Abstract
The objective of this work is to assess the feasibility of successfully repairing the torn ACL. Two major motivators for developing a new treatment for ACL injuries are the recently reported high rates of osteoarthritis after conventional ACL reconstruction as well as the problem of how to safely treat skeletally immature patients. A key factor in developing such a technique was the identification of the main inhibitor of intrinsic ACL healing – the lack of clot formation between the two torn ends of the ligament. A bioactive and biocompatible scaffold which could be placed in the wound site to enhance cellular proliferation and biosynthesis was developed. This biomaterial has shown promising functional outcomes in several large animal models of primary repair of partial and complete ACL transection over 4 to 14 weeks, suggesting potential for a successful, future clinical application.
Keywords: Tissue engineering, ACL, regeneration, PRP, animal model, biomechanics
From replacement to regeneration
Around the beginning of the 19th century, the discovery of antibiotics and new advances in material science set the stage for the development of orthopaedic implants and durable prostheses. This opened the door for new, highly effective, treatments for diseases like arthritis and osteonecrosis by replacing the damaged tissue with synthetic materials, including polyethylene, metals and ceramics. Later in that century, newly acquired knowledge in both biology and material science offered additional instruments for physicians to further advance treatment options for such diseases. The most influential advances were cell culture techniques and the development of processing techniques to create biocompatible, degradable (bio)materials. These technologies have coalesced into what is commonly known as tissue engineering. As the name implies, this new technology combines knowledge from both engineering and the life sciences, and aims at the creation of biological substitutes or restoration of tissue and organ function by enhanced regeneration.
Despite the undeniably large success of the gold standard treatments such as total joint replacement, the advent of tissue engineering precipitated a move from replacement of damage tissue to enhanced repair and regeneration of tissues; for example, autologous chondrocyte implantation for cartilage defects, bioactive plugs for femoral head necrosis, and autologous cell injections for intervertebral discs. A major driving force behind these developments was a shift in the demographics of the orthopaedic patients with an increasing prevalence of patients with high demand and/or of young age1; 2. This population, given its specific requirements, does not lend itself to classic replacement, mostly because these patients are particularly prone to complications such as wear and loosening of implants over time. Tissue engineering methods potentially offer such patients biological regeneration and repair as a procedure which could delay the need replacement by years, if not for decades.
Repair of the ACL: necessary?
Developing a regenerative method for repair of the torn ACL begs the question whether there is need for such a technique at all. Three arguments can be made why there is need for such a technique. First, ACL treatment aims at achieving two things, 1) immediately resolving knee instability and pain and, 2) avoiding long-term complications, primarily osteoarthritis. The current gold standard treatment for complete tears of the ACL, reconstruction using an autologous or allogenic tendon graft, yields excellent results for instability and pain in most patients. However, in adolescents there is a higher failure rate than in other age groups with up to 20% to 25% of patients experiencing problems postoperatively3. This problem suggests a different solution may be more optimal for this patient age group. In addition, as more and more systematically gathered long-term follow-up data on ACL reconstructions is becoming available, evidence shows high rates of osteoarthritis despite treatment with ACL reconstruction4-7. These data suggest other options and techniques could improve outcomes.
Second, there are still no standardized treatment options for skeletally immature patients with ACL tears, and the incidence of such injuries has been steadily rising3. ACL reconstruction with transphyseal grafts is still bevlieved to be a risk factor for limb length and angular deformities8; 9 by many, despite the fact that several studies have shown no increase in the rate of such complications10-12. However, the pre-pubescent population could benefit greatly from a regenerative treatment which does not require violation of the physes, and, as has been shown recently, this group of patients may have the potential to respond most strongly to the biological stimuli employed in current enhanced repair techniques13; 14. A repair procedure which does not involve transphyseal drilling therefore has a lower risk profile of physeal complications15.
Third, regeneration of the ACL, rather than replacement with a similar type of tissue, has the potential to preserve the proprioceptive nerve fibers and the complex architecture of the ligament insertion side, features that are usually not reproduced by tendon grafts16. This could potentially lead to more normal biomechanics of the knee if adequate regeneration is achieved16.
