Abstract
Activation-induced cytidine deaminase (AID) is a key enzyme for antibody-mediated immune responses. Antibodies are encoded by the immunoglobulin genes and AID acts as a transcription-dependent DNA mutator on these genes to improve antibody affinity and effector functions. An emerging theme in field is that many transcribed genes are potential targets of AID, presenting an obvious danger to genomic integrity. Thus there are mechanisms in place to ensure that mutagenic outcomes of AID activity are specifically restricted to the immunoglobulin loci. Cis-regulatory targeting elements mediate this effect and their mode of action is likely a combination of immunoglobulin gene specific activation of AID and a perversion of faithful DNA repair towards error-prone outcomes.
Introduction
Activation-induced cytidine deaminase (AID) is a DNA mutator enzyme that initiates somatic hypermutation (SHM), immunoglobulin gene conversion (GCV), and class switch recombination (CSR) [1–3]. These processes are critical for an efficient immune response as they drive the generation of high-affinity antibodies, mediated by SHM and GCV, and optimal effector function in the case of CSR. The cognate substrates of AID are the immunoglobulin (Ig) genes, but there is an increasing body of evidence that AID on rare occasions drives the generation of mutations and translocations of non-Ig genes [4]. Such misdirected AID activity leads to deleterious outcomes like lymphomas, and thus understanding how beneficial mutagenesis is largely targeted to Ig loci is a key question in the field. Here we review recent progress over the last two years towards addressing this question, in particular the identification of cis-regulatory “targeting” elements that are critical for SHM and GCV. We also briefly discuss similar findings for CSR. Lastly, we present revised models for targeting of SHM, GCV, and CSR, incorporating most of the recent reports in this area.
The basic mechanism of AID dependent sequence diversification
Upon encountering antigen, B cells activate the secondary Ig gene diversification processes SHM, GCV, and CSR. All three processes are initiated by AID, which introduces U:G mismatches within the variable region or the switch repeats of Ig genes, in the case of SHM and GCV, or CSR, respectively. During SHM this U:G mismatch is repaired by direct replication or by error-prone base excision repair (BER) and mismatch repair (MMR) pathways involving low fidelity DNA polymerases [5]. The outcomes are point mutations or small insertions and deletions, all of which can alter the affinity of the encoded antibody. Repeated cycles of hypermutation and selection in the germinal center are referred to as “affinity maturation”. In contrast, during GCV the U:G mismatches are subject to homology-based repair involving upstream pseudo V genes resulting in multiple “templated” mutations in a single event [6]. GCV occurs only in a subset of jawed vertebrates, including chicken and rabbits, and is also thought to contribute to the generation of the pre-immune Ig repertoire in these species. Lastly, CSR requires the conversion of U:G mismatches to DNA double-strand breaks by components of BER and MMR pathways [7,8]. The DNA fragment in between Sμ and downstream Sγ, Sα, or Sε switch repeats is excised, and the subsequent ligation of the chromosomal DNA ends by non-homologous end-joining replaces the IgM constant region exons with those encoding the IgG, IgA, or IgE isotypes.
Biochemical studies demonstrated that the substrates of AID are cytidines presented in the context of single-stranded DNA [9–11]. In vitro, U:G mismatches within double-stranded DNA are only generated in the context of transcription, which transiently exposes unpaired bases in the transcription bubble [11,12]. This requirement holds true for all AID-mediated sequence diversification processes in vivo. Transcription by itself, however, is not sufficient to recruit AID activity to a gene locus in vivo, as promoters [13] and cis-regulatory targeting elements (see sections “Targeting of SHM and GCV” and “Targeting of CSR”) impose a critical additional layer of regulation.
Amongst the three AID-mediated processes, CSR stands out based on its unique DNA substrate, the switch repeats, and its reaction intermediates, double-strand breaks. SHM and GCV, however, are thought to be closely related, and hence will largely be discussed together throughout this review. Interestingly, the deletion of all pseudo V segments and the deletion of factors involved in homology-based DNA repair in chicken B cells both abolish GCV and in turn increase the number of non-templated mutation events reminiscent of SHM [14,15]. This suggests that SHM is the default pathway to resolve U:G mismatches in the variable genes in Ig loci, and that GCV is an add-on in the presence of homologous repair templates. But it is important to note that the pattern of point mutations in murine B cells and pseudo V-deficient chicken B cells differs [14]. Hence it is possible that distinct targeting mechanisms exist for the unique DNA repair factor requirements of each of these three processes, SHM, GCV, and CSR.
