Abstract
Attentional dysfunction is one of the most consistent findings in individuals with autism spectrum disorders (ASD). However, the significance of such findings for the pathophysiology of autism is unclear. In this study, we investigated cellular neurochemistry with proton magnetic resonance spectroscopy imaging (1H-MRS) in brain regions associated with networks subserving alerting, orienting, and executive control of attention in patients with ASD. Concentrations of cerebral N-acetyl-aspartate (NAA), creatinine + phosphocreatinine, choline-containing compounds, myo-inositol (Ins) and glutamate + glutamine (Glx) were determined by 3 T 1H-MRS examinations in 14 high-functioning medication-free adults with a diagnosis of ASD and 14 age- and IQ-matched healthy controls (HC) in the anterior cingulate cortex (ACC), thalamus, temporoparietal junction (TPJ), and areas near or along the intraparietal sulcus (IPS). Compared to HC group, the ASD group showed significantly lower Glx concentrations in right ACC and reduced Ins in left TPJ. This study provides evidence of abnormalities in neurotransmission related to networks subserving executive control and alerting of attention, functions which have been previously implicated in ASD pathogenesis.
Keywords: autism, spectroscopy, glutamate, anterior cingulate cortex, intraparietal sulcus, myo-inositol
1. Introduction
The National Survey of Children's Health reports that autism spectrum disorders (ASD) affect as many as 673,000 children in the United States, with a corresponding cross-sectional prevalence of 1.1% of individuals aged 3 to 17 years (Kogan et al. 2009). ASD have mainly been diagnosed among children and adolescents. However, long-term prospective studies have also shown high diagnostic stability in adulthood (Billstedt et al. 2005;Cederlund et al. 2008). Although some studies showed that cognitive abilities and social interaction skills improve with age (McGovern and Sigman 2005;Sigman et al. 1999), core symptoms of ASD include social deficits, communication abnormalities, and repetitive and stereotyped behaviors with restricted focus (Volkmar and Pauls 2003) in children as well as in adults (Hofvander et al. 2009). The neuropsychology underlying the constellation of ASD symptoms is still debated. Theories suggest contributions of attentional deficits to the development of social communication problems (Gold and Gold 1975;Kanner 1968). Molecular, cellular, and anatomical observations provide insight into the neuropsychology and bases for the conceptual understanding of ASD. Despite the rising number of investigations, there are still critical gaps in knowledge about the neurobiology of ASD (Jarbrink and Knapp 2001).
Functional brain imaging, neurochemistry, and clinical pharmacological studies have implicated impairment in the regulation of the inhibitory/excitatory balance in ASD pathogenesis (Dolen and Bear 2008;Rippon et al. 2007) and led to hypotheses of deficiencies in glutamatergic transmission (Carlsson 1998). For example, the efficacy of combined dopamine receptor 2 (D2) and serotonin receptor 2A (5-HT2A) antagonists (e.g., risperidone) in the treatment of autism has been attributed to D2-mediated glutamate release (Laruelle et al. 2005) and through indirect inhibition of GABA interneurons occurring via 5-HT2A blockade (Carlsson 1998;Carlsson et al. 1999). Other authors have suggested an hyperglutamatergic state in ASD (Blaylock and Strunecka 2009), on the basis of clinical observations and open-label studies suggesting the possible efficacy of N-methyl-d-aspartic acid (NMDA) glutamate receptor antagonists in ASD (Chez et al. 2007;Erickson and Chambers 2006;Niederhofer 2007). Evidence from postmortem (Fatemi et al. 2002;Fatemi et al. 2009;Fatemi et al. 2009;Purcell et al. 2001), in vivo (Aldred et al. 2003;Shinohe et al. 2006), and genetic studies (Jamain et al. 2002;Ramoz et al. 2004;Segurado et al. 2005) also suggest an imbalance of excitatory/inhibitory transmission in ASD.
Proton magnetic resonance spectroscopy (1H-MRS) is a brain imaging technique that permits non-invasive quantification of endogenous brain chemistry and examination of regional cellular activity and function in living subjects. Most of the 1H-MRS studies in autism have been conducted in children and have observed widespread and localized reduction in N-acetyl-aspartate (NAA) concentration (Chugani et al. 1999;DeVito et al. 2007;Endo et al. 2007;Friedman et al. 2003;Friedman et al. 2006;Hisaoka et al. 2001;Kleinhans et al. 2007;Otsuka et al. 1999). NAA concentration is considered a measure of neural density and mitochondrial function (Clark 1998). Reductions in choline-containing compounds (Cho), considered a measure of phosphate membrane turnover, have also been observed in the temporal lobe, the anterior cingulate cortex, and thalamus, while increased Cho concentrations have been reported in the frontal lobes and head of the caudate nucleus in patients with ASD (Friedman et al. 2003;Levitt et al. 2003).