Repair of the ACL: possible?
Historically, simple suture repair re-approximating the distal and proximal was one of the earliest suggested techniques for treating ACL tears. As early as 1895, primary repair was described17, but it wasn't until the 1950s that the technique was documented by O'Donoghue18-20. Feagin21 and Cabaud22 were the first to report long-term outcomes in the 1970s. However, these early techniques were reported to fail in over 90% of patients21 and, after Sandberg showed in a randomized controlled trial that there was no difference in outcomes after primary repair versus conservative treatment23, suture repair was soon abandoned for surgical replacement of the ACL. But the question of why these repairs failed remained unanswered. Only recently, when the attention of investigators shifted from reconstruction to new ways of ACL treatment, have some answers begun to be found16. Comparing the cells of the spontaneously healing collateral ligaments of the knee with the cruciate ligaments, there were no significant differences in three important biologic processes in wound repair and regeneration 24; 25 26.(Table 1) However, the ACL, in contrast to extra-articular ligaments, does not form a fibrin-platelet clot at the site of the defect, a clot which is the primary scaffolding material filling the wound of the medial collateral ligament. Without this clot, the gap between the two torn ends remains open, and in addition, the inflammatory cascade leading to cell immigration and tissue remodeling is disrupted at its earliest stage24. The reason for this lacking clot formation may be the intra-articularly circulating plasmin in the synovial fluid, which prematurely breaks down the fibrin clot27; 28 . The synovial fluid has also been shown to inhibit ACL fibroblasts26. Premature loss of this fibrin clot may be one mechanism explaining why the ACL does not heal, neither in the natural history nor after suture repair.
Table 1.
Response of the ACL and MCL to injury
| Parameter | ACL | MCL |
|---|---|---|
| Cellular proliferation | yes | yes |
| Cellular migration | yes | yes |
| Collagen production | yes | yes |
| Cells inhibted by synovial fluid | yes | no |
| Clot formation at defect site | no | yes |
Identifying this premature loss of scaffold allowed scientists to investigate potential solutions, and one logical answer to the lack of clot formation was to create an artificial substitute for the fibrin-platelet clot that would not be prematurely broken down in the synovial environment. (Figure 2) One suggestion was to use hyaluronic acid, which could be injected into the joint to cover a central defect, but this technique, which does not provide mechanical stabilization with sutures or other methods, does not lend itself to complete tears29. A combination of suturing and a collagen-based construct has also shown some promise. Type-I collagen, the principal constituent of the ACL, is a safe and compatible biomaterial with FDA approved use in human applications and can be delivered in form of a gel or a sponge30. Further research showed that ACL fibroblasts spontaneously migrate into collagen biomaterial and are sustained therein. Hence there is no need for preoperative, ex-vivo cell seeding of a collagen biomaterial, and the material can thus be stored for off-shelf use.
Figure 2.
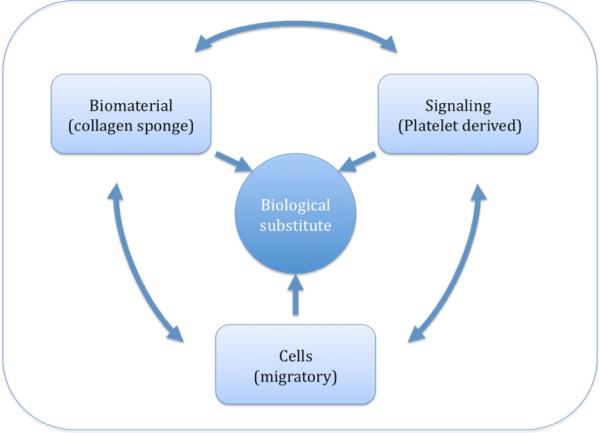
shows the triad of tissue engineering (E. Bell in “Principles of Tissue Engineering). Biomaterials, cells, and signals act together to create a biological substitute. The platelet-concentrate acts as both a source for signals and a biomaterial once clotted. ACL fibroblast from the ACL stump migrate spontaneously into the biomaterial, thus cell seeding is not needed.
