Summary
Lamprey and hagfish are surviving representatives of the most ancient vertebrates. They possess adaptive immune systems based on a vast, somatically diversified repertoire of lymphocyte-bound antigen receptors. Despite these similarities to antibody and T cell receptors (TCR) of later vertebrates, the variable lymphocyte receptors (VLR) are not related to the immunoglobulin (Ig)-superfamily of genes; and instead of V(D)J recombination VLR are somatically assembled by a gene conversion process. However, recent studies have revealed two lamprey lymphocyte subsets so closely resembling B cells and T cells that separate lymphocyte lineages must have already existed in the ancestral vertebrate, before Ig/TCR emergence. VLR and Ig/TCR arose independently, but the convergent evolution they display actually reflects their selection in cells with specialized functions.
Introduction
Lymphocytes in sharks and human beings recognize antigenic determinants through the N-terminal domains of their B cell and T cell receptors (BCR, TCR), called variable (V) regions, whose diversity is by generated V(D)J recombination. For many years in the 20th century comparative immunologists searched for the “primordial” V gene: the antigen receptor genes that existed before the introduction of recombination activating genes (RAG) that mediate V(D)J rearrangement. This was the assumed precursor to immunoglobulin (Ig) and TCR genes, and as such, presumably existed before the emergence of B and T lymphocytes. The candidate animals targeted in this hunt were lamprey and hagfish, the surviving representatives of the most ancient vertebrates (agnathans, jawless fishes) (Fig. 1). They possessed hematopoietic tissues with lymphoid elements and circulating cells that morphologically resembled lymphocytes and plasma cells; they made humoral responses to various injected antigens, although no Ig or Ig-like proteins could be isolated [reviewed in ref. 1, 2].
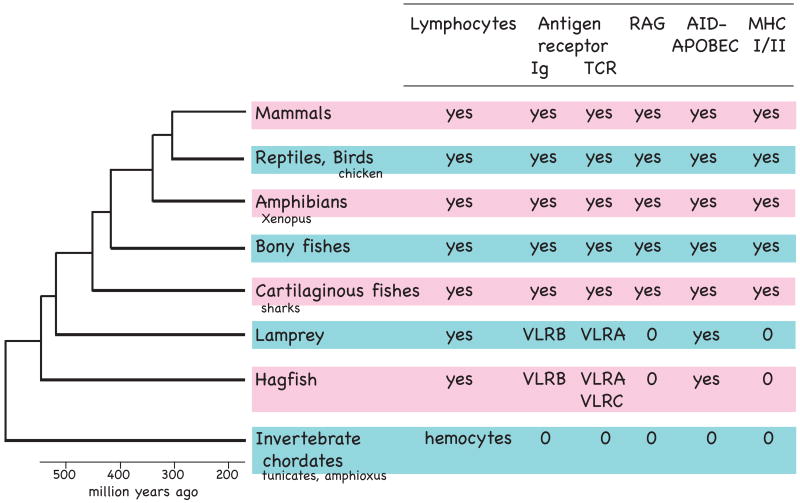
Figure. 1.
Adaptive immune features in vertebrates. The phylum Chordata includes jawed vertebrates (gnathostomes), jawless vertebrates (agnathans like hagfish and lamprey), and invertebrate chordates, such as cephalochordates (amphioxus) and urochordates (tunicates). Animals referred to in the text are indicated. The scale shows when taxa emerged in evolution. A much-debated issue is the phylogenetic position of lampreys, here depicted as sister group of jawed vertebrates [42]. The immune system features include hematopoietic cells and their key gene products that enable antigen recognition (cell surface receptors Ig, TCR, MHC class I, MHC class II) and generate antigen receptor sequence diversity (RAG1/RAG2, AID-APOBEC cytidine deaminase family). The immune systems of the jawed vertebrates are reviewed in ref. [43]. The agnathan characteristics are discussed in the text. The genomes of Ciona (tunicate) and amphioxus have been examined for immune components [44, 45]; it is not clear whether RAG2 exists in amphioxus.