To date, few studies have investigated glutamate concentrations in ASD. In conventional spin echo spectroscopy at 1.5 T, the resonances at 2.35 ppm are mostly assigned to a mixture of Glu, glutamine, and GABA, designated as Glx. No significant differences in Glx levels were identified in the thalamus or elsewhere in the white and gray matter by 1.5 T 1H-MRS comparing children and adolescents with ASD to healthy controls (HC) (Friedman et al. 2006;Hardan et al. 2008). However, in adults with ASD, Glx concentrations were found to be increased in the amygdalohippocampal region (Page et al. 2006). Because of their complex multiplet shapes and overlapping frequency response, accurate measurement of Glx peaks is challenging on a 1.5 T scanner, whereas the interference of the GABA to Glu signal is reduced at 3 T (Schubert et al. 2004). To our knowledge, only one study analyzed Glx with 3 T resolution, and this study had a long TE. The results were a widespread Glx reduction in cerebral lobes of male, medicated, children with ASD (DeVito et al. 2007). To our knowledge, there are no prior in vivo studies of Glx concentrations in medication-free adults with ASD, in regions other than the amygdala and hippocampus (Page et al. 2006).
The present study aims to provide novel data on potential regional differences in Glx concentration by sampling regions of the attentional networks in which Glx has not previously been investigated. This study uses a multivoxel 3 T 1H-MRS approach in a sample of medication-free adults with ASD compared to a sample of age- and IQ-matched controls. Based on the theory that higher-level cognitive deficits in autism may develop as a consequence of fundamental abnormalities of the attentional networks, or as compensatory strategies for such abnormalities (Gold and Gold 1975;Kanner 1968), we investigated the three attentional networks of alerting, orienting, and executive control (Fan et al. 2002;Fan and Posner 2004;Fan et al. 2005;Fan et al. 2007;Gu et al. 2008). Differences in concentrations of NAA, Cr, Cho, Ins, and Glx were measured in voxels drawn bilaterally from the grey matter of the thalamus, the temporoparietal junction (TPJ), anterior cingulate cortex (ACC) and the area near/along the intraparietal sulcus (IPS). These areas have been previously implicated in attentional networks (Fan et al. 2002;Fan and Posner 2004;Fan et al. 2005). The thalamus and TPJ are involved in the alerting network of attention (Fan et al. 2005). The IPS is associated with orienting attention and with the interaction between different functions of attention (Wang et al. 2010). Finally, the ACC is considered part of the network mediating executive control (MacDonald, III et al. 2000) and has been implicated in ASD pathophysiology as well as in the glutamate neurotransmission (Cummings 1995;Di Martino et al. 2009;Haznedar et al. 1997;Purcell et al. 2001). We expected to find a core alteration in the ACC glutamatergic projections, which mediates executive control of attention and may be related to top-down control of behaviors such as social novelty discrimination (Harich et al. 2007) and mental perspective taking (Montag et al. 2008).
