00202.x.
In the spring of 2009, a novel influenza A virus of the H1N1 subtype emerged that transmitted efficiently among humans; by June of 2009, the outbreak reached pandemic status. The pandemic virus possesses six viral RNA segments from so‐called triple reassortant swine viruses that emerged in North American pig populations in the late 1990s and two viral RNA segments from Eurasian avian‐like swine influenza viruses. Most human infections with the virus have been mild; however, severe and fatal infections occurred among certain risk groups, but also among those without any known risk factors. Here, we summarize the evolutionary, epidemiological, clinical, and molecular findings on the pandemic virus. We also discuss the arsenal of antiviral compounds and vaccines available to prevent and treat infections with the virus.
Keywords: Antiviral compounds, H1N1, influenza virus, pandemic, reassortment, vaccines
Introduction
Influenza A viruses (family Orthomyxoviridae) cause respiratory infections with mild to severe symptoms. Based on the antigenicity of their surface glycoproteins hemagglutinin (HA) and neuraminidase (NA), they are subdivided into 16 HA subtypes (H1–16) and nine NA subtypes (N1–9) (reviewed in Refs. 1 , 2 ). Influenza pandemics (i.e., outbreaks on a global scale with sustained human‐to‐human transmission) are caused by viruses to which people have little or no immunity. In 1918, an influenza virus of the H1N1 subtype caused the ‘Spanish influenza’ that killed an estimated 20–50 million people worldwide. Descendants of this virus circulated until 1957, when they were replaced with an avian/human reassortant of the H2N2 subtype that caused a new pandemic (‘Asian influenza’). In 1968, another pandemic was caused by an avian/human reassortant virus of the H3N2 subtype (‘Hong Kong influenza’); this virus replaced the previously circulating viruses of the H2N2 subtype. In 1977, an H1N1 virus reemerged that closely resembled the viruses isolated in the mid‐1950s. The reemergence of this virus did not cause a full‐scale pandemic, likely because of pre‐existing immunity to viruses of this subtype in individuals born before 1957. Since 1977, viruses of the H3N2 and H1N1 subtypes have cocirculated in humans. In 2009, a novel influenza virus of the H1N1 subtype caused the most recent pandemic (reviewed in Refs. 3 , 4 , 5 , 6 ). Notably, this most recent pandemic did not involve a change in the HA subtype. Here, we summarize current knowledge about this novel H1N1 virus, referred to as 2009 H1N1 virus.
The emergence of a new pandemic virus
In late March of 2009, two children in Southern California experienced an influenza‐like illness, 7 , 8 and soon after, similar cases were reported in Mexico. 9 , 10 , 11 , 12 On April 14, the CDC identified the causative agents as swine influenza H1N1 influenza viruses. 7 Ten days later, the CDC reported that the Mexican and Californian cases were caused by a genetically similar virus. 11 , 13 In response to the efficient spread of the novel virus, the World Health Organization (WHO) declared a ‘public health emergency of international concern’ on April 25. 11 Within 2 months, sustained human‐to‐human transmission in several countries on different continents was reported, prompting the WHO to announce the highest alert level (phase 6, pandemic) on June 12, 2009. 14 , 15
The 2009 H1N1 virus swiftly spread around the world and dominated the circulating, seasonal influenza viruses – from August 30, 2009 through March 27, 2010, 99·4% of subtyped influenza A viruses were novel 2009 H1N1 viruses. 16 The United States experienced a first wave in late spring of 2009, followed by a second wave that peaked in mid‐October. As of February 13, 2010, the CDC estimated 42–86 million cases in the United States (mid‐level: 59 million cases) (http://www.cdc.gov/H1N1flu/estimates/April_February_13.htm), with 188 000–389 000 hospitalizations (mid‐level: 265 000) and an estimated 8520–17 620 deaths (mid‐level: 12 000). This number is still less than that of seasonal influenza‐related deaths, which is estimated to be 30 000 per year in the United States. The estimated case‐fatality rate ranges from 0·2% to 1·23% 17 , 18 , 19 ; however, significant differences between age groups were apparent. 17 , 18 , 19 , 20 , 21 , 22 , 23 Children experienced a high attack rate, but a low case‐fatality rate; by contrast, the elderly experienced a low attack rate but a high case‐fatality rate. 17 , 18 , 19 , 20 , 21 , 22 , 23 The relatively low attack rates among the elderly suggest some pre‐existing immunity in this age group (see ‘HA Antigenicity’). In terms of absolute numbers, the age group of 5–59 years was most affected, accounting for close to 90% of deaths and about 70% of cases of severe pneumonia, compared with 17% and 32% for recent outbreaks of seasonal influenza. 19 , 20
Clinical manifestations and risk factors
Most cases of 2009 H1N1 infection presented as mild upper respiratory tract illness. The most common symptoms were fever, cough, sore throat, shortness of breath, headache, and rhinorrhea. 8 , 19 , 22 , 24 , 25 , 26 , 27 , 28 , 29 Gastrointestinal symptoms including diarrhea and vomiting (which are unusual with seasonal influenza infections) have been reported and seem to be associated primarily with mild cases. In some instances, the disease progressed to primary and secondary pneumonia that resulted in multi‐organ failure, respiratory failure, acute respiratory distress symptoms, and sometimes death. 8 , 19 , 22 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Pathological findings included diffuse alveolar damage, hemorrhagic interstitial pneumonitis, and peribronchiolar and perivascular lymphocytic infiltrates, 22 , 27 , 31 reminiscent of infections with highly pathogenic avian H5N1 influenza viruses. 32 , 33 , 34 These findings indicated that 2009 H1N1 viruses cause lower respiratory tract infections, in contrast to seasonal influenza viruses, which typically affect the upper respiratory tract. Secondary bacterial infections have been reported in a substantial number of cases 22 , 27 , 35 , 36 , 37 and may exacerbate the course of the disease.
Certain factors significantly increased the risk of severe disease. 8 , 14 , 19 , 22 , 24 , 25 , 27 , 37 , 38 , 39 , 40 , 41 These factors include chronic conditions such as respiratory diseases (notably asthma), autoimmune diseases, cardiovascular diseases, diabetes, and obesity. In addition, pregnancy, particularly in the last trimester, significantly increased the risk for complications. 8 , 14 , 19 , 27 , 38 , 39 , 40 , 41 Increased risk of severe disease was also reported for indigenous people, probably because of overall lower health status and/or limited access to health care. 39 , 42 In general, early treatment with antiviral compounds (see ‘Antiviral Compounds’) appeared to be critical in mitigating the risks associated with 2009 H1N1 infections.
Pigs – a ‘mixing vessel’ of influenza A viruses?