Genome-wide mutagenesis by AID
SHM was originally described as a process unique to Ig genes, and is essential for affinity maturation and highly effective humoral immune responses. It is, however, becoming increasingly apparent that AID-dependent mutagenesis occurs on a far wider range of genes. Strikingly, AID activity on non-Ig genes contributes to B cell lymphomagenesis, with mutations in the BCL-6 regulatory region and c-myc/IgH translocations being prime examples of the underlying molecular events [16–19]. A recent study by the Schatz lab convincingly showed that AID introduces mutations in a large number of genes in activated B cells albeit at very low levels, and that error-free DNA repair further reduced potentially deleterious outcomes in many of these genes [20]. Similarly, homologous recombination prevents widely-spread DNA breaks and translocations in this scenario [21]. A common denominator of all mutated genes was active transcription [20], consistent with in vitro studies using recombinant AID. In addition, a recent chromatin immunoprecipitation sequencing (ChIPseq) study provided evidence that AID is indeed physically present and widely distributed across almost all transcribed genes in activated B cells [22]. In this context, the association of AID with RNA polymerase II (polII) complexes is mediated by Spt5, a transcription elongation factor, which recruits AID to stalled polymerase complexes [23].
In summary, two patterns of AID-dependent mutagenesis are present in activated B cells: widely distributed basal levels that contribute to lymphomagenesis, and narrowly localized high levels (at least 20- to 50-fold higher than the basal) in the Ig genes required for antibody diversity. The former are suppressed by error-free DNA repair while the latter are exacerbated by error-prone DNA repair. The specific recruitment of high-levels of mutagenic activity is referred to as “targeting”, and this topic is the focus of the next sections of this review.
Targeting of SHM and GCV
Early transgenic studies, in particular of the murine Igκ gene, implicated transcriptional enhancers as important elements for recruiting the SHM machinery to the Ig loci [24]. But the individual deletion of these enhancers, the intronic enhancer and its associated matrix attachment region (ie/MAR), the 3′ enhancer (3′kE), and the distal enhancer (Ed) in respective knock-out mice showed surprisingly little effects on SHM [25,26]. Importantly, the modest reductions in SHM could readily be explained by concomitant decreases in transcription. Redundancy of these enhancers with respect to targeting SHM remains a plausible explanation, but the importance of these elements for early B cell development provides a challenging hurdle for experimental testing [27]. Thus, the identity of targeting elements in murine Ig genes remains elusive, and the experimental challenges prompted the search for a better suited model system.
The IGL gene in the chicken DT40 B cell line is constitutively subjected to SHM and GCV, and the genome of these cells can be manipulated by standard gene-targeting strategies. Thus this cell line emerged as an attractive widely used model system to identify cis-regulatory elements required for AID-mediated sequence diversification. Using DT40 cells, we provided the very first experimental evidence for the existence of such targeting elements for SHM/GCV within an endogenous Ig locus [28], and these findings were later confirmed by others [29,30]. We now narrowed the location of one of these elements to within 650 bp, and showed that it is evolutionarily conserved in terms of sequence and function (Kothapalli et al., unpublished data). In contrast to a prevailing model in the field, these targeting elements are distinct from classic transcription enhancers (Kothapalli et al., unpublished data), and importantly are also active when transferred to non-Ig loci [29,30]. Therefore these elements constitute a novel class of cis-regulatory elements, defined by their ability to influence local genome stability.
The trans-acting factors binding to these regulatory DNA elements and mediating targeting remain elusive. E-box transcription factors (likely E2A) emerged as important candidates [31–34][35], but the importance of their binding sites within endogenous Ig loci has not yet been demonstrated. Additional transcription factors including NFκB have been implicated [30], but the broad role of all these transcription factors in gene expression in B lymphocytes and other cell types argues against a unique role in targeting of SHM and GCV. In summary, despite the recent progress, the identities of individual binding sites critical for targeting and their corresponding trans-acting factors are still the biggest mysteries in the field.