2. Results
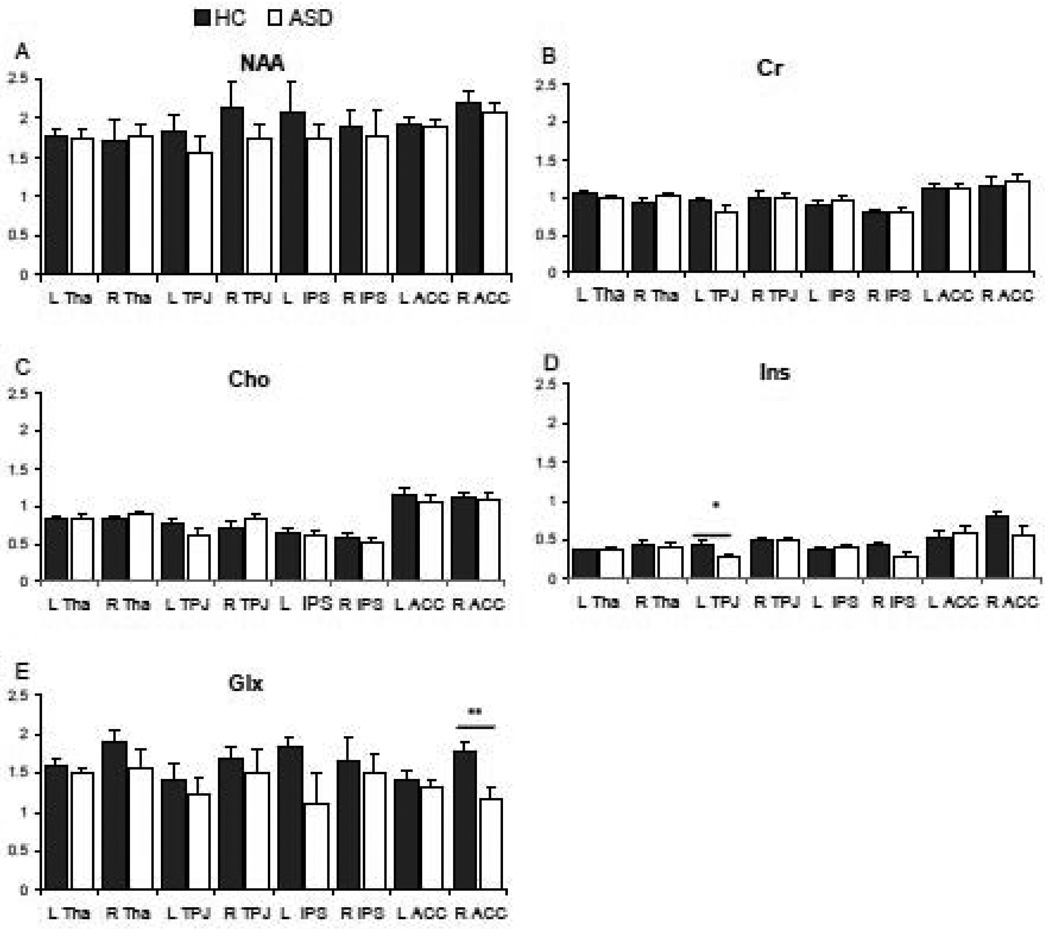
Demographic characteristics of the participating subjects are summarized in Table 1. The groups did not differ significantly in gender or ethnicity. One healthy control was left-handed and two participants with ASD were ambidextrous. The percentage of missing spectra due to exclusion for low fitting was 8.9%, with no differences between conditions. Missing spectra were overrepresented in the right IPS (up to 21.4% of the spectra drawn from the right IPS). The increased difficulty of sampling the IPS provides rationale for the higher localization of missing spectra from this region. Table 2 presents the average metabolite concentrations by region and hemisphere. Linear mixed-model analysis revealed a significant main effect of diagnosis on Glx concentrations (F (1) = 7.23, p < 0.008). The interaction between region, hemisphere and diagnosis was significant on Ins concentrations (F (8) = 2.01, p < 0.047). The main effect of region was also significant for Cr (F (3) = 11.29, p < 0.001), Cho (F (3) = 24.24, p < 0.001). The effect of covariates (IQ and age) was not statistically significant in all cases. Analysis of covariance (ANCOVA) by single region concentrations showed a significant effect of diagnosis in Glx in the right ACC (ASD vs. HC, −33.5%; F (1) = 9.12, p < 0.006) and in Ins concentration in the left TPJ (ASD vs. HC, −38.3%; F (1) = 5.47, p < 0.030) after covariance with a statistical trend toward significance effect of IQ (F (1) = 4.15, p < 0.055) on the Ins model.
Table 1.
Demographic and clinical characteristics of individuals with autism spectrum disorder and healthy controls.
| Measure | ASD (n = 14) |
HC (n = 14) |
Test Statistic |
df | p |
|---|---|---|---|---|---|
| Age (years) | 29.2 (6.1) | 29.7 (8.3) | t = −0.1 | 26 | 0.85 |
| Gender (female/male) | 2/12 | 3/12 | χ2 = 0.24 | 1 | 0.52 |
| Years of education | 15.6 ± 2.2 | 15.8 ± 1.7 | t = −0.20 | 22 | 0.83 |
| Ethnicity (caucasian/afro-american) | 9/5 | 12/2 | χ2 = 1.71 | 1 | 0.19 |
| ASD diagnosis (Autism/Asperger) | 8/6 | - | - | - | - |
| Full Scale IQ | 115 ± 14 | 111 ± 16 | t = −0.70 | 26 | 0.48 |
| Verbal IQ | 116 ± 17 | 120 ± 15 | t = −0.75 | 22 | 0.46 |
| Performance IQ | 112 ± 15 | 116 ± 11 | t = −0.65 | 22 | 0.52 |
| Years of education | 15 ± 2.8 | 15 ± 1.7 | t = −0.20 | 22 | 0.83 |
| ADI-R | 38.4 ± 13.4 | ||||
| Social | 18.8 ± 8.0 | ||||
| Verbal Communication | 12.9 ± 4.0 | ||||
| Repetitive Behavior | 6.7 ± 3.6 | ||||
| ADOS-G | 12.2 ± 4.1 | ||||
| Communication | 3.0 ± 1.8 | ||||
| Social | 7.3 ± 2.5 | ||||
| Imagination | 0.8 ± 0.7 | ||||
| Stereotyped behaviors | 1.3 ± 1.3 |
All continuous data presented as mean ± SD. ASD, autism spectrum disorder; HC, Healthy Control, ADI-R, Autism Diagnostic Interview-Revised; ADOS-G, Autism Diagnostic Observation Schedule.
Table 2.
Regional metabolite concentration in adults with autistic spectrum disorders and healthy controls.
