Introduction
The complexity of the intrarenal renin-angiotensin system (RAS) continues to reveal itself as evidence accumulates demonstrating its robust independent regulation in the interstitial and intratubular compartments within the kidney1–3. Early reports demonstrating the presence of angiotensin II (Ang II) receptors on the brush border of proximal tubules suggested physiological roles4. However, because of the abundance of degradating enzymes on the brush border, the concentrations of angiotensin peptides were considered to be relatively low. Nevertheless, the abundance of luminal Ang II receptors throughout proximal and distal nephron segments sustained interest in its luminal actions5,6. Tubular perfusion studies indicating that luminal Ang II alters tubular sodium and volume reabsorption rate 1,5,7,8 supported an important physiological role for luminal Ang II receptors9.
A paradigm shift occurred when it was discovered that the proximal intratubular concentrations of Ang I and II were much greater than their corresponding plasma concentrations7,10,11. In addition, when proximal tubular fluid was incubated with excess renin, the resultant formation of Ang I indicated very high angiotensinogen (AGT) substrate availability in this segment7,12. Furthermore, tubular fluid collected from downstream segments of perfused tubules also had Ang II concentrations similar to those in non-perfused tubules thus supporting a local origin11. These findings, along with the demonstration that proximal tubule cells express AGT mRNA and protein13,14, established the foundation for the existence of a robust physiologically important tubular RAS.
Intratubular Ang II Receptors and Ang II Concentrations
The principal AT receptor in adult kidneys is the AT1 receptor5, although AT2 receptors are upregulated in certain conditions 15,16 and may also play a role in renin synthesis17. Nevertheless overall renal AT1 receptor abundance far exceeds AT2 receptor levels 5,18 and AT1 receptors are widely distributed on luminal membranes throughout the nephron segments including proximal tubule, thick ascending limb of Loop of Henle, macula densa, distal tubule and collecting ducts (CD)5,19. The regulation of intrarenal AT1 receptors is complex as vascular AT1 receptors are downregulated while tubular AT1 receptors are either sustained or upregulated by elevated Ang II levels1,6,20,21.
The presence of AT1 receptors on luminal membranes of various nephron segments generated interest in the Ang II concentrations available to activate the receptors7,22–24. Proximal tubule fluid concentrations of Ang I and Ang II are in the range of 5–10 pmol/ml 2,7 which are similar to renal interstitial fluid concentrations25. The tubular Ang II concentrations remain elevated in hypertension models including Ang II infused hypertension26, Goldblatt hypertension 27 and TGR(mRen2) rats 28 suggesting their sustained actions on proximal reabsorption rate. The critical importance of kidney AT1 receptors in the regulation of normal blood pressure and development of hypertension has been demonstrated by studies showing that AT1a receptors in the kidneys are essential for normal blood pressure regulation and for mediating the hypertensive response to Ang II infusions29,30. Furthermore, AT1a knockout mice fail to develop hypertension in response to unilateral renal arterial constriction31,32.
The tubular fluid Ang II concentrations in other nephron segments have not been measured due to difficulty in collecting sufficient fluid for analysis. Measurements made from urine samples collected under conditions where the major distal nephron transport systems were pharmacologically blocked, suggest CD concentrations in the range of 0.5 pmol/ml for control mice with about a two fold increase in Ang II infused hypertensive mice33. Increased urinary excretion rates of Ang II also occur in chronic Ang II infused rats 34,35 and these were decreased during treatment with AT1 receptor blockers even though the circulating Ang II concentrations were increased35. These recent studies indicate that distal nephron Ang II is formed locally in the tubules at concentrations that are sufficiently high to influence distal nephron transport function which has been shown to respond to Ang I and Ang II7,22,23. Distal nephron Ang II was recently shown to enhance the sensitivity of the “connecting tubule glomerular feedback mechanism” that communicates signals between the connecting tubule (CNT) and the afferent arteriole36. In contrast to the macula densa tubular glomerular feedback mechanism where Ang II augments its vasoconstriction capability5,37, the effect of Ang II on the CNT feedback mechanism is afferent vasodilatation36.
AT1 receptors are also responsible for internalizing Ang II and the presence of substantial Ang II in endosones in both control and Ang II infused hypertensive rats supports their internalization into a protected compartment that prevents degradation of some of the internalized Ang II20. AT1 receptor blockade prevents the internalization of the Ang II. Intracellular Ang II may activate various signaling pathways and also contribute to fibrogenic proliferative responses and microthrombosis38–40. Internalized Ang II may also migrate to the nucleus to exert transcriptional effects38,41. Ang II binding sites have been shown on nuclear membranes 41,42 and co-localization with nuclear markers suggests migration of the receptor complex to the nucleus38,43.
Augmentation of Intrarenal AGT in Hypertension
The seminal findings that AGT mRNA and protein are present in proximal tubule cells generated a great deal of interest regarding its intrarenal function13,44–46. Chronic Ang II infusions augment intrarenal AGT mRNA and protein in proximal tubule cells in rats and mice13,14,47,48. This effect is mediated via activation of AT1 receptors as it is prevented by treatment with ARBs48,49. Ang II also stimulates AGT production in proximal tubule cell cultures50. Thus, chronic infusions of Ang II in rats and mice lead to an augmentation of AGT expression leading to greater generation and intrarenal production of Ang II (Figure 1). Importantly, this process appears to be self limiting as higher Ang II infusions do not stimulate AGT mRNA 48 and complex signaling mechanisms are activated to prevent uncontrolled positive feedback51,52.