The studies in lamprey and hagfish brought some expected answers (no V(D)J recombination or RAG genes, no Ig or TCR genes, no class I or class II molecules of the major histocompatibility complex (MHC) [3, 4]) and some unanticipated findings -- the antigen receptors expressed on the lymphocytes were highly diverse but not related to the Ig-superfamily (IgSF) that Ig/TCR belong to. They were also somatically assembled to generate a vast immune repertoire, but not by RAG. Thus, during the evolution of vertebrates, adaptive immune systems have twice emerged independently. In recent years it has transpired that the lamprey lymphocyte lineages closely resemble T and B cells, so that lymphocyte specializations already existed in the ancestral vertebrate, more than 500 million years ago, and in point of fact pre-date the emergence of Ig/TCR antigen receptors.
Agnathan antigen receptors
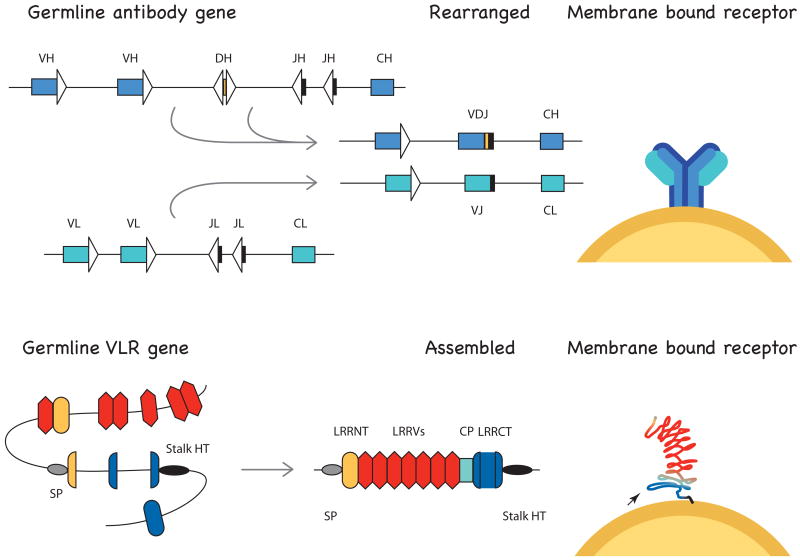
The variable lymphocyte receptors (VLR) of lamprey were first isolated from a cDNA library constructed from activated lymphocytes after injection with antigen/mitogen cocktails [5]. The most abundant set consisted of 239 unique sequences containing leucine-rich-repeat (LRR) elements, and the diverse portion within these sequences encoded an N-terminal LRR (LRRNT), varying numbers of LRR units (LRRV) each of about 24 amino acids, a connecting peptide (CP), a C-terminal LRR (LRR-CT) capping region (Fig. 2). However, they could only have been generated by a single VLR locus whose germline organization consisted of the 5′ half of LRRNT and 5′ and 3′ parts of LRR-CT. During lymphocyte differentiation the interstitial region is replaced/inserted with varying numbers of different LRR units and the LRRNT and LRR-CT are extended to generate the mature, assembled VLR gene.
Figure 2.
Somatically recombined antigen receptors in vertebrates. Top. Immunoglobulin genes are shown in their germline configuration (VH, DH, JH gene segments for H chain, VL and JL gene segments for L chain) (right), rearranged as VDJ (H chain) and VJ (L chain) (center), transcribed with their constant regions (CH in H chain, CL in L chain) and expressed as integral membrane receptors on lymphocytes (right). Triangles indicate recombination signal sequences recognized by RAG. Bottom. The lamprey VLRB gene is shown in germline configuration (left), as assembled VLR with inserted LRR sequences (center), as a horseshoe-shaped receptor whose concave surface forms a ligand-binding β sheet (right) [36]. The germline gene (signal peptide, SP, gray oval; partial 5′LRRNT, yellow; 5′LRRCT, 3′LRRCT, blue; stalk and hydrophobic tail (HT)) and flanking cassettes with LRRNT-LRR1, LRRV, LRRCT, are drawn after ref. [5]; relative distances are not accurate. The successive transfer of LRR sequences is initiated from the 5′ and 3′ ends of the germline gene, culminating in the assembly of the mature VLR. This consists of signal peptide (gray oval), N-terminal LRR (LRRNT, yellow ellipse), LRR1 of 18 amino acids and additional LRRV modules of 24 amino acids (red hexagons), connecting peptide (CP, blue rectangle) of 13 amino acids, C-terminal LRR (LRRCT, in navy) varying in length, and the C terminus (stalk and hydrophobic tail (HT), black oval). The arrow at right points to the LRRCT insert (blue loop in receptor) when long enough to be a protrusion.
