Abstract
Background
In ambulatory patients with heart failure with reduced ejection fraction (HFrEF), high systolic blood pressure (SBP) is associated with better outcomes. However, it is not known whether there is a ceiling beyond which high SBP has a detrimental effect. Thus, our aim was to assess the linearity of association between SBP and mortality.
Methods
We used the External Peer Review Program (EPRP) and Digitalis Investigation Group (DIG) trial databases of HFrEF patients. Linearity of association of SBP with mortality was assessed by plotting Martingale residuals against SBP. To assess the patterns of relationship of SBP with mortality, we used restricted cubic spline analysis with Cox proportional hazards model.
Results
In patients with mild-to-moderate left ventricular systolic dysfunction (LVSD) (30%≤LVEF<50%), SBP had a non-linear association with mortality in both EPRP (n=3,693) and DIG (n=3,263) databases. In these patients, SBP had a significant U shaped association with mortality in EPRP and a trend towards U shaped relationship in DIG database. In patients with severe LVSD (LVEF<30%), SBP had a linear association with mortality in both EPRP (n=2,906) and DIG (n=3,537) databases, with lower SBP being associated with increased mortality.
Conclusions
SBP has a complex non-linear association with mortality in HF patients. Whereas it has a U shaped association in patients with mild-to-moderate LVSD, it has a linear association with mortality in patients with severe LVSD. Recognition of this pattern of association of blood pressure profile may help clinicians in providing better care for their patients and help improve existing prediction models.
Keywords: heart failure, reduced ejection fraction, systolic blood pressure, blood pressure, mortality, spline analysis, Martingale plots
INTRODUCTION
The relationship of systolic blood pressure (SBP) with long term outcomes in patients with heart failure with reduced ejection fraction (HFrEF) is complex. Although, hypertension is associated with development of incident HFrEF, once the HFrEF is established, it has a protective survival effect. Advanced HFrEF is usually associated with low systolic blood pressure (SBP) and ability to generate high SBP in severe HFrEF patients is accepted as an indicator of a relatively preserved pump function in the presence of appropriate peripheral compensation.
Accordingly, SBP is used as one of the parameters for prediction of survival in patients with established HFrEF. Every 10 mmHg rise in SBP has been shown to be associated with a 13% reduction in mortality. This model, however, assumes a continuous linear inverse protective relationship of SBP with mortality, and does not account for a potential increased risk at higher SBP. Although Lee et al recently identified a U shaped relationship between discharge SBP and long-term mortality in acute decompensated HF patients, data on such an association in stable chronic HF are lacking.
We hypothesized that there is a range of SBP associated with better survival in HFrEF patients, and that a SBP above or below this range would be associated with worse outcomes in this population. We further hypothesized that this relationship is different with varying degrees of left ventricular systolic dysfunction (LVSD). Thus, our aims were to assess the linearity of association between SBP and mortality, the profile of the relationship of SBP with mortality and to examine the patterns of association in different degrees of LVSD.
METHODS
Patient cohort
EPRP Database
We performed a retrospective study of a national cohort of veterans with HFrEF treated in ambulatory clinics at Veterans Affairs medical centers using the Veteran Affairs External Peer Review Program (EPRP) data between October 2000 and September 2002 (n=6,608), as described previously. This database contained qualitative LV function assessments of mild to moderate ((30%≤ LVEF<50%), or severe LV systolic dysfunction (LVEF<30%), and not specific LVEF measurements. Only patients for whom LVEF was determined to be <50% within one year prior to or three months after the clinic visit were included. Four patients were excluded due to missing SBP value and five patients were excluded due to missing outcomes. For variables with <20% missing values, imputation procedures were applied and variables with missing values of more than 20% were excluded from the analyses. Missing values for serum sodium (6.1%), hemoglobin (15.9%) and blood urea nitrogen (7.8%) were imputed. For these continuous variables, the missing values were imputed using linear regression with baseline variables as predictors and constraints applied based on observed minimum and maximum values. All analyses were also repeated by excluding observations with imputed values and the results were found to be concordant. Thus, results using imputed data are shown.