It is important to remember that the functions of the clot spanning a wound are not confined to structural support, but that the clot also acts as the major source for a plethora of growth factors and cytokines31-33. Thus, solely implanting a collagen scaffold would be insufficient to recreate conditions permissive for ACL regeneration. To obviate this problem, a source of growth factors is needed. One of the currently investigated growth factor delivery processes is autologous platelet-concentrates32; 33. (Figure 3) This platelet concentrate, which can be adjusted to a desired platelet density, releases a number of crucial growth factors that stimulate cell migration, proliferation, and collagen production. The amount of released peptides is a function of platelet concentration, but, interestingly, the effects of these growth factors on functional healing diminish with age13; 14; 34, suggesting that younger patients would benefit more greatly than older ones.
Figure 3.
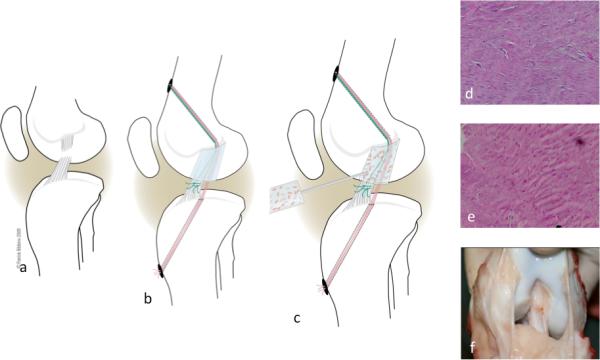
shows the surgical model. (a) The ACL is cut at the junction of the proximal and middle third. An EndoButton loaded with three sutures is passed through a tunnel on the femoral cortex. All sutures are passed through a collagen sponge. Two sutures (red) are passed through a tibial tunnel and tied over a button on the tibial cortex. The third suture (green), using the Endobutton on the femur as a pulley, pulls the tibial ACL stump into the biomaterial (b). Finally, 3cc of platelet-rich plasma are added to soak the collagen sponge (c). Histology after 6 (d) and 14 weeks (e) shows an increase in cellularity, as well as increased collagen deposition and crimp. Macroscopically, the repair appears equivalent to a tendon graft, the current gold standard of care (14 weeks postop, panel f) (modified from Murray MM et al 2010 The effect of skeletal maturity on functional healing of the anterior cruciate ligament. JBJS-Am 92:2039-2049, with permission. www.ejbjs.org)
Combing the platelet concentrate with a collagenous biomaterial offers a delivery system for platelets into the wound site, and, at the same time, protects the platelets from the influence of the abovementioned plasmin. Also, the collagen acts as a platelet activator and initiates the release of a number of growth factors into the sponge material. Thus higher concentrations of growth factors can be reached, sustained, and spatially concentrated.
Repair of the ACL: feasible?
What was needed after the in vitro proof of principle was confirmation in an in vivo model that closely mimicked that human knee joint. Among the available large animal models, canine models have a long track record for ligament repair models, but procedures that are routinely successful in humans fail in canines, making it a less clinically relevant model. Pigs and sheep are closest in anatomy and biomechanics and are validated and economical models for knee surgery and ligament repair and were therefore chosen for this purpose. The development of the model itself, however, was a two-stage procedure. First, the therapeutic potential of enhanced healing had to be tested in a stable, central, defect before a second variable – mechanical stress – could be introduced in the second step, the complete rupture model.
The central defect model, designed to test the biologic healing capacity of enhance repair, was a canine model. In a randomized trial, a standardized. 3.5mm central defect was created in the central portion of the ACL and either left untreated as a control, or treated with a collagen-platelet-composite (CPC)24; 35. Comparing this central defect model to the biological gold standards of ligament and tendon healing, the medial collateral ligament (MCL) and patellar tendon, the model confirmed the poor healing capacity of the untreated central defect of the ACL and excellent healing of the untreated defect in the MCL and patellar tendon24. In addition, a procedure where the defect was filled in with a collagen-platelet composite (CPC) resulted in healing of the ACL defect that was histologically similar to the MCL and patellar tendon. Further histology and immunohistochemistry of the defect site showed a significant increase in tissue filling, and growth factor expression at 3 and 6 weeks post-op with use of CPC24. Finally, MRI analysis and biomechanical testing was done at 6 weeks post-op to complement the description of the repair tissue's histologic profile35. These analyses showed a significant increase in ACL strength after treatment with CPC, and a significant association of this increase in strength with the amount of wound filling on MRI35.