| NAA | Cr | Cho | Ins | Glx | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | ASD | HC | ASD | HC | ASD | HC | ASD | HC | ASD | HC |
| Tha | ||||||||||
| Left | 1.75 (0.4) | 1.77 (0.5) | 0.98 (0.1) | 1.06 (0.2) | 0.84 (0.1) | 0.83 (0.4) | 0.37 (0.1) | 0.37 (0.1) | 1.49 (0.3) | 1.58 (0.2) |
| Right | 1.77 (0.4) | 1.72 (0.4) | 1.02 (0.1) | 0.94 (0.1) | 0.89 (0.1) | 0.84 (0.1) | 0.42 (0.2) | 0.44 (0.2) | 1.57 (0.5) | 1.90 (0.9) |
| TPJ | ||||||||||
| Left | 1.56 (0.4) | 1.83 (0.4) | 0.80 (0.2) | 0.95 (0.3) | 0.62 (0.2) | 0.77 (0.5) | 0.27 (0.1) | 0.45 (0.2) | 1.23 (0.7) | 1.41 (0.7) |
| Right | 1.76 (0.5) | 2.14 (0.6) | 1.01 (0.1) | 1.00 (0.2) | 0.83 (0.3) | 0.72 (0.2) | 0.49 (0.1) | 0.49 (0.1) | 1.49 (0.5) | 1.67 (1.0) |
| IPS | ||||||||||
| Left | 1.76 (0.3) | 2.06 (0.7) | 0.95 (0.2) | 0.90 (0.2) | 0.62 (0.1) | 0.65 (0.2) | 0.40 (0.1) | 0.37 (0.1) | 1.12 (0.5) | 1.82 (1.3) |
| Right | 1.79 (0.7) | 1.88 (0.5) | 0.80 (0.2) | 0.80 (0.1) | 0.52 (0.2) | 0.57 (0.2) | 0.29 (0.1) | 0.43 (0.1) | 1.51 (1.0) | 1.65 (0.7) |
| ACC | ||||||||||
| Left | 1.87 (0.3) | 1.91 (0.4) | 1.12 (0.3) | 1.12 (0.3) | 1.05 (0.2) | 1.17 (0.4) | 0.60 (0.3) | 0.52 (0.2) | 1.31 (0.4) | 1.41 (0.3) |
| Right | 2.07 (0.4) | 2.20 (0.4) | 1.23 (0.3) | 1.17 (0.3) | 1.09 (0.2) | 1.13 (0.3) | 0.57 (0.3) | 0.79 (0.4) | 1.17 (0.4) | 1.76 (0.5) |
NAA, N-acetyl-aspartate; Cr, creatinine+phosphocreatinine, Cho, choline-containing compounds; Glx, glutamate+glutamine; Ins, myo-inositol; ACC, anterior cingulate cortex; IPS, intraparietal sulcus; TPJ, temporoparietal junction; Tha, thalamus; ASD, autism spectrum disorder; HC, healthy control.
3. Discussion
Inviduals with ASD had significantly reduced levels of cerebral Glx and Ins when compared with HC. Specifically, Glx concentrations were significantly reduced in the right ACC. Ins concentrations were significantly reduced in the left TPJ. The finding of significantly lower Glx concentrations in the ASD sample is consistent with previous findings in children with widespread Glx reduction in most cerebral lobes and cerebellum (DeVito et al. 2007). Our results complement the previous study and provide replication in an adult sample not taking psychotropic medications, with the application of a short TE.
The frontal cortex, the temporal cortex, and the basal ganglia develop heterochronically; changes in metabolite concentrations have been reported across different developmental ages (Horska et al. 2002). Thus, by analyzing a sample of adults with ASD we have also provided information about the stability of differences in Glx concentration shown in previous studies conducted in childhood. Only one study analyzed Glx concentration in a similar population to the one recruited in this study (Page et al. 2006), however, the analyses were limited to the amygdalohippocampal region and reported a significantly higher Glx concentration with 1.5 T 1H-MRS (Schumann et al. 2009).
It is important to note that the resonance group attributed to Glx includes contribution from glutamate/GABA and glutamine, and therefore a reduction in any or all of these compounds may be responsible for the reduction in Glx. However, previous studies have cautiously attributed Glx reduction to glutamate, as it constitutes the most abundant central neurotransmitter (DeVito et al. 2007;Hardan et al. 2008;Page et al. 2006). Glutamate plays a critical role in neurodevelopmental processes such as neuronal migration, differentiation, and plasticity (Coyle et al. 2002). Autism is associated with abnormal brain development (Nicolson and Szatmari 2003) and this study provides further evidence of impaired glutamatergic transmission previously implicated in the pathophysiology of ASD (Carlsson 1998;DeVito et al. 2007;Page et al. 2006;Polleux and Lauder 2004).
Our analyses localized the reduction in Glx concentration in the right ACC. Decreased metabolism and smaller volume of the ACC have been reported in individuals with ASD (Haznedar et al. 1997), and a quantitative meta-analysis of imaging studies in autism reported the ACC as the region with higher likelihood of hypoactivation (Di Martino et al. 2009;Haznedar et al. 1997). The imbalance between excitation and inhibition in the cortex of ASD may cause disruption of the synchrony of the ACC neurons that are thought to be necessary for executive control (Fan et al. 2005;MacDonald, III et al. 2000;Posner et al. 2007). A preliminary hypothesis may involve a reduction of the prefrontal glutamate-stimulated release of dopamine from terminals of the ventral tegmental area or substantia nigra. These systems are considered to be important in the processes of movement, learning, reward, motivation (Wise 2008), and error monitoring (Pourtois et al. 2009), all functions subject to top-down control, which is impaired in ASD (Brian et al. 2003;Hughes et al. 1994;Shu et al. 2001). Interestingly, a recent study reported enhancement in the regulation of dopamine release in the substantia nigra in an animal model of attention deficit/ hyperactivity disorder (Warton et al. 2009), another disorder with deficits in attention and impaired executive control (Swanson 2003). Future studies of connectivity may provide further insight into this hypothesis.