Sequence and evolutionary analyses of 2009 pandemic isolates revealed an H1N1 virus that resembles viruses isolated from pigs. 7 , 8 , 43 , 44 , 45 Influenza A viruses are known to replicate in pigs, where they typically cause mild respiratory symptoms (reviewed in Ref. 46 ). While most avian influenza viruses are restricted in their ability to replicate in humans, and vice versa, pigs can be infected by both virus types 47 and were therefore proposed as a ‘mixing vessel’ 48 in which human and avian influenza viruses may reassort, potentially leading to viruses with novel gene combinations against which humans are immunologically naïve. In fact, the pandemic viruses of 1957 and 1968 were human/avian reassortants, but it is not known whether the reassortment occurred in pigs. In 1976, a swine influenza virus of the H1N1 subtype caused an outbreak among soldiers at Fort Dix, New Jersey. 49 , 50 , 51 Since then, multiple human infections with swine influenza viruses have been reported 26 , 52 , 53 , 54 , 55 , 56 ; however, these infections did not cause widespread outbreaks. The finding of a pandemic influenza virus that likely originated from pigs was therefore of great interest.
In North American pig populations, so‐called classical H1N1 influenza viruses (descendants of the 1918 pandemic virus) circulated until 1997/1998, when H3N2 triple human/avian/swine reassortant viruses emerged; these viruses have now spread throughout the swine populations (reviewed in Ref. 46 ) (Figure 1). These viruses possess HA, NA, and PB1 polymerase genes of human virus origin, PB2 and PA polymerase genes of avian virus origin, and matrix (M) and non‐structural (NS) genes of classical H1N1 swine virus origin. Through further reassortment, the H3N2 triple reassortant viruses acquired HA, or HA and NA genes from classical H1N1 viruses, resulting in triple reassortant H1N2 and H1N1 viruses. The 2009 H1N1 viruses possess six genomic RNA segments from triple reassortant swine viruses and acquired their HA and M segments from a Eurasian avian‐like swine virus 7 , 8 , 43 , 44 , 45 (Figure 1).
Figure 1.
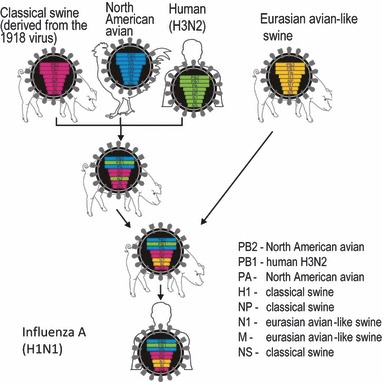
Genesis of pandemic 2009 H1N1 viruses. The NA and M genes were derived from a Eurasian avian‐like swine virus (yellow). The remaining six genes were derived from triple resssortant swine viruses that possessed genes originating from classical H1N1 swine (red), North American avian (blue) and human H3N2 (green) viruses. Reprinted by permission from Macmillan Publishers Ltd: Nature, advance online publication 14 June 2009 (doi: 10.1038/nature08157).
The reassortment event(s) that led to the generation of the novel viruses likely occurred several months to years before the pandemic outbreak, and the novel virus may have circulated in humans for several months before its detection. 17 , 45 , 57 , 58 , 59 , 60 This would explain why no (major) influenza virus activity was reported in Mexican pigs at the time the outbreak was recognized in humans. However, pigs can be experimentally 61 , 62 , 63 , 64 , 65 and naturally 66 , 67 , 68 infected with 2009 H1N1 viruses, resulting in mild respiratory infection, and contact transmission can be demonstrated in an experimental setting. 61 , 62 , 65 The extent of 2009 H1N1 virus circulation in pigs is not known. In contrast to efficient replication in humans and pigs, avian species such as chickens, ducks, and turkeys are resistant to experimental infection with 2009 H1N1 viruses, 69 , 70 , 71 , 72 although infection of two turkey flocks was reported in Chile. 73 These findings suggest that 2009 H1N1 viruses do not replicate efficiently in avian species, possibly with the exception of turkeys.
Determinants of virulence and pathogenicity
Soon after their recognition, 2009 H1N1 viruses were characterized in mice, ferrets, and non‐human primates. 74 , 75 , 76 , 77 , 78 , 79 Although the new pandemic viruses are not uniformly lethal in these animal models, they cause more severe lung pathology than seasonal influenza viruses. In addition, 2009 H1N1 viruses replicate efficiently in the upper and lower respiratory tract of infected animals, unlike seasonal influenza viruses, which are restricted in their growth to the lower respiratory tract. The ability of 2009 H1N1 viruses to replicate efficiently in the lower respiratory tract may explain, at least in part, the viral pneumonia observed in severe human cases of 2009 H1N1 virus infection. In addition, these studies demonstrated the transmission of 2009 H1N1 viruses in ferrets. 74 , 75 , 76 , 77
Sequence analysis revealed that 2009 H1N1 viruses do not possess recognized markers of high virulence (discussed below), 8 , 17 , 43 , 45 leaving in question the mechanisms for the increased virulence of 2009 H1N1 viruses compared to seasonal H1N1 viruses. With the availability of thousands of complete genomic influenza virus sequences, efforts are now underway to identify amino acid changes that may have facilitated virus transmission to and among humans. 80 , 81 , 82 Others studies are focused on identifying host factors critical for efficient viral replication. 83 , 84 , 85 Together, these studies have identified a number of viral mutations and cellular factors that may play a role in the viral life cycle; however, for most of these candidates, experimental confirmation has yet to be obtained.
Role of HA in viral pathogenicity
The HA protein mediates two critical functions in the viral life cycle – binding of the virus to host cells and the fusion of the viral and endosomal membranes for the release of viral ribonucleoprotein complexes into the cytoplasm. HA also plays a critical role in virulence and host range restriction.
Receptor‐binding specificity
Human influenza viruses preferentially bind to receptors that possess sialic acid linked to galactose by an α2,6‐linkage (SAα2,6Gal), whereas avian influenza viruses preferentially recognize SAα2,3Gal. 86 , 87 This receptor preference is matched by SAα2,3Gal on epithelial cells in the intestinal tract of waterfowl (the main replication site of avian influenza viruses) and by SAα2,6Gal on epithelial cells in the human trachea. We now know, however, that SAα2,3Gal (i.e., avian‐type receptors) are also expressed in the lower respiratory tract of humans. 88 , 89 Interestingly, pigs express both receptor types on their respiratory epithelial cells, 87 , 90 , 91 although avian‐type receptors may only be expressed in bronchioli and alveoli. 90 , 91
Differences in receptor‐binding specificity are determined by specific amino acids in HA. For H1 HA, Asp at positions 190 and 225 (H3 numbering) confers binding to SAα2,6Gal, whereas Glu‐190 and Gly‐225 confer binding to SAα2,3Gal. 92 Hence, human and avian influenza viruses typically possess Asp‐190/Asp‐225 or Glu‐190/Gly‐225, respectively. The pandemic 2009 H1N1 viruses possess SAα2,6Gal receptor‐binding specificity, 75 , 93 although one study reported binding of a 2009 H1N1 to both SAα2,3Gal and SAα2,6Gal. 94 Recently, a change in HA (Asp‐to‐Gly at position 225) was reported that appears to correlate with more severe disease outcomes in humans. 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 This variant does not appear to transmit efficiently among humans, as only a few transmission events have been reported to date. 103 Following experimental inoculation of pigs with mixed virus populations encoding Glu‐225 and Gly‐225, the Glu‐225 variant was found in nasal secretions, while the Gly‐225 variant was found in the lower respiratory tract. 65
Hemagglutinin cleavage
The HA protein is synthesized as a precursor protein (HA0) that is post‐translationally cleaved into two disulfide‐linked subunits, HA1 and HA2. This cleavage event exposes the N‐terminus of HA2 (the so‐called fusion peptide), which mediates the fusion between the viral envelope and the endosomal membrane. HA cleavage is therefore essential for viral infectivity. The 2009 H1N1 viruses, like other human and swine influenza viruses, possess a single basic amino acid at the HA cleavage site, which is cleaved by proteases in the respiratory and/or intestinal organs, hence restricting systemic spread. By contrast, highly pathogenic avian influenza viruses (including H5N1 and H7N7) possess multiple basic amino acids at the HA cleavage site. This cleavage motif is recognized by ubiquitous proteases and leads to systemic infection.