DIG Database
The database used for this study was a public-use copy of the main Digitalis Investigation Group (DIG) study from the National Heart, Lung, and Blood Institute (Bethesda, Maryland). The main DIG study recruited patients from February 1991 through September 1993 to test the effects of effects of digoxin on mortality and hospitalization in patients with HFrEF (LVEF≤45%). Of note, the DIG trial did not exclude patients based on their blood pressure profile, although patients with acute HF were excluded. Three patients with missing SBP values were excluded from the analyses. None of the other variables had missing values >0.3% that were addressed as described above. For categorical variables, categories were imputed based on the predicted probability of occurrence of a particular level generated from logistic regression.
Covariates
EPRP Database
Covariates were identified using backward stepwise Cox PH analysis with exclusion set at 0.05 for the end point of all cause mortality. Significant factors included LVSD, categorized as mild-to-moderate (30% ≤ LVEF <50%) and severe systolic dysfunction (LVEF < 30%), age, body mass index, SBP, hemoglobin level, serum blood urea nitrogen levels, serum sodium levels; presence or absence of peripheral arterial disease, cerebrovascular accident, metastatic cancer, dementia, past hospitalization for HF, chronic obstructive pulmonary disease, diabetes mellitus and; use of beta blockers, ACE inhibitors/Angiotensin receptor blockers and statins. Clinically significant variables (gender and history of past MI) were forced into the model.
DIG Database
Covariates were identified as described above. Significant factors included LVEF as a continuous variable, age, BMI, duration of HF, cardiothoracic index, diastolic blood pressure (DBP), gender, NYHA class; presence or absence of edema, third heart sound, congestion on X ray, DM and; use of potassium sparing diuretics such as spironolactone, Non-potassium sparing diuretics, nitrates and hydralazine. Clinically significant variables (SBP, etiology of HFrEF and use of ACEI/ARB) were forced into the model. Data on the use of cardiac resynchronization therapy and or implantable cardioverter defibrillator were not available in either of the databases.
Statistical Analyses
Survival analyses were done using Cox proportional hazards (PH) model with all the covariates described above for the end-point of all cause mortality. Both accelerated failure time models and PH model were assessed, and PH model was found to have a better fit, which has been used throughout the manuscript. Nonlinearity in the relationship between the log hazard and SBP was assessed by plotting Martingale residuals against SBP. Fractional polynomial curve fitting was done for the plot, and non-linearity was described as 95% CI not overlapping 0. In order to evaluate the functional form of the SBP effect on the log-hazard of mortality, restricted cubic spline analysis for Cox PH model was performed with all covariates, using knots at the 5th, 25th, 50th, 75th, and 95th percentiles of SBP. Continuous variables are reported using mean ± standard deviation. Continuous variables were compared using t test and categorical variables were compared using χ2 test. A 2-sided p value <0.05 for comparisons was considered statistically significant. All analyses were performed using SAS statistical software version 9.1.3 (SAS Institute, Cary, NC).
Source(s) of funding
D.A. is a recipient of a NIH Mentored Career Development Award (5K01-HL092585-02). X.H.T.W. is a W.M. Keck Foundation Distinguished Young Scholar in Medical Research, and is also supported by NIH/NHLBI grants R01-HL089598 and R01HL091947. B.B. is supported by NIH 3U01DE017793 and 9K30RR02229. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Patient Characteristics
The baseline demographic, laboratory characteristics and comorbidities of the patients with HFrEF in the EPRP database are summarized in Table I. The median follow-up duration was 631±201 days. All cause mortality was 25% in the overall group, which was significantly higher in patients with severe LVSD (29%), in comparison with patients with mild-to-moderate LVSD (22.6%, p<0.001). Most of the baseline parameters were different between the two groups as detailed in Table I.
Table I.