Targeting of CSR
The DNA sequence of the switch repeat regions in the Igh locus promotes the transcription-dependent formation of stable R-loops [36], and the unpaired DNA strand of such structures provides a perfect substrate for AID. It is unclear, however, whether these R-loops by themselves are sufficient to sequester AID to achieve efficient CSR. Notably, the switch repeats in the igh locus of Xenopus laevis do not form R-loops, but readily serve as AID substrates during CSR [37].
In addition to the nucleotide sequences of the AID substrates themselves, targeting of CSR to the murine Igh locus has also long been thought to involve the 3′ regulatory region, which consists of four DNaseI hypersensitive sites HS1, HS2, HS3A/B, and HS4. The deletion of these hypersensitive sites individually had little effect on CSR [38–40] suggesting redundancy of these elements. The combined deletion of all four sites, however, resulted in a dramatic reduction of CSR to all isotypes, but also in a simultaneous reduction in the levels of CSR-associated transcription of switch repeats [41,42]. Thus it is still unclear whether these hypersensitive sites control CSR by virtue of being enhancers of transcription [43], or also by recruitment of factors critical for AID-initiated DNA recombination. The fine-mapping and subsequent deletion of important binding sites and cross-complementation studies with non-Ig transcription enhancers will likely provide a conclusive answer to this question.
The mechanism of targeting
The molecular mechanism that underlies the targeting of mutagenic AID activity to Ig gene loci remains still elusive. The importance of cis-regulatory elements indicates that critical components of the mutator machinery are recruited to the Ig loci, or, alternatively that these loci are recruited to sub-nuclear compartments that could be simplistically labeled as “mutator factories” (Fig. 1). Such a factory model has been invoked for transcription [44] and V(D)J recombination [45] ; but whether the Ig loci are moving to distinct areas of the nucleus or important factors move to Ig loci, in each case a unique micro-environment is created that supports mutagenesis of Ig gene sequences. As AID is physically present on a large number of transcribed genes, the most parsimonious model stating that AID is specifically recruited to Ig loci is likely invalid. A revised model for targeting now proposes that AID is rendered highly active by post-translational modifications and/or interactions with other proteins (Fig. 2A). This activation would be Ig gene specific and mediated directly (or indirectly) by the trans-acting factors binding to the targeting elements. The recruitment of PKA to phosphorylate AID in the context of CSR might be one example of such regulation [46]. An alternative, but not mutually exclusive model proposes that DNA repair at Ig loci is particularly error-prone (Fig. 2B). This is consistent with the observations that error-free repair is prevalent throughout the genome, thereby protecting non-Ig genes from AID-dependent mutagenesis [20,21]. The recruitment of such error-prone repair to (or the exclusion of error-free repair from) Ig loci in activated B cells could be mediated by distinct chromatin states that are non-permissive for the respective undesired mode of DNA repair. Localized recruitment of error-prone repair would also be an interesting general concept to explain restricted islands of genomic instability including recurrent translocations and DNA recombination breakpoints.
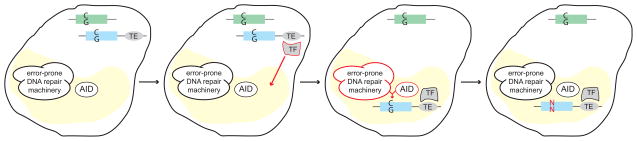
Figure 1. Mutator factories and targeting of AID-mediated mutagenesis.
Schematic representation of the B cell nucleus including a mutator factory (yellow), and a step-by-step depiction of the targeting process. In this model, the cis-regulatory targeting element (TE, grey oval) associated with the Ig gene (blue) is recognized by a targeting factor (TF), and upon binding this factor repositions the Ig gene to the mutator factory. A non-Ig locus (green) lacks such control sequences and remains at its original location. Within the mutator factory, AID deaminates individual cytidines (C), and subsequent error-prone DNA repair introduces a point mutation at or around this site (N). C:G base pairs in the context of the non Ig gene remain unaltered.