Hemagglutinin antigenicity
Sequence analysis, serology data, and experimental studies in animal models demonstrated that the 2009 H1N1 HA proteins more closely resemble the 1918 HA protein than the HA proteins of recent seasonal H1N1 viruses. 43 , 45 , 74 , 93 , 104 , 105 , 106 , 107 , 108 , 109 These findings offer an explanation for the serum cross‐reactivity with 2009 H1N1 viruses observed for some elderly people, but not younger individuals. 20 , 22 , 74 , 110 , 111 , 112 For H1 HA proteins, four antigenic sites (Ca, Cb, Sa, and Sb) have been identified. 113 , 114 The Sa, Sb, and Cb sites of 2009 H1N1 HA proteins differ by one, two, and one amino acid, respectively, from the 1918 HA protein. By contrast, the respective antigenic sites of a recent seasonal H1N1 HA protein differ by five, eight, and four amino acids from the 1918 HA protein. The Ca site is more divergent.
Antigenic sites can be masked by glycans: the 1918 and 2009 H1N1 HA proteins do not possess glycosylation sites near the Sa antigenic site; by contrast, seasonal H1N1 HA proteins possess several oligosaccharides that block the Sa site. Together, these data suggest the following scenario: the 1918 HA remained relatively unchanged in pigs; people infected with the 1918 pandemic virus, or a close descendant thereof, are therefore partially protected against swine‐origin 2009 H1N1 viruses. In humans, however, the pandemic 1918 HA faced significant immune pressure that has led to the accumulation of mutations in the antigenic sites and the circulation of seasonal H1N1 viruses up to 2009. Younger people, who have not been exposed to 1918‐like viruses, are therefore not similarly protected.
Role of PB2 in viral pathogenicity
The polymerase subunit PB2 is critical for influenza virus replication and transcription, and is a determinant of host range restriction in mammalian species 115 , 116 : human influenza viruses (with the exception of the 2009 H1N1 viruses) possess Lys at position 627, which confers efficient replication in mammalian cells. By contrast, most avian influenza viruses possess Glu‐627, which restricts replication in mammals, particularly in the upper respiratory tract. The PB2 gene of 2009 H1N1 viruses originated from an avian influenza virus and possesses Glu (i.e., the avian‐type amino acid) at PB2‐627 43 , 45 ; substitution of Glu with Lys does not increase viral growth rates in cell culture or virulence in mice. 117 , 118 Two recent studies now offer an explanation for the efficient replication of 2009 H1N1 influenza viruses in mammals despite the lack of lysine at position 627. 119 , 120 A Lys at position 591 of PB2 (which replaces the Glu typically found at this position) confers efficient replication in mammalian cells in the background of Glu‐627. 119 , 120 X‐ray crystallography of a 2009 H1N1 PB2 protein demonstrated that Lys‐591 alters the surface charge and structure of PB2, 120 which may then prevent the binding of an inhibitory factor that suppresses the activity of avian‐type polymerases (possessing PB2‐627‐Glu) in mammalian cells, as suggested by Mehle and Doudna. 121
Another study found that the amino acid at position 701 of PB2 affects the replicative ability of an H5N1 virus in mammals. 122 2009 H1N1 viruses possess the ‘low‐pathogenic’ amino acid (Asp) at this position, and its replacement with the ‘high‐pathogenic’ amino acid (Asn) did not increase virus replication in mammalian cells or virulence in mice and ferrets. 117 Recent data also suggest a role for the amino acid at position 271 of PB2 in adaptation to mammals. 123 The novel pandemic viruses possess the human‐type amino at this position, which contributes to the high replicative ability of the 2009 H1N1 virus replication complex. 123
Role of NS1 in viral pathogenicity
The NS1 protein is an interferon antagonist 124 that executes its role through several mechanisms (reviewed in Refs. 125 , 126 ); it counteracts virus‐stimulated RIG‐I signaling, binds to double‐stranded RNA to prevent the activation of 2′‐5′ oligo(A)synthetase and RNaseL, and interferes with the activation of transcription factors and IFN‐β‐stimulated gene products. The amino acid at position 92 may play a role in regulating interferon responses, with Glu (found in highly pathogenic avian influenza viruses) resulting in high virus pathogenicity, and Asn resulting in low virus pathogenicity. 127 2009 H1N1 viruses possess the ‘low‐pathogenic’ amino acid at this position. The four C‐terminal amino acids of NS1 form a PDZ ligand domain motif 128 that may affect virulence. 129 The NS1 protein of the novel pandemic viruses lacks the 11 C‐terminal amino acids, 43 , 45 yet restoration of the PDZ ligand domain motif does not increase the virulence of 2009 H1N1 viruses, 130 suggesting that this motif is not a major determinant of virulence in the genetic background of the 2009 pandemic viruses.
Several studies have assessed the cytokine/chemokine levels induced by 2009 H1N1 viruses; while some reported similar induction of cytokines/chemokines by seasonal and pandemic H1N1 viruses, 131 , 132 one study found higher levels of cytokine/chemokine induction upon infection with 2009 H1N1 viruses 74 ; these differences likely reflect differences in the test systems used. Comparisons of mild and severe cases of 2009 virus infection demonstrate higher levels of proinflammatory cytokine/chemokines in the latter group. 133 , 134 , 135
Role of PB1‐F2 in viral pathogenicity
The PB1‐F2 protein, expressed from the +1 reading frame of the polymerase PB1 gene, induces apoptosis, retains PB1 protein in the nucleus for efficient replication, and increases the frequency and severity of secondary bacterial infections (reviewed in Ref. 136 ). 2009 H1N1 viruses encode a PB1‐F2 peptide of only 11 amino acids, 43 , 45 because of stop codons in the open reading frame. Reverse genetics allowed the generation of pandemic viruses with full‐length PB1‐F2 proteins. 137 In mice and ferrets, these variants were comparable in their virulence to wild‐type virus encoding a PB1‐F2 peptide of 11 amino acids, 137 suggesting that PB1‐F2 is not a major factor in the virulence and pathogenicity of 2009 H1N1 viruses.