Baseline profile of patients in the EPRP database.
| Mild-to-Moderate LVSD (n=3,693) | Severe LVSD (n=2,906) | P value | |
|---|---|---|---|
| Age | 70.4 ± 10.0 | 68.4 ± 10.6 | <0.001 |
| Male (%) | 95.5 | 97.6 | <0.001 |
| SBP | 127.8 ± 20.8 | 120.4 ± 20.3 | <0.001 |
| BMI | 29.3 ± 6.1 | 27.6 ± 5.5 | <0.001 |
| DM (%) | 42.1 | 37.3 | <0.001 |
| H/O MI (%) | 40 | 41 | 0.42 |
| COPD (%) | 29.3 | 23.2 | <0.001 |
| PAD (%) | 28.1 | 27.5 | 0.58 |
| CVA (%) | 21.1 | 21.5 | 0.74 |
| Past HF hospitalization (%) | 19.5 | 27.2 | <0.001 |
| Dementia (%) | 3.0 | 2.1 | 0.03 |
| Metastatic cancer (%) | 1.4 | 1.6 | 0.68 |
| BUN (mg/dl) | 26.2 ± 14.8 | 26.7 ± 14.4 | 0.19 |
| Beta-blockers (%) | 64.6 | 65.1 | 0.69 |
| Hemoglobin (gm/dl) | 13.3 ± 1.9 | 13.5 ± 1.9 | <0.001 |
| Serum sodium (meq/l) | 139.2 ± 3.3 | 138.8 ± 3.7 | <0.001 |
| ACEI/ARB (%) | 82.6 | 89.3 | <0.001 |
| Statins (%) | 64.6 | 65.1 | 0.68 |
ACEI/ARB: angiotensin converting enzyme inhibitors/angiotensin receptor blockers; BMI: body mass index; BUN: blood urea nitrogen; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; DM: diabetes mellitus; EPRP: External Peer Review Program; HF: Heart failure; LVSD: left ventricular systolic dysfunction; MI: myocardial infarction; PAD: peripheral arterial disease; SBP: systolic blood pressure. Data are represented as mean±SD for continuous variables and as percentage for categorical variables.
The baseline demographic, laboratory characteristics and comorbidities of the patients with HFrEF in the DIG database are summarized in Table II. The median follow-up duration was 1064±455 days. All cause mortality was 35% in the overall group, which was significantly higher in patients with severe LVSD (41.9%), in comparison with patients with mild-to-moderate LVSD (27.4%, p<0.001). Most of the baseline parameters were different between the two groups as detailed in Table II.
Table II.
Baseline characteristics in DIG database
| Mild-to-Moderate LVSD (n=3,263) | Severe LVSD (n=3,537) | P value | |
|---|---|---|---|
| Age (years) | 64.0 | 63.0 | 0.001 |
| Male (%) | 73.7 | 81.3 | <0.001 |
| EF (%) | 36.2 ± 4.6 | 21.5 ± 5.2 | <0.001 |
| Duration of HFrEF (months) | 29.5 ± 37.3 | 30.8 ± 36.4 | 0.13 |
| SBP (mm Hg) | 129.4 ± 19.9 | 122.5 ± 19.4 | <0.001 |
| DBP (mm Hg) | 75. 6± 11.0 | 74.3 ± 11.4 | <0.001 |
| BMI | 27.4 ± 5.3 | 26.8 ± 5.1 | <0.001 |
| DM (%) | 29.3 | 27.6 | 0.13 |
| Edema (%) | 18.8 | 21.1 | 0.02 |
| Third heart sound (%) | 20.2 | 30.7 | <0.001 |
| Congestion on X-ray (%) | 11.5 | 17.9 | <0.001 |
| Cardio-thoracic index | 0.52 ± 0.07 | 0.54 ± 0.07 | <0.001 |
| NYHA class | 2.1 ± 0.7 | 2.3 ± 0.7 | <0.001 |
| Ischemic etiology (%) | 73.2 | 68.6 | <0.001 |
| ACEI/ARB (%) | 93.3 | 95.5 | <0.001 |
| Potassium sparing diuretics (%) | 7.7 | 7.5 | 0.82 |
| Non-potassium sparing diuretics (%) | 73.7 | 82.6 | <0.001 |
| Nitrates (%) | 41.8 | 43.4 | 0.18 |
| Hydralazine (%) | 2.1 | 2.1 | 0.93 |
ACEI/ARB: angiotensin converting enzyme inhibitors/angiotensin receptor blockers; BMI: body mass index; DBP: diastolic blood pressure; DIG: Digitalis Investigation Group; DM: diabetes mellitus; EF: ejection fraction; HFrEF: heart failure with reduced ejection fraction; LVSD: left ventricular systolic dysfunction; NYHA: New York Heart Association; SBP: systolic blood pressure. Data are represented as mean±SD for continuous variables and as percentage for categorical variables.