The stable defect model showed that treatment with CPC resulted in more defect fill and a stronger repair tissue, similar to the tissue seen in the healing MCL. However, a more clinically relevant model would be a complete ACL rupture. In the second stage of in vivo testing, a complete ACL transection model was validated in pigs. A new problem in this model was how to deliver and maintain the CPC in the defect site. In the most recent modification of the technique, a specially processed collagen scaffold soaked with PRP is used as a delivery system.
Briefly, for descriptive purposes, the procedure can be divided into two aspects. The central mechanism is suture bridge which is stabilized proximally with an Endobutton (Smith and Nephew, Andover, MA) on the proximal lateral femoral cortex and then sutures from the Endobutton are brought through the knee and tied over a extracortical button on the anteromedial aspect of the tibia to stabilize the knee during the early post-op period A second set of sutures coming from the Endobutton are ties to the tibial stump of the ACL in an attempt to recreate the initial trajectory of the torn ACL. To accomplish this, six suture limbs, run from the Endobutton through a 4.5 mm tunnel into the knee joint. Four of the suture limbs are threaded through the collagen scaffold and continue to leave the knee through a 2.4 mm tunnel from the tibial ACL insertion onto the medial tibial cortex where they are tied, under tension control, over an extracortical button. These sutures serve to pull the scaffold, soaked with PRP hence malleable, into the knee and to afford initial antero-posterior stability of the joint. Since these sutures are absorbable and lose roughly 25% of their initial strength per week, there is an increasing amount of mechanical stress, or stimulus, on the repair tissue over time until the suture is completely absorbed after approximately 63 days on average (Ethicon, Somerville, NJ). This is the first aspect of the procedure, creating a temporarily stable scaffold for repair in perfect alignment with the original ACL. With two of the three sutures used for scaffold fixation and antero-posterior stability, the remaining two suture limbs running from the femoral Endobutton are tied to a whipstitch running up and down the anterior and posterior side of the tibial ACL stump, pulling the ACL tibial stump into the scaffold. This second aspect of the repair procedure with aligns and stabilizes the distal ACL stump to allow for cell migration into the collagen sponge. At this point, the repair construct is left untouched for 10 minutes before wound closure to allow for complete clotting of the PRP.
The use of three sutures begs the question whether stability in biomechanical testing is confounded by this material, rather than afforded by the repair tissue. However, when compared with suture repair alone, suture repair augmented with a collagen-platelet composite resulted in significant improvement in repair strength at 4 weeks (the time when suture strength approaches 0), and 3 months36; 37. At the same time, there was a significant increase in cellularity with the use of CPC, i.e. a strong, ongoing regenerative response, suggesting even better biomechanical outcomes once these cells produced an organized extracellular matrix36.
A potential shortcoming of these studies is that the porcine model builds on an ACL transection rather than a complete, traumatic rupture. However, it should be remembered that even in a clinically complete rupture some fiber bundles as well as the synovial sheath are oftentimes preserved. Also, given the anatomy of the porcine ACL, which is a broad ligament that flatly lies on the tibial plateau rather than obliquely crossing the notch, the “transection” is more of a staged process than a single cut, usually resulting in a combination of numerous cuts at various levels of height as well as quite a few ruptured fibers. Finally, it seems worthwhile to consider that tunnels are drilled through both physes, femoral and tibial, to pass sutures. Such tunnels potentially might jeopardize the growth plate and affect growth. However, the tunnels used for this technique are rather small both in absolute diameter and in relation to physeal area, centered, and bear only little tension, i.e. as much tension as four 0.35mm thick sutures are able to withstand. All these parameters have been shown to reduce the risk of growth disturbances38-43.