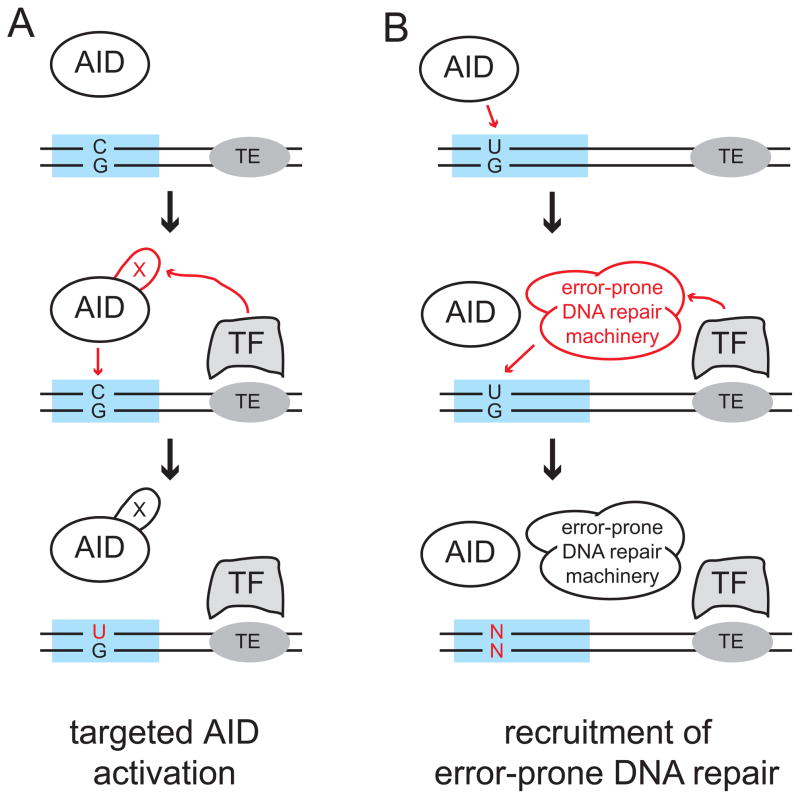
Figure 2. Immunoglobulin gene specific activation of AID or recruitment of error-prone repair.
Two models are widely entertained to explain the mechanism of targeting of AID-mediated mutagenesis to Ig loci, and it is important to point out that these are not mutually exclusive. (A) AID is physically present on a large number of transcribed genes, including Ig genes (blue). The binding of a targeting factor (TF) to a cis-regulatory targeting element (TE, grey oval) initiates a post-translational modification of AID that dramatically increases its activity. This introduces a level of U:G mismatches in Ig genes that is much higher than that observed in the rest of the genome, and the resolution of these lesion results in SHM, GCV, and CSR. Note, within the limits of this model it also is possible that a cofactor of AID is uniquely recruited, or that the modification does not occur on AID but rather on such cofactor of AID. (B) AID is physically present and active as a deaminase on a large set of genes, including Ig genes (blue). The cognate interaction between the cis-regulatory targeting element and its corresponding trans-acting targeting factor activates (or recruits) error-prone DNA processes. These convert the U:G mismatches to random point mutations within the Ig loci, and such lesions in non-Ig genes are fixed by faithful repair.
Conclusions
The emerging picture in the field is that cis-regulatory elements are critical to direct the AID-mediated sequence diversification processes to the Ig loci. This targeting likely involves the recruitment of AID co-factors or modifying enzymes that activate AID specifically on Ig genes. A two-pronged approach, the generation of systematic deletions in non-coding regions of Ig loci and studies of proteins interacting with AID, will likely bring dramatic advances towards this decades old question of SHM, GCV, and CSR targeting in the near future. Lastly, we hope such studies will also provide new insights into the molecular basis of mis-directed AID activity that causes B cell lymphomas, and towards understanding general principles that govern genomic instability.