Antiviral compounds
As vaccine development and production typically takes more than 3 months, compounds with antiviral activity are the first line of defense to newly emerging viruses. Two classes of antiviral compounds are available to treat influenza virus infections: (a) amino adamantanes, such as amantadine hydrochloride and rimantadine, that block the ion channel formed by the viral M2 protein and (b) inhibitors of the viral NA activity, such as oseltamivir and zanamivir. The new pandemic viruses are resistant to ion channel inhibitors, 7 , 8 , 138 because of a Ser‐to‐Ala mutation at position 31 of the M2 protein, 8 , 43 , 45 which is known to confer resistance to amino adamantanes. However, 2009 H1N1 viruses are sensitive to NA inhibitors, 8 , 138 although oseltamivir‐resistant viruses have now been reported, both in oseltamivir‐treated and oseltamivir‐untreated individuals. 139 , 140 , 141 , 142 , 143 , 144 These viruses possess a histidine‐to‐tyrosine mutation at position 275 of their NA protein (N1 numbering), a known source of oseltamivir resistance. Currently, oseltamivir‐resistant 2009 H1N1 viruses do not appear to transmit efficiently among humans, although isolated cases of transmission have been reported. 143 , 144 In contrast to 2009 H1N1 viruses, almost all seasonal H1N1 viruses are now resistant to oseltamivir. Concern therefore exists that the pandemic viruses may acquire this trait through mutation. Zanamivir 145 and peramivir (a NA inhibitor in Phase III clinical trials that recently received ‘Emergency Use Authorization’) 146 would remain as treatment options.
Vaccines
The antigenic differences and the resulting lack of serum cross‐reactivity between seasonal and pandemic H1N1 2009 viruses in younger people 43 , 45 , 74 , 93 , 104 , 105 , 106 , 107 , 108 , 109 (see also ‘HA Antigenicity’) necessitated the development of a new vaccine to the pandemic viruses. Based on antigenic, genetic, and phylogenetic characterization, the WHO recommended on May 26, 2009, that vaccines contain an A/California/07/2009‐like virus (http://www.who.int/csr/resources/publications/swineflu/H1N1Vaccinevirusrecommendation26May2009.pdf). Several candidate vaccines were soon developed in which (at least) the HA and NA genes of A/California/07/2009 virus were combined with the remaining genes of A/Puerto Rico/8/34 (H1N1) virus, the virus commonly used for human influenza vaccine production 147 (http://www.who.int/csr/resources/publications/swineflu/summary_candidate_vaccine.pdf). Reassortants were generated by classic reassortment techniques or reverse genetics. Early findings of poor growth of the vaccine candidates (http://www.thelancet.com/H1N1‐flu/egmn/0c03c805) and the potential need for two doses 148 , 149 sparked concerns over a vaccine shortage. Antigen‐sparing strategies, such as the use of adjuvants, were therefore considered. Clinical trials tested one‐ and two‐dose regimens of different amounts of adjuvanted or non‐adjuvanted split‐virion vaccines, or adjuvanted whole virion vaccine. 147 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 These studies demonstrated high seroconversion rates with hemagglutination inhibition titers of 1:40 or more [which are considered protective (http://www.fda.gov/cber/gdlns/panfluvac.htm)] in healthy adults after a single dose of vaccine. The strong immune responses induced in adults by a single dose of 2009 H1N1 vaccine suggested a certain level of pre‐existing immunity that cross‐reacts with the 2009 pandemic virus. By contrast, seroconversion rates were generally lower in children, 155 , 156 , 157 , 158 suggesting that two doses may be necessary. A clinical study also demonstrated that the vaccines to pandemic and seasonal influenza viruses can be administered simultaneously without negatively affecting the efficacy of either. 154
The first vaccines against 2009 H1N1 viruses were approved in September of 2009 159 , 160 , 161 (http://www.tga.gov.au/alerts/medicines/h1n1vaccine.htm) (http://www.ema.europa.eu/influenza/vaccines/home.htm) (http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm182399.htm). In the United States, non‐adjuvanted, inactivated split‐virus or subunit vaccines for intramuscular injection, or a live attenuated vaccine for intranasal administration was approved with one dose for adults or two doses for children between the age of 6 months and 9 years. In Europe, only inactivated vaccines were approved and two doses are recommended with one exception, these vaccines are adjuvanted 161 (http://www.ema.europa.eu/influenza/vaccines/home.htm). In Australia, an inactivated split‐virus vaccine was approved for use in adults and children 10 years of age and older (http://www.tga.gov.au/alerts/medicines/h1n1vaccine.htm).
Vaccine safety has been monitored closely through the Vaccine Adverse Event Reporting System (VAERS). As of April 30, 2010, the percentage of serious adverse reactions was similar to that observed with seasonal influenza vaccines (http://vaers.hhs.gov/resources/2010H1N1Summary_May07.pdf). Particular focus has been on cases of Guillain–Barré syndrome (GBS), a neurologic disease that occurred at a higher incidence with the A/New Jersey/1/76 (H1N1) vaccine and contributed to the cessation of that vaccination program. Currently, there is no indication that the 2009 H1N1 vaccines elevated the number of GBS cases when compared to seasonal influenza vaccines (http://vaers.hhs.gov/resources/2010H1N1Summary_May07.pdf).
Outlook
Since their appearance in the spring of 2009, the 2009 H1N1 viruses have largely replaced seasonal H1N1 viruses. This fact was anticipated by WHO and has been recognized with the recent recommendation by the WHO to replace seasonal H1N1 viruses with pandemic H1N1 viruses in the 2010 (Southern Hemisphere; http://www.who.int/csr/disease/influenza/vaccinerecommendations1/en/index.html) and 2010/2011 (Northern Hemisphere) vaccine formulations. 16 The pandemic of 2009 tested our ability to react to outbreaks of novel influenza viruses that spread efficiently in human populations. Fortunately, the 2009 H1N1 virus was not as pathogenic as originally feared, and low levels of immunity primed by seasonal H1N1 infection provided protection with a single dose of vaccine. The need for two doses, together with the fact that almost all influenza vaccines are still produced in embryonated chicken eggs (a production system that is difficult to scale up rapidly), would have resulted in severe vaccine shortages. Future efforts should therefore be directed toward further evaluation of antigen‐sparing strategies and alternative methods of influenza vaccine production such as cell culture.
Acknowledgements
We thank Susan Watson for editing the manuscript. Original work was supported by National Institute of Allergy and Infectious Diseases Public Health Service research grants, by an NIAID‐funded Center for Research on Influenza Pathogenesis (CRIP, HHSN266200700010C), by Grant‐in‐Aid for Specially Promoted Research, by a contract research fund for the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, by grants‐in‐aid from the Ministry of Health, Japan, and by ERATO (Japan Science and Technology Agency).