Effect of Systolic Blood Pressure on Survival in Patients with Mild-to-Moderate LVSD
In the EPRP database, SBP was found to have a non-linear association with all-cause mortality both at lower and upper ranges of SBP. On restricted cubic spline analysis adjusted for covariates, SBP was found to have a significant U shaped association with all cause mortality (Fig 1A). The lowest morality was found at around 130 mmHg, which increased significantly both below 120 and above 150 mmHg. Based on the spline curves, we divided the database into four groups: SBP<110 mmHg, 110 mmHg ≤SBP <130 mmHg, 130 mmHg ≤ SBP<150 and SBP≥150 mmHg. Based on Martingale plots, we found that SBP was linearly associated with the log-hazard of all cause mortality in each of the four subgroups.
Figure 1.
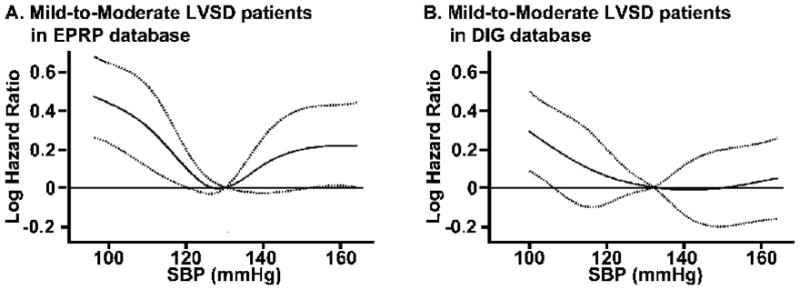
Restricted cubic spline analysis for Cox proportional hazards model, in patients with heart failure with mild-to-moderate left ventricular systolic dysfunction (LVSD), showing A. significantly increased all-cause mortality for systolic blood pressure (SBP) below 120 mm Hg and above 150 mm Hg in External Peer Review Program (EPRP) database and B. significantly increased mortality for SBP below 110 mmHg and a non-significant increase above 150 mmHg in Digitalis Investigation Group (DIG) trial. Solid line represents the estimated logarithmic hazard ratio of all cause mortality with 95% pointwise confidence band represented by broken lines.
In the DIG database, SBP was found to have a non-linear association with all-cause mortality at the lower range of SBP but not at the upper range of SBP. On restricted cubic spline analysis SBP was found to have a significantly increased mortality at pressures below 110 mmHg but only a trend towards an increase at pressures above 150 mmHg (Fig 1B). Based on the spline curves, we divided the database into three groups: SBP<110 mmHg, 110 mmHg ≤ SBP<140 mmHg and SBP≥140 mmHg. Based on Martingale plots, we found that SBP was linearly associated with the log-hazard of all cause mortality in the second and third subgroups but in the first subgroup, there was a non-linear association of SBP with all cause mortality below 90 mmHg.
When the analyses were repeated with further sub-categorization of the mild/moderate LV systolic dysfunction into two groups of patients with mild LVSD (LVEF ≥40 %) and moderate LVSD (40 % > LVEF ≥30 %); the U shaped profile of the association of SBP with mortality in these subgroups (i.e. patients with mild or moderate LV systolic dysfunction) remained similar to the overall mild/moderate LV systolic dysfunction group. But this relationship did not reach significance by restricted cubic spline analysis or by Martingale plots.
Effect of Systolic Blood Pressure on Survival in Patients with Severe LVSD
In both the databases, SBP was found to have a linear association with all-cause mortality throughout the range. This was also confirmed by restricted cubic spline analysis, where SBP was found to have a linear association, with lower SBP being associated with worse mortality in both databases (Fig 2). In the EPRP database, SBP had a linear association with mortality at SBP<120 mmHg and SBP>140 mmHg, with no association with mortality between 120 and 140 mmHg.