Comparison of the LRRV in 517 unique sequences, analyzed in terms of repeated modules and order, produced a potential repertoire estimate of 1014 VLR [6], more than equal to the IgM repertoire of >3.5 × 1010 as measured in humans [7]. The content of LRRV derive from >300 modules flanking the VLR gene [3]. These units are incorporated in a stepwise manner, and in-frame and in tandem with other LRR, as observed from noncompleted intermediates. The insertion process was initially proposed to take place through the use of short homology sequences between modules [8]. A fine analysis of mature VLR compared to a large pool of germline modules suggested that the majority of LRRV are chimeric for two or more different modules, and that intramodular homology is most frequently utilized [3].
The mechanism of the assembly process is not known. The absence of motifs like recombination signals, the lack of reciprocal exchanges, and the involvement of homology-based pairing suggest a donor template copied by the recipient in a gene conversion pathway. Activation-induced cytidine deaminase (AID)-APOBEC family DNA cytosine deaminase sequences are expressed in lymphocytic tissues, raising the possibility that VLR assembly processes may be initiated by AID-induced DNA lesions [3, 9**]. Pathways involving AID include gene conversion, the mechanism by which the primary antibody repertoire is generated in chicken precursor lymphocytes [10-12].
Two lymphocyte subpopulations
Two types of VLR sequences were subsequently identified by differences in the non-modified portions of the genes [13, 3]. They are encoded by two loci, VLR-A and VLR-B, whose mature assembled genes are similar in structure although the germline organizations differ somewhat between lamprey and hagfish. Immunization of lampreys with heterologous erythrocytes or bacteria induced a humoral response consisting of agglutinins that proved to be mainly multivalent VLRB molecules in the form of 8-10 disulfide-bonded VLRB subunits. Comparison with the monomer showed greater avidity of the multimer, reminiscent of pentameric IgM. After immunization, VLRB+ populations transformed into large plasmacytoid cells that secreted VLRB and expressed surface VLRB [14, 15]. Altogether these findings establish VLRB+ lymphocytes as the agnathan B cell equivalent.
Monoclonal antibodies and rabbit antisera specific for lamprey VLRA and VLRB demonstrated the existence of two separate lymphocyte populations that assemble and express one gene while retaining the other in germline configuration [9]. Whereas both VLRA and VLRB are membrane bound receptors, tethered by a glycosyl phosphatidyl inositol anchor, only the VLRB become secreted. Recombinant VLR transfected into the 293T human embryonic kidney cell line retained these characteristics. Moreover, whereas VLRB+ cells expressed B cell lineage-related genes (e.g. B cell receptor signal transduction components Syk and BCAP, Toll-like receptors TLR2a, TLR2b, TLR2c, TLR7, TLR10, chemokine IL-8, cytokine IL-17 receptor) VLRA+ cell populations are distinguished by transcripts found typically in T lymphocytes (e.g. transcription factors GATA2/3, c-Rel, aryl hydrocarbon receptor and BCL11b, chemokine receptor CCR9, T cell differentiation promoter Notch1, tyrosine phosphatase CD45, cytokines MIF and IL-17, chemokine IL-8 receptor).
After VLRA+ cells were stimulated by the classical T cell mitogen phytohemagglutinin, they upregulated transcripts for IL-17 and MIF, an observation that suggests cytokine secretion may be among their functions [9]. Whether VLRB+ and VLRA+ cells interact is not known, but there is now reported a third VLR locus in lamprey, also clonally expressed. In sequence and expression patterns VLRC is more closely related to VLRA, eliciting speculation on another T cell-like subset [16**].
Is there direct interaction between the VLRA/B/C subpopulations? The presence of cytokine and cytokine receptor transcripts in lymphocytes suggest crosstalk involving other cell types. It is not known if there is associative recognition and whether agnathan MHC analogs exist, in the absence of a true thymus (and thymic selection), or by what process lampreys and hagfish reject second-set allografts in an accelerated fashion [17, 18].