We also found a reduced concentration of Ins in the left TPJ. Myo-inositol is a metabolic compound located mostly in astrocytes. High Ins levels are thought to indicate cell growth and have also been directly associated with performance IQ scores in ASD (Gabis et al. 2008). The difference in Ins concentrations in left TPJ was also the only model to demonstrate a statistical trend toward a main effect of IQ. TPJ has been associated with orienting function of attention (Fan and Posner 2004), and also with phasic response and tonic maintenance of the alert state to a warning signal (Fan et al. 2005). The TPJ has also been repeatedly implicated in mechanisms underlying empathy in healthy individuals (Jackson et al. 2006), in autism (Williams et al. 2006), and in other diseases as well (Benedetti et al. 2009).
Contrary to previous findings in children (Friedman et al. 2003;Levitt et al. 2003), there were no significant differences in Cho. Given that Cho compounds are thought to be related to membrane turnover, differences in age-related cellular metabolisms may explain the incongruence with findings in childhood, although this may also be due to type II errors due to small sample size.
Several limitations are important to consider in interpreting these results. First, the small sample size and exclusion of patients with nonverbal IQ ≤ 80 limit generalization of the conclusions. Yet, the main effect of IQ as covariate on Glx concentrations was not significant and did not alter the significance of the model, suggesting that the results of this study are not solely due to the effect of IQ. Second, the MRS protocol employed in the present study did not allow for quantitative determination of glutamate concentration, which has been shown to appear well separated from glutamine at 3 T with an echo time of 80 ms (Schubert et al. 2004). However, the TE employed was short, as is generally required to maximize signal yield by reducing the effects of scalar coupling on the Glx signal. Future studies employing spectral editing techniques to fit glutamate and glutamine spectra separately are needed to confirm these results and to determine if the changes in Glx are due to glutamine, glutamate, or both. Furthermore, future 1H-MRS studies may employ editing techniques to examine the GABAergic concentrations in those regions to shed further light on the etiological hypothesis of ASD as imbalance of excitatory and inhibitory neurotransmission.
In conclusion, high-functioning adults with ASD had a significant reduction of Glx concentration in the ACC, suggesting abnormalities in neurotransmission involved in the executive control of attention previously implicated in ASD pathogenesis.
Furthermore, our results demonstrate a reduced concentration of Ins in the left TPJ, suggesting a role for a region previously implicated in orienting functions of attention. Future studies of the connectivity of the ACC and ventral tegmental area/substantia nigra may provide further insight to the role of glutamate in the executive control of attention.
4. Experimental procedures
4.1 Participants
Fourteen adults with an Autistic or Asperger Disorder and a full scale Intelligence Quotient [IQ] ≥80 between the ages of 21 and 50 were recruited from the local community. All participants were evaluated by physicians and clinical psychologists at the Seaver Autism Center for Research and Treatment at the Mount Sinai School of Medicine, New York. Diagnosis was made using the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria and supported by the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), and the Autism Diagnostic Observation Schedule (ADOS-G) (Lord et al. 2000). Exclusion criteria included: non verbal IQ score < 80 as measured by the Wechsler Adult Intelligence Scale-III (WAIS-III), other DSM-IV Axis I disorder, seizure disorder, other neurological conditions, known cytogenetic abnormality or genetic syndrome, and use of psychotropic medications. Handedness was determined through clinical observation and administration of the Edinburgh Handedness Inventory (Oldfield 1971).
Fourteen healthy controls (HC) were matched to subjects with ASD on non-verbal IQ (within 15 points, 1 SD) and age (birth date within 24 months). Healthy controls underwent the same screening procedure as the potential participants with ASD, with the exception of ADI-R and ADOS-G administration. Healthy controls were also assessed with the Structured Clinical Interview for DSM-IV (SCID I) (First et al. 1996) to rule out other AXIS-I disorders. Other exclusion criteria were identical to that of the ASD group except HC with a positive family history of developmental disorders, learning disabilities, autism, affective disorders, and anxiety disorders in first degree relatives were also excluded. We also excluded potential HC participants with a lifetime history of substance/ethyl alcohol (EtOH) dependence and or substance/EtOH abuse within the last year. All participants were able to provide written informed consent as approved by the Mount Sinai School of Medicine Institutional Review Board for participation.
4.2 MRS Acquisition
All images were acquired with a 3 T head-only MRI scanner (Siemens Medical Solutions USA, Malvern, Pennsylvania). T1-weighted localizer images for each MRI slides were acquired, followed by one pair of T1-weighted transverse slices (TR = 500 ms, TE = 10 ms, Thickness = 10 mm, field-of-view [FOV] = 180×240 mm, matrix size = 384×512) and T2-weighted transverse slice (TR = 5000 ms, TE = 94 ms, Thickness = 10 mm, FOV = 180×240 mm, matrix size = 384×512).