Acknowledgments
We thank Drs. Nina Papavasiliou and Shu Yuan Yang for helpful comments and suggestions. This work was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 2.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Schatz DG. Balancing AID and DNA repair during somatic hypermutation. Trends Immunol. 2009;30:173–181. doi: 10.1016/j.it.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Maul RW, Gearhart PJ. AID and somatic hypermutation. Adv Immunol. 2010;105:159–191. doi: 10.1016/S0065-2776(10)05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arakawa H, Buerstedde JM. Immunoglobulin gene conversion: insights from bursal B cells and the DT40 cell line. Dev Dyn. 2004;229:458–464. doi: 10.1002/dvdy.10495. [DOI] [PubMed] [Google Scholar]
- 7.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri J, Basu U, Zarrin A, Yan C, Franco S, Perlot T, Vuong B, Wang J, Phan RT, Datta A, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 9.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci U S A. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 12.Sohail A, Klapacz J, Samaranayake M, Ullah A, Bhagwat AS. Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations. Nucleic Acids Res. 2003;31:2990–2994. doi: 10.1093/nar/gkg464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SY, Fugmann SD, Schatz DG. Control of gene conversion and somatic hypermutation by immunoglobulin promoter and enhancer sequences. J Exp Med. 2006;203:2919–2928. doi: 10.1084/jem.20061835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa H, Saribasak H, Buerstedde JM. Activation-induced cytidine deaminase initiates immunoglobulin gene conversion and hypermutation by a common intermediate. PLoS Biol. 2004;2:E179. doi: 10.1371/journal.pbio.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sale JE, Calandrini DM, Takata M, Takeda S, Neuberger MS. Ablation of XRCC2/3 transforms immunoglobulin V gene conversion into somatic hypermutation. Nature. 2001;412:921–926. doi: 10.1038/35091100. [DOI] [PubMed] [Google Scholar]
- *16.Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–1038. doi: 10.1016/j.cell.2008.09.062. In this elegant study the authors provide definitive evidence that AID activity is the primary source of DNA double-strand breaks at the c-Myc locus that resulting in c-Myc/Igh translocations during aberrant class switch recombination. This suggests that AID-dependent breaks could be the initial event of many translocations in B cell lymphomagenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Pasqualucci L, Bhagat G, Jankovic M, Compagno M, Smith P, Muramatsu M, Honjo T, Morse HC, 3rd, Nussenzweig MC, Dalla-Favera R. AID is required for germinal center-derived lymphomagenesis. Nat Genet. 2008;40:108–112. doi: 10.1038/ng.2007.35. Numerous mouse models for B cell lymphomas have been developed over the last decades. In this systematic study the authors show that Bcl-6 dependent, germinal center derived B-cell lymphomas are strongly dependent on AID, while Myc driven pre-B cell lymphomas form independent of AID. [DOI] [PubMed] [Google Scholar]
- 18.Shen HM, Peters A, Baron B, Zhu X, Storb U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science. 1998;280:1750–1752. doi: 10.1126/science.280.5370.1750. [DOI] [PubMed] [Google Scholar]
- 19.Pasqualucci L, Migliazza A, Fracchiolla N, William C, Neri A, Baldini L, Chaganti RS, Klein U, Kuppers R, Rajewsky K, et al. BCL-6 mutations in normal germinal center B cells: evidence of somatic hypermutation acting outside Ig loci. Proc Natl Acad Sci USA. 1998;95:11816–11821. doi: 10.1073/pnas.95.20.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. This thorough study provided the first conclusive evidence that low levels of AID activity are found on large numbers of genes in B cells isolated from the Peyer’s patches of mice. In addition, the analysis of DNA repair defective mouse strains indicated that non-Ig genes are protected from deleterious outcomes of such mutations by error-free DNA repair. This suggests that the outcome of AID-dependent process is at least in part controlled by the gene locus specific differences in DNA repair. [DOI] [PubMed] [Google Scholar]