Repair of the ACL: patient-related variables
Many of the initial studies demonstrating the efficacy of CPC enhanced suture repair were performed in juvenile animals. The next series of studies evaluated the effect of age on both intrinsic ACL healing (with no treatment) and suture repair enhanced with CPC44. That study found important effects of age on ligament healing, with skeletally immature animals having a significantly higher return of preoperative ACL strength with intrinsic healing compared to the adult animals. The adolescent animals had less return of ACL strength than the skeletally immature animals with no treatment. However, use of a suture repair enhanced with a collagen-platelet composite resulted in a nearly doubling of the adolescent ACL strength after transection. The skeletally mature animals had less return of ACL strength than immature animals even with the use of a collagen-platelet composite.
In an effort to determine a mechanism for the disparity in healing with age, a histological study of the 3 age groups was performed to examine the early healing response in the ACL after 1, 2, and 4 weeks in three age groups45; 46. The major finding in that study was that the cell density within the wound site was highest in the immature animals. Cellular population of the scaffold occurred in all age groups, but cell density in the adult ligament wound sites lagged 2 weeks behind that in the immature animals. Follow-up in-vitro studies demonstrated the cells from immature ACL both migrate and proliferate more quickly than those from adult ACL, which may represent possible etiologies for the differences observed in vivo. The reason for such differences in cell behavior across age groups, despite the considerable and constant amount of stimulation provided by PRP, might be the cells’ ability to respond to the growth factors released by PRP. Recent studies have shown that expression levels of growth factor receptors depend on age, and that biomechanical outcomes are associated with receptor expression levels47.
In summary, animal age appears to affect the ability of the ACL to repair itself or to respond positively to suture repair. In addition, the preliminary studies performed in juvenile animal were also immediate repairs – that is, the ligament was transected and then repaired right away. The more likely scenario in clinical practice is that 2 to 6 weeks elapse between injury and surgical treatment. Therefore, an additional study was performed to determine the effect of a 2- or 6-week delay on ACL repair strength48. In that study, a delay of either 2 or 6 weeks resulted in a significant loss of ACL strength after 3 months of healing. Possible reasons for this finding include a loss of ACL tissue due to degradation, inflammatory changes in the intra-articular environment and synovial fluid, and loss of secondary structures. Further studies to investigate these hypotheses are needed.
Conclusion
Recent changes in patient populations have supported the principles of tissue engineering and regenerative medicine in orthopedic surgery. Younger and more active patients benefit from such approaches that encourage biological repair since they obviate complications provoked by the high stresses due to high physical demand over a prolonged time after classic replacement with synthetic materials. Currently, methods to enhance biological repair of the torn anterior cruciate ligament are intensively investigated. Such investigations have elucidated the reasons behind the lack of intrinsic healing in the ACL and opened the door for methods to compensate for this lack. A series of in vitro and in vivo studies have resulted in the development of a technique for tissue engineering, enhanced primary ACL repair, using a collagenous scaffold and autologous platelet-rich plasma, allowing for off-shelf availability. Histological assessment has shown a high level of structural equivalence of the repair tissue after such a procedure with that of ligaments and tendons that heal spontaneously, such as the medial collateral ligament. Biomechanical testing has revealed ongoing improvement in repair strength over 3 months. Most recently it was observed that both age and the amount of time between rupture and repair both may play an important role in outcomes.
Figure 1.
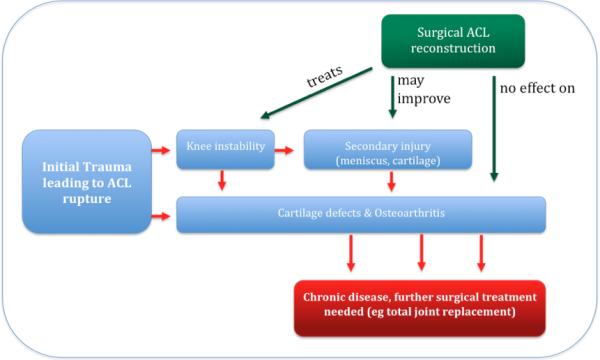
shows the cascade of events after a complete ACL tear.