References
- 1. Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication; in Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B. et al. (eds). Fields Virology, 5th edn Philadelphia, Wolters Kluwer: Lippincott Williams & Wilkins, 2007; 1647–1689. [Google Scholar]
- 2. Wright PF, Neumann G, Kawaoka Y. Orthomyxoviuses; in Knipe DM, Howley PM, Griffin DE. et al. (eds): Fields Virology, 5th edn Philadelphia: Wolters Kluwer: Lippincott Williams & Wilkins, 2007; 1727–1740. [Google Scholar]
- 3. Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine‐origin H1N1 influenza virus. Nature 2009; 459:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peiris JS, Poon LL, Guan Y. Emergence of a novel swine‐origin influenza A virus (S‐OIV) H1N1 virus in humans. J Clin Virol 2009; 45:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peiris JS, Tu WW, Yen HL. A novel H1N1 virus causes the first pandemic of the 21st century. Eur J Immunol 2009; 39:2946–2954. [DOI] [PubMed] [Google Scholar]
- 6. Brockwell‐Staats C, Webster RG, Webby RJ. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1). Influenza Other Respi Viruses 2009; 3:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swine influenza A (H1N1) infection in two children‐Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep 2009;58:400–402. [PubMed] [Google Scholar]
- 8. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 9. Cohen J, Enserink M. Infectious diseases. As swine flu circles globe, scientists grapple with basic questions. Science 2009; 324:572–573. [DOI] [PubMed] [Google Scholar]
- 10. Outbreak of swine‐origin influenza A (H1N1) virus infection – Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep 2009;58:467–470. [PubMed] [Google Scholar]
- 11. Cohen J. Swine flu outbreak. Out of Mexico? Scientists ponder swine flu’s origins. Science 2009; 324:700–702. [DOI] [PubMed] [Google Scholar]
- 12. Update: novel influenza A (H1N1) virus infection – Mexico, March–May, 2009. MMWR Morb Mortal Wkly Rep 2009;58:585–589. [PMC free article] [PubMed] [Google Scholar]
- 13. Update: swine influenza A (H1N1) infections – California and Texas, April 2009. MMWR Morb Mortal Wkly Rep 2009;58(16):435–437. [PubMed] [Google Scholar]
- 14. Zarocostas J. World Health Organization declares A (H1N1) influenza pandemic. BMJ 2009; 338:b2425. [DOI] [PubMed] [Google Scholar]
- 15. Cohen J, Enserink M. Swine flu. After delays, WHO agrees: the 2009 pandemic has begun. Science 2009; 324:1496–1497. [DOI] [PubMed] [Google Scholar]
- 16. Update: influenza activity – United States, August 30, 2009–March 27, 2010, and composition of the 2010–11 influenza vaccine. MMWR Morb Mortal Wkly Rep 2010;59:423–430. [PubMed] [Google Scholar]
- 17. Fraser C, Donnelly CA, Cauchemez S et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009; 324:1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Y, Sugimoto JD, Halloran ME et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science 2009; 326:729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Echevarria‐Zuno S, Mejia‐Arangure JM, Mar‐Obeso AJ et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet 2009; 374:2072–2079. [DOI] [PubMed] [Google Scholar]
- 20. Chowell G, Bertozzi SM, Colchero MA et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679. [DOI] [PubMed] [Google Scholar]
- 21. Presanis AM, De Angelis D, Hagy A et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med 2009; 6:e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec 2009;84:185–189. [PubMed] [Google Scholar]
- 23. Presanis AM, Lipsitch M, Daniela De A et al. The severity of pandemic H1N1 influenza in the United States, April–July 2009. PLoS Curr 2009:RRN1042, e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Update: novel influenza A (H1N1) virus infections – worldwide, May 6, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:453–458. [PubMed] [Google Scholar]
- 25. Hospitalized patients with novel influenza A (H1N1) virus infection – California, April–May, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:536–541. [PubMed] [Google Scholar]
- 26. Shinde V, Bridges CB, Uyeki TM et al. Triple‐reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009; 360:2616–2625. [DOI] [PubMed] [Google Scholar]
- 27. Human infection with pandemic A (H1N1) 2009 influenza virus: clinical observations in hospitalized patients, Americas, July 2009 – update. Wkly Epidemiol Rec 2009;84:305–308. [PubMed] [Google Scholar]
- 28. Perez‐Padilla R, de la Rosa‐Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–689. [DOI] [PubMed] [Google Scholar]
- 29. Cao B, Li XW, Mao Y et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med 2009; 361:2507–2517. [DOI] [PubMed] [Google Scholar]
- 30. Zarocostas J. H1N1 pandemic flu found to cause viral pneumonia in severe cases, says WHO. BMJ 2009; 339:b4313. [DOI] [PubMed] [Google Scholar]
- 31. Soto‐Abraham MV, Soriano‐Rosas J, Diaz‐Quinonez A et al. Pathological changes associated with the 2009 H1N1 virus. N Engl J Med 2009; 361:2001–2003. [DOI] [PubMed] [Google Scholar]
- 32. Tran TH, Nguyen TL, Nguyen TD et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 2004; 350:1179–1188. [DOI] [PubMed] [Google Scholar]
- 33. Beigel JH, Farrar J, Han AM et al. Avian influenza A (H5N1) infection in humans. N Engl J Med 2005; 353:1374–1385. [DOI] [PubMed] [Google Scholar]
- 34. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis 2005; 11:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) – United States, May–August 2009. MMWR Morb Mortal Wkly Rep 2009;58:1071–1074. [PubMed] [Google Scholar]
- 36. Palacios G, Hornig M, Cisterna D et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE 2009; 4:e8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lockman JL, Fischer WA, Perl TM, Valsamakis A, Nichols DG. The critically ill child with novel H1N1 influenza A: a case series. Pediatr Crit Care Med 2010; 11:173–178. [DOI] [PubMed] [Google Scholar]
- 38. Jamieson DJ, Honein MA, Rasmussen SA et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451–458. [DOI] [PubMed] [Google Scholar]
- 39. Baker M, Kelly H, Wilson N. Pandemic H1N1 influenza lessons from the southern hemisphere. Euro Surveill 2009; 14:19370. [DOI] [PubMed] [Google Scholar]
- 40. Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010; 362:27–35. [DOI] [PubMed] [Google Scholar]
- 41. Novel influenza A (H1N1) virus infections in three pregnant women – United States, April–May 2009. MMWR Morb Mortal Wkly Rep 2009;58:497–500. [PubMed] [Google Scholar]
- 42. Zarychanski R, Stuart TL, Kumar A et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 2010; 182:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garten RJ, Davis CT, Russell CA et al. Antigenic and genetic characteristics of swine‐origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trifonov V, Khiabanian H, Rabadan R. Geographic dependence, surveillance, and origins of the 2009 influenza A (H1N1) virus. N Engl J Med 2009; 361:115–119. [DOI] [PubMed] [Google Scholar]
- 45. Smith GJ, Vijaykrishna D, Bahl J et al. Origins and evolutionary genomics of the 2009 swine‐origin H1N1 influenza A epidemic. Nature 2009; 459:1122–1125. [DOI] [PubMed] [Google Scholar]