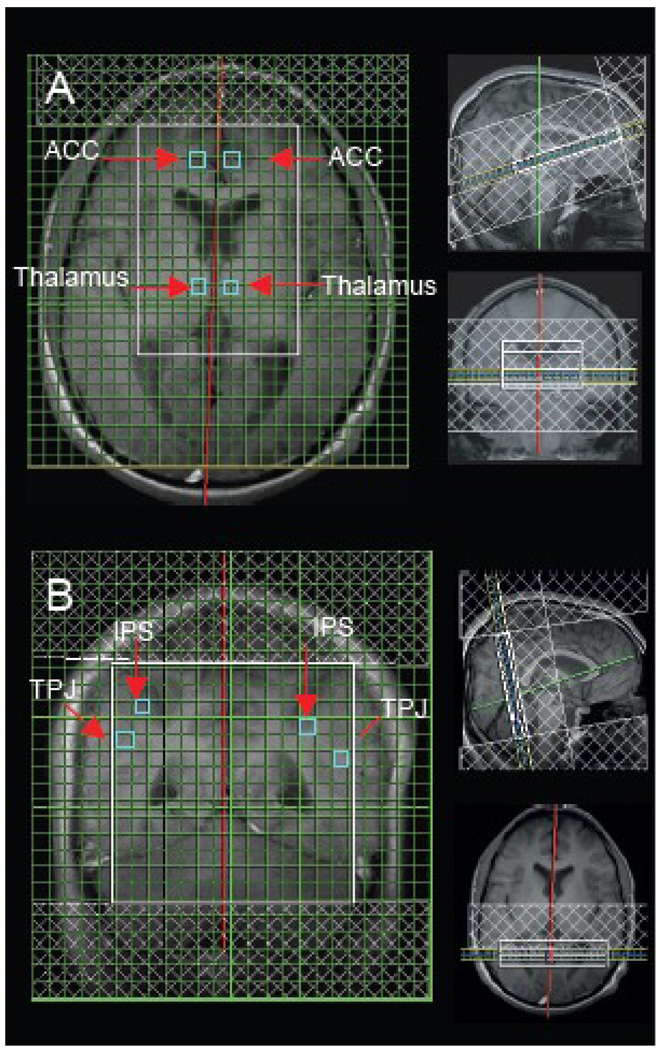
Two sets of 1H spectroscopic imaging (SI) data were recorded using the phase-encoded version of the standard PRESS volume localization sequence. A short TE (30 ms) protocol was applied in order to maximize Glx signal yield with TR = 2000 ms, TE = 30 ms, 24×24 phase-encoding steps over a FOV of 240 mm (zero filled to 32×32 phase-encoding steps before 3D Fourier transformation), a slice thickness of 10 mm, 1 average per phase-encoding step and circular k-space sampling, to obtain voxels having a nominal size of 0.5625 cm3 (1.0×0.75×0.75 cm3). Outer volume saturation bands were prescribed to coincide with all 6 sides of the PRESS box. Water suppression and magnet shimming were performed automatically by the host computer and adjusted manually by the operator. Two slices in total were acquired to obtain the MRS data. The first slice was axial to sample the ACC and thalamus (see Fig. 1A). The second slice was coronal (Talairach coordinate y = −45 approximately) to sample the area near/along the IPS and TPJ (see Fig. 1B). Two-dimensional proton chemical shift imaging (CSI) was acquired to yield the spatial distribution and levels of the metabolites. Raw spectral data were processed for Fourier transformation, and phase and baseline corrections using Syngo MR vr. 2002 software Spectroscopy Task Card during data acquisition. Peak areas of the reference metabolites was calculated with automatic integration and evaluated in individual single voxel of the Hybrid CSI matrix. Corrected metabolite amplitudes are derived as integrals and reported in arbitrary institutional units. Deviation between the theoretical and measured spectrum calculated using the least squares method was controlled. Ten HC and 10 ASD out of a total of 224 spectra did not yield significant fits and were discarded from the analysis. Quantitative analysis of spectra was confined to NAA (chemical shift 2.0 ppm), Cr (3.0 ppm), Cho (3.2 ppm), Ins (3.5 ppm) and Glx (2.35 ppm).
Figure 1.
Slice localization (A) for voxel location in the anterior cingulate cortex (ACC) and thalamus (Tha) and slide localization (B) for voxel location in the intraparietal sulcus (IPS) and temporoparietal junction (TPJ) with representative proton magnetic resonance single spectrum from voxels.
4.3 Statistical Analyses
Analyses were carried out with SPSS 17.0 software (SPSS, Chicago, Illinois). Age and IQs were compared with t tests; race and gender were compared with χ2 analyses or the Fisher Exact test. Distribution of missing values was analyzed separately by condition per hemisphere per region. A type IV sum-of-square method, which is more suitable to the construct model in the presence of empty cells, was applied in all tests to deal with missing data. Group differences in regional metabolite concentration were investigated with linear mixed-model analysis of covariance. Given the hypothesis driven nature of the analyses, separate analyses were performed for each of the five metabolites. Metabolite concentration was the dependent variable, diagnosis was the between-subject factor, and region (ACC, thalamus, IPS, TPJ) and hemispheres (left and right) were the within-subjects factors. Full scale IQ and age were covariates. In addition to the main effect of the independent variables, the model for each metabolite included all of the 2- and 3-ways interactions between diagnosis, region and hemisphere. For each metabolite that showed a significant main effect of diagnosis or of interaction involving diagnosis, univariate ANCOVA with the metabolite concentration of each one of the 4 regions for both hemispheres as dependent variables, diagnosis as fixed factor and IQ and age as covariate was performed to identify the specific region(s) that contributed to the significant main effect or interaction.