Acknowledgments
This work was supported by a grant from NIH R01 AR052772.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McIntosh AL, Dahm DL, Stuart MJ. Anterior cruciate ligament reconstruction in the skeletally immature patient. Arthroscopy. 2006;22:1325–1330. doi: 10.1016/j.arthro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Micheli, Rask, Gerberg Anterior cruciate ligament reconstruction in patients who are prepubescent. Clin Orthop Relat Res. 1999:40–47. doi: 10.1097/00003086-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Mohtadi N, Grant J. Managing anterior cruciate ligament deficiency in the skeletally immature individual: a systematic review of the literature. Clin J Sport Med. 2006;16:457–464. doi: 10.1097/01.jsm.0000248844.39498.1f. [DOI] [PubMed] [Google Scholar]
- 4.Daniel DM, Stone ML, Dobson BE, et al. Fate of the ACL-injured patient. A prospective outcome study. The American journal of sports medicine. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Roos EM. Knee ligament injury, surgery and osteoarthrosis: truth or consequences? Acta Orthop Scand. 1994;65:605–609. doi: 10.3109/17453679408994613. [DOI] [PubMed] [Google Scholar]
- 6.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmander LS, Englund M, Dahl L, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 8.Wester W, Canale ST, Dutkowsky JP, et al. Prediction of angular deformity and leg-length discrepancy after anterior cruciate ligament reconstruction in skeletally immature patients. J Pediatr Orthop. 1994;14:516–521. doi: 10.1097/01241398-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Koman, Sanders Valgus deformity after reconstruction of the anterior cruciate ligament in a skeletally immature patient. A case report. J Bone Joint Surg Am. 1999;81:711–715. doi: 10.2106/00004623-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Streich NA, Barie A, Gotterbarm T, et al. Transphyseal reconstruction of the anterior cruciate ligament in prepubescent athletes. Knee Surg Sports Traumatol Arthrosc. 2010 doi: 10.1007/s00167-010-1057-9. [DOI] [PubMed] [Google Scholar]
- 11.Henry, Chotel, Chouteau, et al. Rupture of the anterior cruciate ligament in children: early reconstruction with open physes or delayed reconstruction to skeletal maturity? Knee Surg Sports Traumatol Arthrosc. 2009;17:748–755. doi: 10.1007/s00167-009-0741-0. [DOI] [PubMed] [Google Scholar]
- 12.Trentacosta, Vitale, Ahmad The effects of timing of pediatric knee ligament surgery on short-term academic performance in school-aged athletes. Am J Sports Med. 2009;37:1684–1691. doi: 10.1177/0363546509332507. [DOI] [PubMed] [Google Scholar]
- 13.Mastrangelo AN, Haus BM, Vavken P, et al. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010 doi: 10.1002/jor.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastrangelo AN, Magarian EM, Palmer MP, et al. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28:644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seil R, Pape D, Kohn D. The risk of growth changes during transphyseal drilling in sheep with open physes. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2008;24:824–833. doi: 10.1016/j.arthro.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Vavken P, Murray MM. Translational Studies in ACL repair. Tissue Eng Part A. 2009 doi: 10.1089/ten.teb.2009.0147. [DOI] [PubMed] [Google Scholar]
- 17.Mayo Robson A. Ruptured cruciate ligaments and their repair by operatoin. Ann Surg. 1903;37:716–718. [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donoghue DH. A Method For Replacement Of The Anterior Cruciate Ligament Of The Knee. J Bone Joint Surg Am. 1963;45:905–924. [PubMed] [Google Scholar]
- 19.O'Donoghue DH, Frank GR, Jeter GL, et al. Repair and reconstruction of the anterior cruciate ligament in dogs. Factors influencing long-term results. J Bone Joint Surg Am. 1971;53:710–718. [PubMed] [Google Scholar]
- 20.O'Donoghue DH, Rockwood CA, Jr., Frank GR, et al. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48:503–519. [PubMed] [Google Scholar]
- 21.Feagin JA, Jr., Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 22.Cabaud H, WG R, Feagin J. Experimental studies of acute anterior cruciate ligament injury and repair. Am J Sports Med. 1979;7:18–22. doi: 10.1177/036354657900700105. [DOI] [PubMed] [Google Scholar]