- 46. Olsen CW. The emergence of novel swine influenza viruses in North America. Virus Res 2002; 85:199–210. [DOI] [PubMed] [Google Scholar]
- 47. Kida H, Ito T, Yasuda J et al. Potential for transmission of avian influenza viruses to pigs. J Gen Virol 1994; 75:2183–2188. [DOI] [PubMed] [Google Scholar]
- 48. Scholtissek C, Burger H, Kistner O, Shortridge KF. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 1985; 147:287–294. [DOI] [PubMed] [Google Scholar]
- 49. Hodder RA, Gaydos JC, Allen RG, Top FH Jr, Nowosiwsky T, Russell PK. Swine influenza A at Fort Dix, New Jersey (January–February 1976). III. Extent of spread and duration of the outbreak. J Infect Dis 1977; 136(Suppl):S369–S375. [DOI] [PubMed] [Google Scholar]
- 50. Gaydos JC, Hodder RA, Top FH Jr et al. Swine influenza A at Fort Dix, New Jersey (January–February 1976). II. Transmission and morbidity in units with cases. J Infect Dis 1977; 136(Suppl):S363–S368. [DOI] [PubMed] [Google Scholar]
- 51. Gaydos JC, Hodder RA, Top FH Jr et al. Swine influenza A at Fort Dix, New Jersey (January–February 1976). I. Case finding and clinical study of cases. J Infect Dis 1977; 136(Suppl):S356–S362. [DOI] [PubMed] [Google Scholar]
- 52. Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dacso CC, Couch RB, Six HR, Young JF, Quarles JM, Kasel JA. Sporadic occurrence of zoonotic swine influenza virus infections. J Clin Microbiol 1984; 20:833–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wentworth DE, Thompson BL, Xu X et al. An influenza A (H1N1) virus, closely related to swine influenza virus, responsible for a fatal case of human influenza. J Virol 1994; 68:2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bastien N, Antonishyn NA, Brandt K et al. Human infection with a triple‐reassortant swine influenza A(H1N1) virus containing the hemagglutinin and neuraminidase genes of seasonal influenza virus. J Infect Dis 2010; 201:1178–1182. [DOI] [PubMed] [Google Scholar]
- 56. Van Reeth K, Nicoll A. A human case of swine influenza virus infection in Europe – implications for human health and research. Euro Surveill 2009;14:19124. [PubMed] [Google Scholar]
- 57. Cohen J. Swine flue outbreak. Flu researchers train sights on novel tricks of novel H1N1. Science 2009; 324:870–871. [DOI] [PubMed] [Google Scholar]
- 58. Rambaut A, Holmes E. The early molecular epidemiology of the swine‐origin A/H1N1 human influenza pandemic. PLoS Curr 2009:RRN1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ding N, Wu N, Xu Q, Chen K, Zhang C. Molecular evolution of novel swine‐origin A/H1N1 influenza viruses among and before human. Virus Genes 2009; 39:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Solovyov A, Greenbaum B, Palacios G, Lipkin WI, Rabadan R. Host dependent evolutionary patterns and the origin of 2009 H1N1 pandemic influenza: Alexander Solovyov*, Benjamin Greenbaum*, Gustavo Palacios, W. Ian Lipkin and Raul Rabadan (*Joint First Authors). PLoS Curr 2010:RRN1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lange E, Kalthoff D, Blohm U et al. Pathogenesis and transmission of the novel swine‐origin influenza virus A/H1N1 after experimental infection of pigs. J Gen Virol 2009; 90:2119–2123. [DOI] [PubMed] [Google Scholar]
- 62. Brookes SM, Irvine RM, Nunez A et al. Influenza A (H1N1) infection in pigs. Vet Rec 2009; 164:760–761. [DOI] [PubMed] [Google Scholar]
- 63. Weingartl HM, Berhane Y, Hisanaga T et al. Genetic and pathobiologic characterization of pandemic H1N1 2009 influenza viruses from a naturally infected swine herd. J Virol 2010; 84:2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vincent AL, Lager KM, Faaberg KS et al. Experimental inoculation of pigs with pandemic H1N1 2009 virus and HI cross‐reactivity with contemporary swine influenza virus antisera. Influenza Other Respi Viruses 2010; 4:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brookes SM, Nunez A, Choudhury B et al. Replication, pathogenesis and transmission of pandemic (H1N1) 2009 virus in non‐immune pigs. PLoS ONE 2010; 5:e9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hofshagen M, Gjerset B, Er C et al. Pandemic influenza A(H1N1)v: human to pig transmission in Norway? Euro Surveill 2009;14:19406. [DOI] [PubMed] [Google Scholar]
- 67. Howden KJ, Brockhoff EJ, Caya FD et al. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can Vet J 2009; 50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- 68. Pasma T, Joseph T. Pandemic (H1N1) 2009 infection in swine herds, Manitoba, Canada. Emerg Infect Dis 2010; 16:706–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Babiuk S, Albrecht R, Berhane Y et al. 1918 and 2009 H1N1 influenza viruses are not pathogenic in birds. J Gen Virol 2010; 2:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Swayne DE, Pantin‐Jackwood M, Kapczynski D, Spackman E, Suarez DL. Susceptibility of poultry to pandemic (H1N1) 2009 virus. Emerg Infect Dis 2009; 15:2061–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Terregino C, De Nardi R, Nisi R et al. Resistance of turkeys to experimental infection with an early 2009 Italian human influenza A(H1N1)v virus isolate. Euro Surveill 2009; 14:19360. [PubMed] [Google Scholar]
- 72. Ilyushina NA, Kim JK, Negovetich NJ et al. Extensive mammalian ancestry of pandemic (H1N1) 2009 virus. Emerg Infect Dis 2010; 16:314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mathieu C, Moreno V, Retamal P et al. Pandemic (H1N1) 2009 in Breeding Turkeys, Valparaiso, Chile. Emerg Infect Dis 2010; 16:709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Itoh Y, Shinya K, Kiso M et al. In vitro and in vivo characterization of new swine‐origin H1N1 influenza viruses. Nature 2009; 460:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maines TR, Jayaraman A, Belser JA et al. Transmission and pathogenesis of swine‐origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 2009; 325:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Munster VJ, de Wit E, van den Brand JM et al. Pathogenesis and transmission of swine‐origin 2009 A(H1N1) influenza virus in ferrets. Science 2009; 325:481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Perez DR, Sorrell E, Angel M et al. Fitness of pandemic H1N1 and seasonal influenza A viruses during co‐infection: evidence of competitive advantage of pandemic H1N1 influenza versus seasonal influenza. PLoS Curr 2009:RRN1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van den Brand JM, Stittelaar KJ, van Amerongen G et al. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J Infect Dis 2010; 201:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Belser JA, Wadford DA, Pappas C et al. Pathogenesis of pandemic influenza A (H1N1) and triple‐reassortant swine influenza A (H1) viruses in mice. J Virol 2010; 84:4194–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen GW, Shih SR. Genomic signatures of influenza A pandemic (H1N1) 2009 virus. Emerg Infect Dis 2009; 15:1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Furuse Y, Suzuki A, Oshitani H. Reassortment between swine influenza A viruses increased their adaptation to humans in pandemic H1N1/09. Infect Genet Evol 2010; 10:569–574. [DOI] [PubMed] [Google Scholar]