Agnathans do not possess true lymphoid organs like spleen, lymph nodes or thymus [1], but in pre-metamorphic lampreys lymphocytes appear to collect around the gill basket [19, 20]. The majority are VLRA+, and cells expressing AID-APOBEC were restricted to the tips of the gill filaments [21**]. Inspection of the pharyngeal epithelium also revealed the presence of Foxn1 and Delta-like orthologs [21, 22], genes essential in thymopoiesis and providing the microenvironment specific for T lymphocyte differentiation. If AID-expressing cells are assembling VLRA at the gill filaments, the lamprey thymus equivalent is not one lymphoid entity but numerous discrete sites in the gills. Lymphoid progenitors would migrate to the gill branch from the kidney, the site of hematopoiesis in lamprey and in bony fishes, or the typhlosole, an invagination in the gut in larvae, which regresses and is replaced in function by the supraneural/fat body in adults [1]. Agnathan lymphocytes express Notch receptors, whose ligand may be the Delta-like ortholog found on the pharyngeal epithelium, and it is speculated that in lamprey the Notch signaling induces T lineage specification and development, as in mammals [23].
VLRA+ and γδ-TCR lymphocytes
Immunization with Bacillus anthracis exosporium caused comparable proliferation levels in responsive VLRB+ and VLRA+ lymphocytes, although the latter could not be found to bind spores before or after immunization [9]. However both VLRA and VLRB specific for a soluble protein antigen like hen egg lysozyme (HEL) have been isolated and the VLR-antigen complexes determined [24, 25]. It is not clear whether VLRA have different recognition or binding requirements, but their direct binding of antigen [26**] has prompted comparison to γδ-TCR, in contrast to αβ-TCR that require antigen processing and presentation for recognition. VLRA may undergo somatic mutation, like TCRγ in sharks [27**]. Thirteen unique HEL-binding VLRA, isolated from a yeast surface display library constructed from immunized lamprey, varied in affinity by 100-fold range but they were all the same length and differed in only 15 of 244 positions, which suggested their descent from 1-2 mature VLRA progenitors [26].
The TCRγ substitutions in sandbar shark include blocks of 2-5 bp of contiguous changes previously seen only in Ig and Ig-like sequences of cartilaginous fishes [28], bespeaking a common hypermutation pathway in T and B cells. As in the VLRA, it is not clear whether the somatic mutations in shark TCRγ occurred as a result of antigen encounter. The common features of the shark and agnathan receptors suggest that “primordial” lymphocytes expressed receptors that directly bound antigen and may be diversified by mutation. The distinction between BCR and TCR has been further eroded in the amphibian Xenopus, where a number of the germline V gene segments at the TCRδ locus appear to have been paralogously co-opted from IgH. This demonstrates a need for antibody-like binding for some T cell function – perhaps one that is driven by antigen recognition [29].
Immunity based on clonally expressed antigen receptors
With somatically diversified antigen receptors that number in the many millions, selection against unwanted, autoreactive specificities must take place in the individual. Tolerance may be achieved by deletion or inactivation of the cells when they interact with self components, but the receptor must be expressed at sufficient levels to allow such a selection to take place [reviewed in 30]. Ig receptors, even when encoded by 20-200 independently rearranging Ig H chain genes in cartilaginous fishes, are clonally expressed [reviewed in 31].
Activation of the VLRA and VLRB loci is strictly mutually exclusive and expression of the receptor largely monoallelic [9, 8, 32*]. In single cell analysis of hagfish peripheral blood leukocytes, Kishishita and coworkers [32] found that in 90% of VLRA- and 95% of VLRB-assembled cells where both alleles of that locus were detected, one allele was functionally assembled while the other was in germline configuration. In many cells where two assembled VLR alleles were detected, one was defective and contained frameshifts or a germline-based stop codon; about 5% of VLRA- and 1% of VLRB- assembled cells carry two apparently functional VLR. The frequency of these presumed double expressers seems on a par with allelic inclusion of kappa light chains in mouse B cells [33], but interpretation awaits a better understanding of VLR receptor selection. Since both alleles at an activated VLR locus are transcribed, the results suggest that in the VLRA- or B-committed progenitor, assembly was asynchronously initiated. If a functional VLR was not expressed by the first allele, the absence of a feedback signal may have led to assembly activation at the second [32].