- * 21.Hasham MG, Donghia NM, Coffey E, Maynard J, Snow KJ, Ames J, Wilpan RY, He Y, King BL, Mills KD. Widespread genomic breaks generated by activation-induced cytidine deaminase are prevented by homologous recombination. Nat Immunol. 2010;11:820–826. doi: 10.1038/ni.1909. This paper provides further evidence for the promiscuity of AID and shows that AID-mediated DNA breaks cause severe genomic instability at a genome-wide level in the absence of homologous recombination based DNA repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Yamane A, Resch W, Kuo N, Kuchen S, Li Z, Sun H-W, Robbiani DF, McBride K, Nussenzweig MC, Casellas R. Deep-sequencing Reveals the Genomic Targets of AID and RPA in B lymphocytes. Nat Immunol. 2010 doi: 10.1038/ni.1964. in press. This landmark paper reports the genome-wide distribution of AID as determined by chromatin immunoprecipitation followed by high-throughput sequencing. The surprising conclusion is that AID is far more widely distributed than previously anticipated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Pavri R, Gazumyan A, Jankovic M, Di Virgilio M, Klein I, Ansarah-Sobrinho C, Resch W, Yamane A, San-Martin BR, Barreto V, et al. Activation-Induced Cytidine Deaminase Targets DNA at Sites of RNA Polymerase II Stalling by Interaction with Spt5. Cell. 2010;143:122–133. doi: 10.1016/j.cell.2010.09.017. Using a RNAi screen in CH12 B cells, the authors identify the transcription elongation factor Spt5 as an important AID interaction partner for CSR. Spt5 is critical to re-start stalled RNA polymerase II complexes, and its genome-wide distribution correlates well with the observed presence of AID-dependent mutations in respective sites. Thus Spt5 is likely the long-sought link between transcription and AID-mediated sequence diversification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- 25.Inlay MA, Gao HH, Odegard VH, Lin T, Schatz DG, Xu Y. Roles of the Ig kappa light chain intronic and 3' enhancers in Igk somatic hypermutation. J Immunol. 2006;177:1146–1151. doi: 10.4049/jimmunol.177.2.1146. [DOI] [PubMed] [Google Scholar]
- 26.Xiang Y, Garrard WT. The Downstream Transcriptional Enhancer, Ed, positively regulates mouse Ig kappa gene expression and somatic hypermutation. J Immunol. 2008;180:6725–6732. doi: 10.4049/jimmunol.180.10.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inlay M, Alt FW, Baltimore D, Xu Y. Essential roles of the kappa light chain intronic enhancer and 3' enhancer in kappa rearrangement and demethylation. Nat Immunol. 2002;3:463–468. doi: 10.1038/ni790. [DOI] [PubMed] [Google Scholar]
- **28.Kothapalli N, Norton DD, Fugmann SD. Cutting Edge: A cis-Acting DNA Element Targets AID-Mediated Sequence Diversification to the Chicken Ig Light Chain Gene Locus. J Immunol. 2008;180:2019–2023. doi: 10.4049/jimmunol.180.4.2019. This study provides the first experimental evidence for the existence of cis-acting DNA elements essential for targeting AID-mediated sequence diversification. A systematic deletion analysis of the IGL locus in chicken DT40 cells leads to the identification of the 3' regulatory region, a non-coding DNA sequence that contains a targeting element for SHM and GCV as well as a classic transcriptional enhancer. [DOI] [PubMed] [Google Scholar]
- **29.Blagodatski A, Batrak V, Schmidl S, Schoetz U, Caldwell RB, Arakawa H, Buerstedde JM. A cis-acting diversification activator both necessary and sufficient for AID-mediated hypermutation. PLoS Genet. 2009;5:e1000332. doi: 10.1371/journal.pgen.1000332. The authors confirm previous findings of targeting elements for SHM and GCV (see [28]) in the chicken IGL locus, and make the striking observation that AID-mediated mutability can be transferred when these elements are inserted in non-Ig loci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Kim Y, Tian M. NF-kappaB family of transcription factor facilitates gene conversion in chicken B cells. Mol Immunol. 2009;46:3283–3291. doi: 10.1016/j.molimm.2009.07.027. The third report (see [28] and [29]) reporting the existence of cis-regulatory targeting elements in the IGL locus of chicken DT40 cells. The authors also show that these sequences are functional outside the IGL locus, and implicate NFκ B and other transcription factors in its function. [DOI] [PubMed] [Google Scholar]
- 31.Michael N, Shen HM, Longerich S, Kim N, Longacre A, Storb U. The E box motif CAGGTG enhances somatic hypermutation without enhancing transcription. Immunity. 2003;19 :235–242. doi: 10.1016/s1074-7613(03)00204-8. [DOI] [PubMed] [Google Scholar]