- 82. Pan C, Cheung B, Tan S et al. Genomic signature and mutation trend analysis of pandemic (H1N1) 2009 influenza A virus. PLoS ONE 2010; 5:e9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Karlas A, Machuy N, Shin Y et al. Genome‐wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 2010; 7282:818–822. [DOI] [PubMed] [Google Scholar]
- 84. Shapira SD, Gat‐Viks I, Shum BO et al. A physical and regulatory map of host‐influenza interactions reveals pathways in H1N1 infection. Cell 2009; 139:1255–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carette JE, Guimaraes CP, Varadarajan M et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science 2009; 326:1231–1235. [DOI] [PubMed] [Google Scholar]
- 86. Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983; 127:361–373. [DOI] [PubMed] [Google Scholar]
- 87. Ito T, Couceiro JN, Kelm S et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 1998; 72:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature 2006; 440:435–436. [DOI] [PubMed] [Google Scholar]
- 89. van Riel D, Munster VJ, de Wit E et al. H5N1 virus attachment to lower respiratory tract. Science 2006; 312:399. [DOI] [PubMed] [Google Scholar]
- 90. Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res 2010; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 2010; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science 2004; 303:1866–1870. [DOI] [PubMed] [Google Scholar]
- 93. Yang H, Carney P, Stevens J Structure and receptor binding properties of a pandemic H1N1 virus hemagglutinin. PLoS Curr 2010:RRN1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Childs RA, Palma AS, Wharton S et al. Receptor‐binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotechnol 2009; 27:797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kilander A, Rykkvin R, Dudman SG, Hungnes O Observed association between the HA1 mutation D222G in the 2009 pandemic influenza A(H1N1) virus and severe clinical outcome, Norway 2009–2010. Euro Surveill 2010;15(9). [DOI] [PubMed] [Google Scholar]
- 96. Mak GC, Au KW, Tai LS et al. Association of D222G substitution in haemagglutinin of 2009 pandemic influenza A (H1N1) with severe disease. Euro Surveill 2010;15:19534. [PubMed] [Google Scholar]
- 97. Glinsky GV. Genomic analysis of pandemic (H1N1) 2009 reveals association of increasing disease severity with emergence of novel hemagglutinin mutations. Cell Cycle 2010; 9:958–970. [DOI] [PubMed] [Google Scholar]
- 98. Potdar VA, Chadha MS, Jadhav SM, Mullick J, Cherian SS, Mishra AC. Genetic characterization of the influenza A pandemic (H1N1) 2009 virus isolates from India. PLoS ONE 2010; 5:e9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Miller RR, MacLean AR, Gunson RN, Carman WF. Occurrence of haemagglutinin mutation D222G in pandemic influenza A(H1N1) infected patients in the West of Scotland, United Kingdom, 2009–10. Euro Surveill 2010; 15:19546. [PubMed] [Google Scholar]
- 100. Preliminary review of D222G amino acid substitution in the haemagglutinin of pandemic influenza A (H1N1) 2009 viruses. Wkly Epidemiol Rec 2010;85:21–22. [PubMed] [Google Scholar]
- 101. Anton A, Marcos MA, Martinez MJ et al. D225G mutation in the hemagglutinin protein found in 3 severe cases of 2009 pandemic influenza A (H1N1) in Spain. Diagn Microbiol Infect Dis 2010; 67:207–208. [DOI] [PubMed] [Google Scholar]
- 102. Chen H, Wen X, To KK et al. Quasispecies of the D225G substitution in the hemagglutinin of pandemic influenza A(H1N1) 2009 virus from patients with severe disease in Hong Kong, China. J Infect Dis 2010; 201:1517–1521. [DOI] [PubMed] [Google Scholar]
- 103. Puzelli S, Facchini M, Spagnolo D et al. Transmission of hemagglutinin D222G mutant strain of pandemic (H1N1) 2009 virus. Emerg Infect Dis 2010; 16:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Igarashi M, Ito K, Yoshida R, Tomabechi D, Kida H, Takada A. Predicting the antigenic structure of the pandemic (H1N1) 2009 influenza virus hemagglutinin. PLoS ONE 2010; 5:e8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE Jr, Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010; 328:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Greenbaum JA, Kotturi MF, Kim Y et al. Pre‐existing immunity against swine‐origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A 2009; 106:20365–20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Krause JC, Tumpey TM, Huffman CJ et al. Naturally occurring human monoclonal antibodies neutralize both 1918 and 2009 pandemic influenza A (H1N1) viruses. J Virol 2010; 84:3127–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Manicassamy B, Medina RA, Hai R et al. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918‐like and classical swine H1N1 based vaccines. PLoS Pathog 2010; 6:e1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wei CJ, Boyington JC, Dai K et al. Cross‐neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci Transl Med 2010; 2:24ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hancock K, Veguilla V, Lu X et al. Cross‐reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–1952. [DOI] [PubMed] [Google Scholar]
- 111. Ikonen N, Strengell M, Kinnunen L et al. High frequency of cross‐reacting antibodies against 2009 pandemic influenza A(H1N1) virus among the elderly in Finland. Euro Surveill 2010;15:19478. [PubMed] [Google Scholar]
- 112. Serum cross‐reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep 2009;58:521–524. [PubMed] [Google Scholar]
- 113. Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 1982; 31:417–427. [DOI] [PubMed] [Google Scholar]
- 114. Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 1981; 290:713–717. [DOI] [PubMed] [Google Scholar]
- 115. Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001; 293:1840–1842. [DOI] [PubMed] [Google Scholar]
- 116. Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol 1993; 67:1761–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Herfst S, Chutinimitkul S, Ye J et al. Introduction of virulence markers in PB2 of pandemic swine‐origin influenza virus does not result in enhanced virulence or transmission. J Virol 2010; 84:3752–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhu H, Wang J, Wang P et al. Substitution of lysine at 627 position in PB2 protein does not change virulence of the 2009 pandemic H1N1 virus in mice. Virology 2010; 401:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mehle A, Doudna JA. Adaptive strategies of the influenza virus polymerase for replication in humans. Proc Natl Acad Sci U S A 2009; 106:21312–21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yamada S, Hatta M, Staker B et al. Biological and structural characterization of a host‐adapting amino acid in influenza virus. PLoS Pathog, 2010; 5: e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mehle A, Doudna JA. An inhibitory activity in human cells restricts the function of an avian‐like influenza virus polymerase. Cell Host Microbe 2008; 4:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li Z, Chen H, Jiao P et al. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J Virol 2005; 79:12058–12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bussey KA, Bousse TL, Desmet EA, Kim B, Takimoto T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J Virol 2010; 84:4395–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Garcia‐Sastre A, Egorov A, Matassov D et al. Influenza A virus lacking the NS1 gene replicates in interferon‐deficient systems. Virology 1998; 252:324–330. [DOI] [PubMed] [Google Scholar]