The novelty of sequence length diversity
The selected innovations of VLR and Ig/TCR lie in the extent to which ligand-combining sites can be varied in topology as well as sequence. The diverse topology of the Ig/TCR combining site is largely governed by the CDR3 loops created during the V(D)J rearrangement process [34], and the loop length spectrum of human H chain CDR3 encompasses 2-26 amino acids [35]. The ligand-binding site of VLR is formed by a continuous β sheet of LRR modules [36**] where the surface area size variation in VLRA can be 1-5 LRRV modules and in VLRB 1-8 LRRV modules [6]. No pre-vertebrate mutational pathway provided this kind of diversity over and above sequence variation [28].
The LRRV modules are each the same size except for the C-terminal module, LRRCT, which is generated with diverse insertions of varying sequence and length [3, 24, 25, 36] (Fig. 2, arrow). In crystallographic studies the antigen has key interactions with the concave LRR surface and the LRRCT insert [36, 24, 25]. The mobile flexibility of LRRCT may provide for a degree of induced binding not possible with the rigid LRR β sheet surface [36, 24, 25]. Although the VLRA and VLRB molecules appear overall structurally similar, the LRRCT insert diversity distinguishes them. The LRRCT inserts range between 0-13 and 2-12 amino acids for hagfish and lamprey VLRB, in contrast to the VLRA insert sizes of 3-4 and 10-13 amino acids, a considerably more restricted 2-3 residue range [24]. How the LRRCT diversity influences recognition capabilities has yet to be elucidated, but this distinction between VLRA and VLRB is a selected feature because it exists in both lamprey and hagfish, which have long diverged from each other (Fig. 1). If two lymphocyte subpopulations with different functions expressed overlapping repertoires they would directly compete for the same set of epitopes. If the two cell types expanded unequally, subsequent re-exposures to that antigen would exacerbate the imbalance, perhaps to the point of eliminating either the cellular or humoral component of the response.
Conclusions
The current reports collectively demonstrate that by the time of hagfish divergence, lymphocyte subsets were present in stem vertebrates and preceded emergence of Ig/TCR. This unexpected conclusion informs us that it is not the tail (receptor) that wags the dog (lymphocyte) in antigen receptor evolution; selection on the receptors depended on the efficaciousness they brought the cell's immune functions. The similarities of VLR and Ig/TCR reflect convergent evolution, perhaps not unexpectedly so, as they were molded in cells already capable of specialized roles. Did Ig/TCR and VLR evolve independently and in parallel or did Ig/TCR replace some version of VLR [37]? The phylogenetic position of lampreys, as depicted in Fig. 1, would suggest the latter; but whether lampreys are sister group of gnathostomes or of hagfish is a controversial issue [38].
Although efforts to understand the evolution of the adaptive immune system have focused on the origins of antigen receptors and their recombination pathways, the spotlight ought to shift from the receptors to the cell that they serve. Key issues such as the evolution of cellular interactions (and associative recognition of antigen) and the functions of the leukocyte subsets require investigation in not only agnathans but also protochordates. In the latter, morphology [39] and expression of hemocyte-specific genes have revealed several tunicate blood cell subsets [40*, 41] and a more complex picture of their cellular immunity. The relationship of these protochordate immune cells to lymphocytes needs clarification. After all, it is the invention of lymphocytes that made possible adaptive immune systems.