- 23.Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am. 1987;69:1120–1126. [PubMed] [Google Scholar]
- 24.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligamentwith a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 25.Murray MM, Martin SD, Martin TL, Spector M. Histological Changes in the Human Anterior Cruciate Ligament After Rupture. J Bone Joint Surg Am. 2000;82:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979:279–283. [PubMed] [Google Scholar]
- 27.Rosc D, Powierza W, Zastawna E, et al. Post-traumatic plasminogenesis in intraarticular exudate in the knee joint. Med Sci Monit. 2002;8:CR371–378. [PubMed] [Google Scholar]
- 28.Brommer EJ, Dooijewaard G, Dijkmans BA, Breedveld FC. Depression of tissue-type plasminogen activator and enhancement of urokinase-type plasminogen activator as an expression of local inflammation. Thromb Haemost. 1992;68:180–184. [PubMed] [Google Scholar]
- 29.Wiig ME, Amiel D, VandeBerg J, et al. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res. 1990;8:425–434. doi: 10.1002/jor.1100080314. [DOI] [PubMed] [Google Scholar]
- 30.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B Appl Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 31.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 32.El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 33.Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 34.Vavken P, Saad FA, Murray MM. Age dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. doi: 10.1002/jor.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 36.Joshi SM, Mastrangelo AN, Magarian EM, et al. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 38.Shea KG, Apel PJ, Pfeiffer RP. Anterior cruciate ligament injury in paediatric and adolescent patients: a review of basic science and clinical research. Sports Med. 2003;33:455–471. doi: 10.2165/00007256-200333060-00006. [DOI] [PubMed] [Google Scholar]
- 39.Shea KG, Apel PJ, Pfeiffer RP, Traughber PD. The anatomy of the proximal tibia in pediatric and adolescent patients: implications for ACL reconstruction and prevention of physeal arrest. Knee Surg Sports Traumatol Arthrosc. 2007;15:320–327. doi: 10.1007/s00167-006-0171-1. [DOI] [PubMed] [Google Scholar]
- 40.Guzzanti, Falciglia, Stanitski Physeal-sparing intraarticular anterior cruciate ligament reconstruction in preadolescents. Am J Sports Med. 2003;31:949–953. doi: 10.1177/03635465030310063401. [DOI] [PubMed] [Google Scholar]
- 41.Guzzanti, Falciglia, Stanitski Preoperative evaluation and anterior cruciate ligament reconstruction technique for skeletally immature patients in Tanner stages 2 and 3. Am J Sports Med. 2003;31:941–948. doi: 10.1177/03635465030310063301. [DOI] [PubMed] [Google Scholar]
- 42.Houle JB, Letts M, Yang J. Effects of a tensioned tendon graft in a bone tunnel across the rabbit physis. Clin Orthop Relat Res. 2001:275–281. doi: 10.1097/00003086-200110000-00032. [DOI] [PubMed] [Google Scholar]
- 43.Edwards TB, Greene CC, Baratta RV, et al. The effect of placing a tensioned graft across open growth plates. A gross and histologic analysis. J Bone Joint Surg Am. 2001;83-A:725–734. doi: 10.2106/00004623-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Murray M, Magarian E, Harrison S, et al. Skeletal maturity significantly affects functional healing of the anterior cruciate ligament. JBJS-Am. 2010 doi: 10.2106/JBJS.I.01368. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mastrangelo A, Haus B, Vavken P, et al. Immature animals have denser anterior cruciate ligament wound site cell repopulation than adolescent or adult animals. J Orthop Res. 2009 doi: 10.1002/jor.21070. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastrangelo A, Magarian E, Palmer M, et al. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2009 doi: 10.1002/jor.21018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vavken P, Saad F, Murray M. Age-dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. 2010 doi: 10.1002/jor.21111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magarian E, Harrison S, Mastrangelo A, et al. Delay of 2 or 6 weeks adversely affects the functional outcome of augemented primary repair of the anterior cruciate ligament. Am J Sports Med. doi: 10.1177/0363546510377416. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]