- 125. Krug RM, Yuan W, Noah DL, Latham AG. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology 2003; 309:181–189. [DOI] [PubMed] [Google Scholar]
- 126. Hale BG, Randall RE, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 2008; 89:2359–2376. [DOI] [PubMed] [Google Scholar]
- 127. Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti‐viral cytokine responses. Nat Med 2002; 8:950–954. [DOI] [PubMed] [Google Scholar]
- 128. Obenauer JC, Denson J, Mehta PK et al. Large‐scale sequence analysis of avian influenza isolates. Science 2006; 311:1576–1580. [DOI] [PubMed] [Google Scholar]
- 129. Jackson D, Hossain MJ, Hickman D, Perez DR, Lamb RA. A new influenza virus virulence determinant: the NS1 protein four C‐terminal residues modulate pathogenicity. Proc Natl Acad Sci U S A 2008; 105:4381–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hale BG, Steel J, Manicassamy B et al. Mutations in the NS1 C‐terminal tail do not enhance replication or virulence of the 2009 pandemic H1N1 influenza A virus. J Gen Virol 2010; 91:1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Woo PC, Tung ET, Chan KH, Lau CC, Lau SK, Yuen KY. Cytokine profiles induced by the novel swine‐origin influenza A/H1N1 virus: implications for treatment strategies. J Infect Dis 2010; 201:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chan MC, Chan RW, Yu WC et al. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol 2010; 176:1828–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. To KK, Hung IF, Li IW et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 2010; 50:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. de Castro IF, Guzman‐Fulgencio M, Garcia‐Alvarez M, Resino S. First evidence of a pro‐inflammatory response to severe infection with influenza virus H1N1. Crit Care 2010; 14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bermejo‐Martin JF, Ortiz de Lejarazu R, Pumarola T et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care 2009; 13:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Conenello GM, Palese P. Influenza A virus PB1‐F2: a small protein with a big punch. Cell Host Microbe 2007; 2:207–209. [DOI] [PubMed] [Google Scholar]
- 137. Hai R, Schmolke M, Varga ZT et al. PB1‐F2 expression by the 2009 pandemic H1N1 influenza virus has minimal impact on virulence in animal models. J Virol 2010; 84:4442–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Update: drug susceptibility of swine‐origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep 2009;58:433–435. [PubMed] [Google Scholar]
- 139. Oseltamivir‐resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients – Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep 2009;58:893–896. [PubMed] [Google Scholar]
- 140. Leung TW, Tai AL, Cheng PK, Kong MS, Lim W. Detection of an oseltamivir‐resistant pandemic influenza A/H1N1 virus in Hong Kong. J Clin Virol 2009; 46:298–299. [DOI] [PubMed] [Google Scholar]
- 141. Chen H, Cheung CL, Tai H et al. Oseltamivir‐resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg Infect Dis 2009; 15:1970–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Baz M, Abed Y, Papenburg J, Bouhy X, Hamelin ME, Boivin G. Emergence of oseltamivir‐resistant pandemic H1N1 virus during prophylaxis. N Engl J Med 2009; 361:2296–2297. [DOI] [PubMed] [Google Scholar]
- 143. Gulland A. First cases of spread of oseltamivir resistant swine flu between patients are reported in Wales. BMJ 2009; 339:b4975. [DOI] [PubMed] [Google Scholar]
- 144. Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P. A community cluster of oseltamivir‐resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87. [DOI] [PubMed] [Google Scholar]
- 145. Gaur AH, Bagga B, Barman S et al. Intravenous zanamivir for oseltamivir‐resistant 2009 H1N1 influenza. N Engl J Med 2010; 362:88–89. [DOI] [PubMed] [Google Scholar]
- 146. Birnkrant D, Cox E. The Emergency Use Authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med 2009; 361:2204–2207. [DOI] [PubMed] [Google Scholar]
- 147. Valdespino‐Gomez JL, Garcia‐Garcia L, de Leon‐Rosales SP. Vaccines against influenza A (H1N1) pandemic. Arch Med Res 2009; 40:693–704. [DOI] [PubMed] [Google Scholar]
- 148. Dormitzer PR, Rappuoli R, Casini D et al. Adjuvant is necessary for a robust immune response to a single dose of H1N1 pandemic flu vaccine in mice. PLoS Curr. 2009:RRN1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kobinger GP, Meunier I, Patel A et al. Assessment of the efficacy of commercially available and candidate vaccines against a pandemic H1N1 2009 virus. J Infect Dis 2010; 201:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Willyard C. Pandemic vaccine enters clinical trials. Nat Med 2009; 15:978. [DOI] [PubMed] [Google Scholar]
- 151. Greenberg ME, Lai MH, Hartel GF et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med 2009; 361:2405–2413. [DOI] [PubMed] [Google Scholar]
- 152. Clark TW, Pareek M, Hoschler K et al. Trial of 2009 influenza A (H1N1) monovalent MF59‐adjuvanted vaccine. N Engl J Med 2009; 361:2424–2435. [DOI] [PubMed] [Google Scholar]
- 153. Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A‐adjuvant: preliminary report of an observer‐blind, randomised trial. Vaccine 2010; 28:1740–1745. [DOI] [PubMed] [Google Scholar]
- 154. Vajo Z, Tamas F, Sinka L, Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–10 influenza season: a multicentre, randomised controlled trial. Lancet 2010; 375:49–55. [DOI] [PubMed] [Google Scholar]
- 155. Liang XF, Wang HQ, Wang JZ et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet 2010; 375:56–66. [DOI] [PubMed] [Google Scholar]
- 156. Plennevaux E, Sheldon E, Blatter M, Reeves‐Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2010; 375:41–48. [DOI] [PubMed] [Google Scholar]
- 157. Zhu FC, Wang H, Fang HH et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 2009; 361:2414–2423. [DOI] [PubMed] [Google Scholar]
- 158. Nolan T, McVernon J, Skeljo M et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 2010; 303:37–46. [DOI] [PubMed] [Google Scholar]
- 159. Safety of influenza A (H1N1) 2009 monovalent vaccines – United States, October 1–November 24, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1351–1356. [PubMed] [Google Scholar]
- 160. Update on influenza A (H1N1) 2009 monovalent vaccines. MMWR Morb Mortal Wkly Rep. 2009; 58:1100–1101. [PubMed] [Google Scholar]
- 161. Johansen K, Nicoll A, Ciancio BC, Kramarz P. Pandemic influenza A(H1N1) 2009 vaccines in the European Union. Euro Surveill 2009; 14:19361. [PubMed] [Google Scholar]