Acknowledgments
I thank Louis Du Pasquier and Chris Amemiya for their invaluable comments on the manuscript. The author's laboratory was supported by the National Institutes of Health (R01-GM068095).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Zapata A, Amemiya CT. Phylogeny of Lower Vertebrates and Their Immunological Structures. Curr Top Microbiol Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 2.Litman GW, Rast JP, Hulst MA, Litman RT, Shamblott MJ, Haire RN, Hinds-Frey KR, Buell RD, Margittai M, Ohta Y, Zilch AC, Good RA, Amemiya CT. Evolutionary Origins of Immunoglobulin Gene Diversity. Progress in immunology. Proceedings of the 8th International Congress of Immunology, Budapest. 1992;8:107–114. [Google Scholar]
- 3.Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Shin-I T, Kohara Y, Kasahara M. Transcriptome analysis of hagfish leukocytes: a framework for understanding the immune system of jawless fishes. Dev Comp Immunol. 2004;28:993–1003. doi: 10.1016/j.dci.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Pancer Z, Amemiya CT, Ehrhardt GRA, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. [Google Scholar]
- 6.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and Function of Adaptive Immune Receptors in a Jawless Vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 7.Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, Ni I, Mei L, Sundar PD, Day GMR, Cox D, Rajpal A, Pons J. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc Natl Acad Sci U S A. 2009;106:20216–20221. doi: 10.1073/pnas.0909775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagawa F, Kishishita N, Shimizu K, Hirose S, Miyoshi M, Nezu J, Nishimura T, Nishizumi H, Takahashi Y, Hashimoto SI, Takeuchi M, Miyajima A, Takemori T, Otsuka AJ, Sakano H. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8:206–213. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]
- **9.Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]; With the use of anti-VLRA and anti-VLRB antibodies, distinct and exclusive lymphocyte populations are revealed in jawless fishes. Activated VLRB+ cells secrete multimeric VLRB; VLRA remain membrane bound. Sorted cells show respective B and T cell-like gene expression profiles.
- 10.Reynaud CA, Dahan A, Anquez V, Weill JC. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59:171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- 11.Harris RS, Sale JE, Petersen-Mahrt SK, Neuberger MS. AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr Biol. 2002;12:435–438. doi: 10.1016/s0960-9822(02)00717-0. [DOI] [PubMed] [Google Scholar]
- 12.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 13.Pancer Z, Saha NR, Kasamatsu J, Suzuki T, Amemiya CT, Kasahara M, Cooper MD. Variable lymphocyte receptors in hagfish. Proc Natl Acad Sci U S A. 2005;102:9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, Gartland GL, Boydston JA, Turnbough CL, Jr, Cooper MD. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 15.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GRA, Boydston JA, Turnbough CL, Jr, Cooper MD. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci U S A. 2008;105:2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci U S A. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]; A third VLR locus, VLRC, is established in hagfish. Its products are expressed on lymphocytes exclusively of VLRA and VLRB. VLRC is phylogenetically more closely related to VLRA, but by comparison harbor more structural restrictions.
- 17.Perey DYE, MD, Finstad J, MS, Pollara B, MD, PhD, Good RA., MD, PhD Evolution of the Immune Response VI. First and Second Set Skin Homograft Rejections in Primitive Fishes. Laboratory Investigation. 1968;19:591–597. [PubMed] [Google Scholar]
- 18.Hildemann WH, Thoenes GH. Immunological responses of Pacific hagfish. I. Skin transplantation immunity. Transplantation. 1969;7:506–521. doi: 10.1097/00007890-196906000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Good RA, Finstad J, Pollara B, Gabrielsen AE. Morphologic studies on the evolution of the lymphoid tissues among the lower vertebrates. In: Smith RT, Miescher PA, Good RA, editors. Phylogeny of Immunity. University of Florida Press; 1966. pp. 149–170. [Google Scholar]
- 20.Salkind J. Contributions histologiques à la biologie comparée du thymus. Arch Zool Exper. 1915;55:81–322. [Google Scholar]
- **21.Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, McCurley N, Bockman DE, Schorpp M, Cooper MD, Boehm T. Identification of a thymus candidate in lampreys. Nature. 2010 doi: 10.1038/nature09655. in press. [DOI] [PubMed] [Google Scholar]; This paper shows that cells in the tips of larval lamprey gill filaments express AID-APOBEC. The overlap of Foxn1 signal and abundance of VLRA+ cells in this area suggest that VLRA lineage induction takes place in this microenvironment, which the authors call the “thymoid”. No AID-APOBEC signal was observed in the pharyngeal lymphocyte aggregations previously speculated to be thymic-like niches (see Supplementary Text and Fig. 1).