Figure 2.
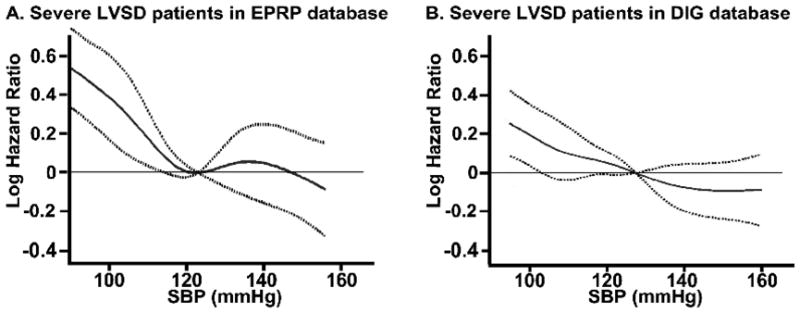
Restricted cubic spline analysis for Cox proportional hazards model, in patients with heart failure with severe left ventricular systolic dysfunction (LVSD), showing a relatively linear association of systolic blood pressure with all cause mortality in A. External Peer Review Program (EPRP) database and B. Digitalis Investigation Group (DIG) trial. Solid line represents the estimated logarithmic hazard ratio of all cause mortality with 95% pointwise confidence band represented by broken lines.
DISCUSSION
This study shows that in ambulatory HFrEF patients with mild-to-moderate LVSD, SBP had a non-linear relationship with mortality. The lowest mortality was in the SBP range of 130–140 mmHg with a significant increase of mortality in patients with SBP below 110 mmHg. In the more recent EPRP database, there was a significantly increased mortality above 150 mmHg, whereas in the DIG database, there was only a trend towards increased mortality at similar SBP range. On the other hand, in HFrEF patients with severe LVSD SBP had a linear association with mortality with higher SBP being associated with better outcomes.
Previous studies have shown that in patients with established HFrEF, low SBP is associated with worse outcomes. In a recent meta-analysis, there was a 13% reduction in the mortality for every 10 mmHg rise in SBP in stable chronic HF patients. However, there is lack of information, whether this relationship is linear or not. Although, a recent study showed that in patients admitted with acute HFrEF, discharge SBP had a U shaped relationship with long-term mortality, such data for chronic HFrEF patients is lacking. Additionally, even though Lee et al, demonstrated that SBP has a significant non-linear association with mortality but this study did not address the characteristics or shape of this non-linearity. Also, in some of these studies, the inverse relationship of SBP with mortality was demonstrated in patient population with relatively low SBP (which is an established risk factor for higher mortality), rather than normal SBP ranges.
The second important finding of our study is that the association of SBP with mortality varies with the severity of LVSD. We found a U shaped relationship of SBP with mortality in patients with mild-to-moderate LVSD. In contrast, in patients with severe LVSD, higher SBP was associated with decreased mortality throughout the range of SBP. In the absence of any previous literature on the same, our study is the first to reveal different patterns of relationship of SBP with mortality, according to the severity of LVSD.
We believe that the U shaped relationship of SBP with mortality may be potentially attributable to two interrelated mechanisms. Whereas on one hand hypertension is a well-known risk factor for worse outcomes, SBP is also dependent on cardiac output and thus lower SBP indicates pump failure or poor contractile reserve in the setting of decompensated or advanced LVSD. In severe LVSD, the second factor may be more dominant resulting in higher SBP having a protective effect on HF outcomes. On the other hand, in the general population the first factor is more dominant resulting in higher SBP having a detrimental effect on survival. Mild-to-moderate LVSD straddles both these populations and, as shown by us, has a symmetrical U shaped relationship of SBP with mortality. When we examined the subgroups of patients with mild LVSD (LVEF≥40) and moderate LVSD (40 % > LVEF ≥30 %) separately; the U shaped profile of the association of SBP with mortality persisted, but did not reach significance probably due to smaller sample size and limited power in these subgroups.