Highlights.
There is the lower glutamate + glutamine concentrations in right anterior cingulate cortex and reduced myo-inositol in left temporoparietal junction in patients with autism spectrum disorders.
The findings suggest abnormalities in neurotransmission in the networks subserving attentional functions
Figure 2.
Metabolites amplitude in adults with autism spectrum disorder and healthy controls. Note: * p < 0.03 significance evaluated with ANCOVA with age and IQ as covariates; ** p < 0.006 with ANCOVA with age and IQ as covariates; data are expressed as means and error bars represent standard errors; Glx, glutamate+glutamine; L, left; R, right; ACC, anterior cingulate cortex; IPS, intraparietal sulcus; TPJ, temporoparietal junction; ASD, autism spectrum disorder; HC, healthy control.
Acknowledgements
The study described was supported by National Center for Research Resources Grant M01 RR000071. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. This work was also supported in part by a Young Investigator Award from the NARSAD and by a NIMH grant MH083164 to JF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. Jack M. Gorman for his support. Thank Kevin G. Guise, Laura Martin, and Dr. Cheuk Y. Tang for assistance with data collection. We thank the Beatrice and Samuel A. Seaver Foundation.
Abbreviations
- NAA
N-acetyl-aspartate
- Cr
creatinine+phosphocreatinine
- Cho
choline-containing compounds
- Glx
glutamate+glutamine
- Ins
myo-inositol
- ACC
anterior cingulate cortex
- IPS
intraparietal sulcus
- TPJ
temporoparietal junction
- ASD
autism spectrum disorder
- HC
healthy control
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. J Autism Dev Disord. 2003;33:93–97. doi: 10.1023/a:1022238706604. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, Poletti S, Radaelli D, Riccaboni R, Scotti G, Smeraldi E. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–160. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C. Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord. 2005;35:351–360. doi: 10.1007/s10803-005-3302-5. [DOI] [PubMed] [Google Scholar]
- Blaylock RL, Strunecka A. Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr Med Chem. 2009;16:157–170. doi: 10.2174/092986709787002745. [DOI] [PubMed] [Google Scholar]
- Brian JA, Tipper SP, Weaver B, Bryson SE. Inhibitory mechanisms in autism spectrum disorders: typical selective inhibition of location versus facilitated perceptual processing. J Child Psychol Psychiatry. 2003;44:552–560. doi: 10.1111/1469-7610.00144. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. Hypothesis: is infantile autism a hypoglutamatergic disorder? Relevance of glutamate - serotonin interactions for pharmacotherapy. J Neural Transm. 1998;105:525–535. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- Carlsson ML, Martin P, Nilsson M, Sorensen SM, Carlsson A, Waters S, Waters N. The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice. J Neural Transm. 1999;106:123–129. doi: 10.1007/s007020050144. [DOI] [PubMed] [Google Scholar]
- Cederlund M, Hagberg B, Billstedt E, Gillberg IC, Gillberg C. Asperger syndrome and autism: a comparative longitudinal follow-up study more than 5 years after original diagnosis. J Autism Dev Disord. 2008;38:72–85. doi: 10.1007/s10803-007-0364-6. [DOI] [PubMed] [Google Scholar]
- Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J Child Neurol. 2007;22:574–579. doi: 10.1177/0883073807302611. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Sundram BS, Behen M, Lee ML, Moore GJ. Evidence of altered energy metabolism in autistic children. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:635–641. doi: 10.1016/s0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Coyle J, Leski M, Morrison J. The diverse roles of L-glutamic acid in brain signal transduction. In: David K, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology, The Fifth Generation of Progress. Philadelphia: Lippincott, Williams, and Wilkins; 2002. pp. 71–90. [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- DeVito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, Nicolson R. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61:465–473. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Shioiri T, Kitamura H, Kimura T, Endo S, Masuzawa N, Someya T. Altered chemical metabolites in the amygdala-hippocampus region contribute to autistic symptoms of autism spectrum disorders. Biol Psychiatry. 2007;62:1030–1037. doi: 10.1016/j.biopsych.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Chambers JE. Memantine for disruptive behavior in autistic disorder. J Clin Psychiatry. 2006;67:1000. doi: 10.4088/jcp.v67n0619h. [DOI] [PubMed] [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI. The relation of brain oscillations to attentional networks. J Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Posner M. Human attentional networks. Psychiatr Prax. 2004;31 Suppl 2:S210–S214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: 1996. [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Dawson G, Petropoulos H, Dager SR. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63:786–794. doi: 10.1001/archpsyc.63.7.786. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, Posse S, Dager SR. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Gabis L, Wei H, Azizian A, DeVincent C, Tudorica A, Kesner-Baruch Y, Roche P, Pomeroy J. 1H-magnetic resonance spectroscopy markers of cognitive and language ability in clinical subtypes of autism spectrum disorders. J Child Neurol. 2008;23:766–774. doi: 10.1177/0883073808315423. [DOI] [PubMed] [Google Scholar]