- 22.Bajoghli B, Aghaallaei N, Hess I, Rode I, Netuschil N, Tay BH, Venkatesh B, Yu JK, Kaltenbach SL, Holland ND, Diekhoff D, Happe C, Schorpp M, Boehm T. Evolution of Genetic Networks Underlying the Emergence of Thymopoiesis in Vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Radtke F, Fasnacht N, MacDonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Velikovsky CA, Deng L, Tasumi S, Iyer LM, Kerzic MC, Aravind L, Pancer Z, Mariuzza RA. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nature Struct Mol Biol. 2009;16:725–731. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, Kerzic MC, Flajnik MF, Aravind L, Pancer Z, Mariuzza RA. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc Natl Acad Sci U S A. 2010;107:13408–13413. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Tasumi S, Velikovsky CA, Xu G, Gai SA, Wittrup KD, Flajnik MF, Mariuzza RA, Pancer Z. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc Natl Acad Sci U S A. 2009;106:12891–12896. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]; VLRA and VLRB binders to a soluble protein, hen egg lysozyme, were cloned into a yeast surface-display library. The expressed sequences can be isolated by ligand-binding and the dissociation constants measured. This paper demonstrates that both types of receptors can directly recognize ligand.
- **27.Chen H, Kshirsagar S, Jensen I, Lau K, Covarrubias R, Schluter SF, Marchalonis JJ. Characterization of arrangement and expression of the T cell receptor γ locus in the sandbar shark. Proc Natl Acad Sci U S A. 2009;106:8591–8596. doi: 10.1073/pnas.0811283106. [DOI] [PMC free article] [PubMed] [Google Scholar]; The content and organization of sandbar shark TCR γ locus were determined. Comparison of expressed cDNA sequences with the five germline V gene segments from a single shark indicate diversification by somatic mutation.
- 28.Lee SS, Tranchina DS, Ohta Y, Flajnik MF, Hsu E. Hypermutation in shark immunoglobulin light chain genes results in contiguous substitutions. Immunity. 2002;16:571–582. doi: 10.1016/s1074-7613(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 29.Parra ZE, Ohta Y, Criscitiello MF, Flajnik MF, Miller RD. The dynamic TCRδ: TCRδ chains in the amphibian Xenopus tropicalis utilize antibody-like V genes. Eur J Immunol. 2010;40:1–11. doi: 10.1002/eji.201040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nature Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 31.Hsu E. V(D)J recombination: of mice and sharks. Adv Exp Med Biol. 650:166–179. doi: 10.1007/978-1-4419-0296-2_14. [DOI] [PubMed] [Google Scholar]
- *32.Kishishita N, Matsuno T, Takahashi Y, Takaba H, Nishizumi H, Nagawa F. Regulation of antigen-receptor gene assembly in hagfish. EMBO reports. 2010;11:126–132. doi: 10.1038/embor.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single cell genomic and RT-PCR of 1000 leukocytes from hagfish showed that VLRA and VLRB assembly is exclusive. ≥90% of cells committed to one VLR type showed primarily monoallelic assembly, although both alleles were transcribed. This is a comprehensive study where the presence of the non-assembled allele was assayed at the same time. In cells where both alleles were assembled many carried a nonfunctional VLR.
- 33.Velez MG, Kane M, Liu S, Gauld SB, Cambier JC, Torres RM, Pelanda R. Ig Allotypic Inclusion Does Not Prevent B Cell Development or Response. J Immunol. 2007;179:1049–1057. doi: 10.4049/jimmunol.179.2.1049. [DOI] [PubMed] [Google Scholar]
- 34.Chothia C, Lesk AM, Tramontano A, Levitt M, Smith-Gill SJ, Air G, Sheriff S, Padlan EA, Davies D, Tulip WR, Colman PM, Spinelli S, Alzari PM, Poljak RJ. Conformations of immunoglobulin hypervariable regions. Nature. 1989;342:877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- 35.Wu TT, Johnson G, Kabat EA. Length distribution of CDRH3 in antibodies. Proteins. 1993;16:1–7. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]
- **36.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen Recognition by Variable Lymphocyte Receptors. Science. 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first crystallographic study on lamprey VLRB complexed with H-antigen trisaccharide from human erythrocytes. The key contacts of the ligand involve the VLR concave surface and the LRRCT insert, whose varying lengths, sequence and secondary structures were compared among different VLRs.