With the use of spline analysis, we found that in patients with mild-to-moderate LVSD, the trough of association of SBP with mortality to be lower in EPRP database than in DIG trial. Specifically, we found that SBP associated with the lowest mortality in the EPRP database to be around 130 mmHg, whereas in the DIG trial it was around 140 mmHg. At the time of the DIG trial, beta blocker therapy was not widespread, but during the EPRP database collection more HF patients were being treated with beta-blockers (Table II). We believe that the addition of beta-blocker therapy may have affected the optimal SBP range that is associated with survival in patients with HFrEF. We would like to clarify though that although this particular range is associated with better prognosis, the goal of this study was not to prove a causal relationship between SBP and mortality and/or define a target for treatment. Our results do underline the need for prospective trials addressing safety and efficacy of different SBP targets in patients with HFrEF, with or without hypertension.
To improve generalizability of our results, we examined two databases. Whereas on one hand DIG trial offered us patients who were prospectively enrolled and underwent close scrutiny during enrollment and follow-up, it represented a historically older HFrEF population enrolled in a clinical trial with certain inclusion and exclusion criteria. In contrast, the EPRP database represents a more recent HFrEF population, examining real world and not clinical trial patients, including all ranges of comorbidities and renal function. However, this database also has the limitations of representing a predominantly male veteran population, and data compilation by chart review. Importantly, in both the EPRP database and DIG trial, there were no exclusions based on SBP levels. This offers unique possibilities to explore the effect of SBP on outcomes as the SBP ranged from 74 mmHg to 220 mmHg in DIG trial and from 66 mmHg to 214 mmHg in EPRP database. Many of the HF trials since then have excluded patients based on their blood pressure profile, and thus by using EPRP database and DIG trial, we could explore the relationship of SBP profile with outcomes throughout the range.
LIMITATIONS
It is important to note that these were post hoc analyses of two databases. Our analyses were limited by covariates collected by design in the two databases, and residual unmeasured confounding factors may exist. Among the variables significantly associated with mortality in HFrEF, we did not have information on important variables such as the beta-blocker use, diuretic dose, hemoglobin, sodium; and BNP levels in DIG database; and NYHA class and specific numeric EF measurements in EPRP database. In addition, DIG trial did not document other non-cardiac comorbidities which could have contributed to all-cause mortality. We believe that our results were strengthened by validating our results in the EPRP database, which included non-cardiac comorbidities and had beta blocker use, hemoglobin levels and sodium levels that were not available in DIG database. Also, the cut off for diagnosing HFrEF in the two databases was slightly different. Although, this introduces heterogeneity, but by conducting analyses in both the databases separately, we believe our results are both reproducible and generalizable. Furthermore, spline analysis has an inherent limitation of over fitting the data. Thus, we used two large databases to confirm our findings and moreover limited the fitting to 5th and 95th percentile.
CONCLUSION
SBP has a complex relationship with outcomes in HFrEF patient that varies with the severity of systolic dysfunction. Specifically, SBP has a U shaped association with mortality in mild-to-moderate LVSD patients, with better outcomes in the range of 130–140 mmHg. On the other hand, in severe LVSD patients, SBP has a relatively linear association with mortality with higher SBP portending better prognosis. Identification of these patterns of association of SBP with mortality may help in better prognostication and management of these patients.
Acknowledgments
The Digitalis Investigation Group (DIG) study is conducted and supported by the NHLBI in collaboration with the Digitalis Investigation Group (DIG) Investigators. This manuscript was not prepared in collaboration with investigators of the Digitalis Investigation Group (DIG) and the views expressed in this article are those of the authors and do not necessarily reflect the opinions or views of the Digitalis Investigation Group (DIG), NHLBI or Department of Veteran Affairs. The authors thank the Office of Quality and Performance of the Veterans Health Administration for Providing External Peer Review Program data and NHLBI for providing the DIG database.
ABBREVIATIONS
- COPD
Chronic obstructive pulmonary disease
- DBP
Diastolic blood pressure
- DIG
Digitalis Investigation Group
- LVEF
Left ventricular ejection fraction
- EPRP
External Peer Review Program
- HFrEF
Heart Failure with reduced ejection fraction
- HR
hazard ratio
- LVSD
Left ventricular systolic dysfunction
- PH
proportional hazards
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.