- *32.Tanaka A, Shen HM, Ratnam S, Kodgire P, Storb U. Attracting AID to targets of somatic hypermutation. J Exp Med. 2010;207:405–415. doi: 10.1084/jem.20090821. This study strengthens previous claims of the authors that CAGGTG motifs in context of murine Ig enhancers are sufficient to promote AID-mediated sequence diversification using a transgenic approach in DT40 cells. The authors suggest that the lymphocyte-specific transcription factor E2A mediates this effect without altering transcription per se. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yabuki M, Ordinario EC, Cummings WJ, Fujii MM, Maizels N. E2A acts in cis in G1 phase of cell cycle to promote Ig gene diversification. J Immunol. 2009;182:408–415. doi: 10.4049/jimmunol.182.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conlon TM, Meyer KB. The chicken Ig light chain 3'-enhancer is essential for gene expression and regulates gene conversion via the transcription factor E2A. Eur J Immunol. 2006;36:139–148. doi: 10.1002/eji.200535219. [DOI] [PubMed] [Google Scholar]
- 35.Schoetz U, Cervelli M, Wang YD, Fiedler P, Buerstedde JM. E2A expression stimulates Ig hypermutation. J Immunol. 2006;177:395–400. doi: 10.4049/jimmunol.177.1.395. [DOI] [PubMed] [Google Scholar]
- 36.Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 37.Zarrin AA, Alt FW, Chaudhuri J, Stokes N, Kaushal D, Du Pasquier L, Tian M. An evolutionarily conserved target motif for immunoglobulin class-switch recombination. Nat Immunol. 2004;5:1275–1281. doi: 10.1038/ni1137. [DOI] [PubMed] [Google Scholar]
- 38.Manis JP, van der Stoep N, Tian M, Ferrini R, Davidson L, Bottaro A, Alt FW. Class switching in B cells lacking 3' immunoglobulin heavy chain enhancers. J Exp Med. 1998;188:1421–1431. doi: 10.1084/jem.188.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bebin AG, Carrion C, Marquet M, Cogne N, Lecardeur S, Cogne M, Pinaud E. In vivo redundant function of the 3' IgH regulatory element HS3b in the mouse. J Immunol. 2010;184:3710–3717. doi: 10.4049/jimmunol.0901978. [DOI] [PubMed] [Google Scholar]
- 40.Vincent-Fabert C, Truffinet V, Fiancette R, Cogne N, Cogne M, Denizot Y. Ig synthesis and class switching do not require the presence of the hs4 enhancer in the 3' IgH regulatory region. J Immunol. 2009;182:6926–6932. doi: 10.4049/jimmunol.0900214. [DOI] [PubMed] [Google Scholar]
- *41.Dunnick WA, Collins JT, Shi J, Westfield G, Fontaine C, Hakimpour P, Papavasiliou FN. Switch recombination and somatic hypermutation are controlled by the heavy chain 3' enhancer region. J Exp Med. 2009;206:2613–2623. doi: 10.1084/jem.20091280. One of two studies (see [40]) that demonstrates a critical role of the murine IgH 3′ regulatory region for CSR using a BAC transgene approach in mice. The authors also conclude a respective role for SHM in the same locus. It remains, however, unclear whether the effects are solely caused by dramatically reduced transcription or also result from a lack of cis-regulatory targeting elements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Vincent-Fabert C, Fiancette R, Pinaud E, Truffinet V, Cogne N, Cogne M, Denizot Y. Genomic deletion of the whole IgH 3' regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood. 2010;116:1895–1898. doi: 10.1182/blood-2010-01-264689. This study confirms the necessity of murine Igh 3’ regulatory region for CSR (see [39]) now using traditional knock-out strategies. This study also shows that the enhancers in the 3’ regulatory region are not essential for B cell maturation. [DOI] [PubMed] [Google Scholar]
- 43.Cogne M, Birshtein BK. Regulation of class switch recombination. In: Honjo T, Alt FWS, editors. Molecular Biology of B Cells. NM: Academic Press; 2004. pp. 289–305. [Google Scholar]
- 44.Bentley D. The mRNA assembly line: transcription and processing machines in the same factory. Curr Opin Cell Biol. 2002;14:336–342. doi: 10.1016/s0955-0674(02)00333-2. [DOI] [PubMed] [Google Scholar]
- 45.Ji Y, Resch W, Corbett E, Yamane A, Casellas R, Schatz DG. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–431. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Vuong BQ, Lee M, Kabir S, Irimia C, Macchiarulo S, McKnight GS, Chaudhuri J. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nat Immunol. 2009;10:420–426. doi: 10.1038/ni.1708. In this study the authors present the provocative finding that protein kinase A is recruited to switch repeats in the murine Igh locus where it leads to localized phosphorylation of AID and this in turn recruits replication protein A. This set of events provides evidence for a novel pathway that regulates CSR efficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]