- 37.Saha NR, Smith J, Amemiya CT. Evolution of adaptive immune recognition in jawless vertebrates. Semin Immunol. 2010;22:25–33. doi: 10.1016/j.smim.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Near TJ. Conflict and Resolution Between Phylogenies Inferred From Molecular and Phenotypic Data sets for Hagfish, Lampreys, and Gnathostomes. J Exp Zool (Mol Dev Evol) 2009;312B:749–761. doi: 10.1002/jez.b.21293. [DOI] [PubMed] [Google Scholar]
- 39.Rowley AF. Ultrastructural and cytochemical studies on the blood cells of the sea squirt, Ciona intestinalis. I. Stem cells and amoebocytes. Cell Tissue Res. 1982;223:403–414. doi: 10.1007/BF01258497. [DOI] [PubMed] [Google Scholar]
- *40.Zucchetti I, Santis RD, Grusea S, Pontarotti P, Du Pasquier L. Origin and evolution of the vertebrate leukocyte receptors: the lesson from tunicates. Immunogenetics. 2009;61:463–481. doi: 10.1007/s00251-009-0373-z. [DOI] [PubMed] [Google Scholar]; Tunicates possess the basal chordate genome, before the two rounds of whole-genome duplication that occurred during early vertebrate evolution. This paper established that a syntenic group of genes involved in leukocyte biology is conserved from tunicates to human beings and expressed in hemocytes. The discussion speculates on the evolutionary history of and role played by such genes and some agnathan IgSF molecules recently reported to be directly related to Ig/TCR.
- 41.Ogasawara M, Nakazawa N, Azumi K, Yamabe E, Satoh N, Satake M. Identification of Thirty-four Transcripts Expressed Specifically in Hemocytes of Ciona intestinalis and Their Expression Profiles throughout the Life Cycle. DNA Res. 2006;13:25–35. doi: 10.1093/dnares/dsi025. [DOI] [PubMed] [Google Scholar]
- 42.Janvier P. Modern look for ancient lamprey. Nature. 2006;443:921–924. doi: 10.1038/443921a. [DOI] [PubMed] [Google Scholar]
- 43.Flajnik MF, Du Pasquier L. Evolution of the Immune System. In: Paul WE, editor. Fundamental Immunology. Lippincott Williams & Wilkins; 2008. pp. 57–124. [Google Scholar]
- 44.Azumi K, Santis RD, Tomaso AD, Rigoutsos I, Yoshizaki F, Pinto MR, Marino R, Shida K, Ikeda M, Ikeda M, Arai M, Inoue Y, Shimizu T, Satoh N, Rokhsar DS, Du Pasquier L, Kasahara M, Satake M, Nonaka M. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: “waiting for Godot.”. Immunogenetics. 2003;55:570–581. doi: 10.1007/s00251-003-0606-5. [DOI] [PubMed] [Google Scholar]
- 45.Holland LZ, Albalat R, Azumi K, Benito-Gutierrez E, Blow MJ, Bronner-Fraser M, Brunet F, Butts T, Candiani S, Dishaw LJ, Ferrier DEK, Garcia-Fernandez J, Gibson-Brown JJ, Gissi C, Godzik A, Hallbook F, Hirose D, Hosomichi K, Ikuta T, Inoko H, Kasahara M, Kasamatsu J, Kawashima T, Kimura A, Kobayashi M, Kozmik Z, Kubokawa K, Laudet V, Litman GW, McHardy AC, Meulemans D, Nonaka M, Olinski RP, Pancer Z, Pennacchio LA, Pestarino M, Rast JP, Rigoutsos I, Robinson-Rechavi M, Roch G, Saiga H, Sasakura Y, Satake M, Satou Y, Schubert M, Sherwood N, Shiina T, Takatori N, Tello J, Vopalensky P, Wada S, Xu A, Ye Y, Yoshida K, Yoshizaki F, Yu JK, Zhang Q, Zmasek CM, de Jong PJ, Osoegawa K, Putnam NH, Rokhsar DS, Satoh N, Holland PWH. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 2008;18:1100–1111. doi: 10.1101/gr.073676.107. [DOI] [PMC free article] [PubMed] [Google Scholar]