- Gold MS, Gold JR. Autism and attention: theoretical considerations and a pilot study using set reaction time. Child Psychiatry Hum Dev. 1975;6:68–80. doi: 10.1007/BF01438301. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Fossella J, Wang K, Fan J. Alexithymic trait and voluntary control in healthy adults. PLoS One. 2008;3:e3702. doi: 10.1371/journal.pone.0003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, Keshavan MS, Stanley JA. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008;163:97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harich S, Gross G, Bespalov A. Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology (Berl) 2007;192:511–519. doi: 10.1007/s00213-007-0742-y. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Metzger M, Solimando A, Spiegel-Cohen J, Hollander E. Anterior cingulate gyrus volume and glucose metabolism in autistic disorder. Am J Psychiatry. 1997;154:1047–1050. doi: 10.1176/ajp.154.8.1047. [DOI] [PubMed] [Google Scholar]
- Hisaoka S, Harada M, Nishitani H, Mori K. Regional magnetic resonance spectroscopy of the brain in autistic individuals. Neuroradiology. 2001;43:496–498. doi: 10.1007/s002340000520. [DOI] [PubMed] [Google Scholar]
- Hofvander B, Delorme R, Chaste P, Nyden A, Wentz E, Stahlberg O, Herbrecht E, Stopin A, Anckarsater H, Gillberg C, Rastam M, Leboyer M. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. doi: 10.1186/1471-244X-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J Magn Reson Imaging. 2002;15:137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T. Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbrink K, Knapp M. The economic impact of autism in Britain. Autism. 2001;5:7–22. doi: 10.1177/1362361301005001002. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35:100–136. [PubMed] [Google Scholar]
- Kleinhans NM, Schweinsburg BC, Cohen DN, Muller RA, Courchesne E. N-acetyl aspartate in autism spectrum disorders: regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97. doi: 10.1016/j.brainres.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A. Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther. 2005;27 Suppl A:S16–S24. doi: 10.1016/j.clinthera.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Levitt JG, O'Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, Guthrie D, Toga AW, Alger JR. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol Psychiatry. 2003;54:1355–1366. doi: 10.1016/s0006-3223(03)00688-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le CA. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. J Child Psychol Psychiatry. 2005;46:401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Montag C, Schubert F, Heinz A, Gallinat J. Prefrontal cortex glutamate correlates with mental perspective-taking. PLoS One. 2008;3:e3890. doi: 10.1371/journal.pone.0003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Szatmari P. Genetic and neurodevelopmental influences in autistic disorder. Can J Psychiatry. 2003;48:526–537. doi: 10.1177/070674370304800804. [DOI] [PubMed] [Google Scholar]
- Niederhofer H. Glutamate antagonists seem to be slightly effective in psychopharmacologic treatment of autism. J Clin Psychopharmacol. 2007;27:317–318. doi: 10.1097/01.jcp.0000270082.30500.69. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Harada M, Mori K, Hisaoka S, Nishitani H. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: an 1H-MR spectroscopy study. Neuroradiology. 1999;41:517–519. doi: 10.1007/s002340050795. [DOI] [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, Ambery F, McAlonan GM, Murphy KC, Murphy DG. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163:2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Vocat R, N'diaye K, Spinelli L, Seeck M, Vuilleumier P. Errors recruit both cognitive and emotional monitoring systems: Simultaneous intracranial recordings in the dorsal anterior cingulate gyrus and amygdala combined with fMRI. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161:662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the "new psychophysiology". Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21:1762–1771. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segurado R, Conroy J, Meally E, Fitzgerald M, Gill M, Gallagher L. Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. Am J Psychiatry. 2005;162:2182–2184. doi: 10.1176/appi.ajp.162.11.2182. [DOI] [PubMed] [Google Scholar]
- Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Minabe Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Shu BC, Lung FW, Tien AY, Chen BC. Executive function deficits in non-retarded autistic children. Autism. 2001;5:165–174. doi: 10.1177/1362361301005002006. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E, Arbeile S, Corona R, Dissanayake C, Espinosa M, Kim N, Lopez A, Zierhut C. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monogr Soc Res Child Dev. 1999;64:1–114. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Role of executive function in ADHD. J Clin Psychiatry. 2003;64 Suppl 14:35–39. [PubMed] [Google Scholar]
- Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar j, Fan J. Effective Connectivity of the Fronto-parietal Network during Attentional Control. Journal of Cognitive Neuroscience. 2010 doi: 10.1162/jocn.2009.21210. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- Warton FL, Howells FM, Russell VA. Increased glutamate-stimulated release of dopamine in substantia nigra of a rat model for attention-deficit/hyperactivity disorder--lack of effect of methylphenidate. Metab Brain Dis. 2009;24:599–613. doi: 10.1007/s11011-009-9166-1. [DOI] [PubMed] [Google Scholar]
- Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and 'mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]