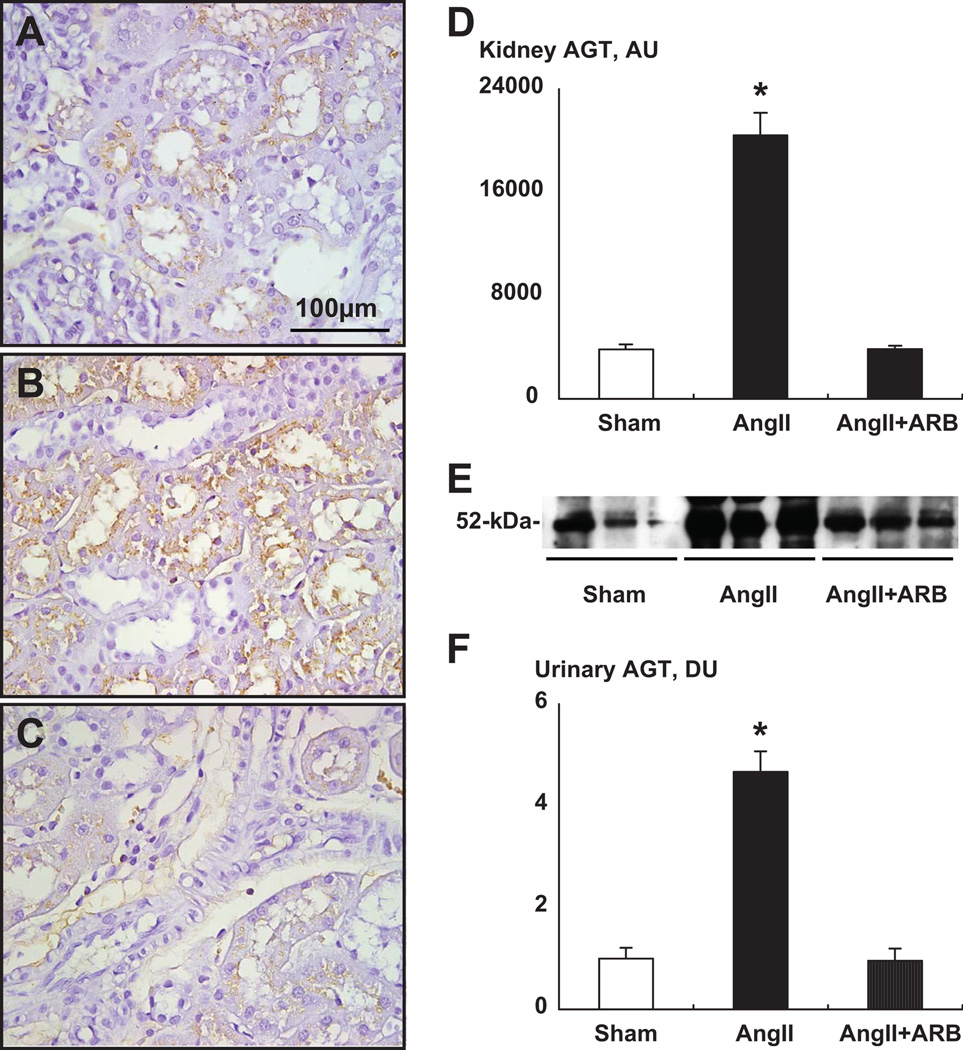
Figure 1. A–C, Representative immunohistochemical analysis of rat kidney AGT from Sham, Ang II infused and Ang II infused plus AT1 receptor blockade groups.
The immunoreactive areas were restricted only to proximal tubular cells. Vascular structures were negative. D, Kidney AGT immunostaining showed a significant enhancement in Ang II group (B) compared with sham group (A). ARB treatment prevented this augmentation (C). E, Representative Western blot analysis of urinary AGT levels among groups showing the stimulation in Ang II-infused group. F, Urinary excretion rates of AGT were enhanced 4.7-fold in Ang II-infused animals. ARB treatment prevented this augmentation. Ang II indicates angiotensin II; ARB, angiotensin II type1 receptor blocker, olmesartan; AGT, angiotensinogen. * P < 0.05 compared with the sham group. Data from Kobori et al. Hypertension 43:1126–1132, 200449.
Because the level of AGT is close to the Michaelis-Menten constant for renin, AGT levels can also control RAS activity; thus, upregulation of AGT levels may lead to elevated angiotensin peptide levels53. Studies on rat and mouse models of hypertension have documented the effect of augmented AGT in the activation of the RAS54–58. Genetic manipulations that lead to overexpression of the AGT gene cause hypertension55,59. In human genetic studies, a linkage has been established between the AGT gene and hypertension60–63. Enhanced intrarenal AGT mRNA and/or protein levels occur in experimental models of hypertension and diabetes including Ang II-dependent hypertensive rats 13,14,47,49 and mice48,56,64, Dahl salt-sensitive hypertensive rats65, and spontaneously hypertensive rats66, as well as in kidney diseases including diabetic nephropathy67–69, IgA nephropathy70–72, and radiation nephropathy73. Thus, increased intrarenal AGT contributes to the development and progression of hypertension and may be useful as a predictor of developing kidney disease1,74. While clearly related to activation of AT1 receptors49, the mechanism by which Ang II stimulates AGT mRNA and protein is complex and appears to require interactions with inflammatory factors including interleukin 6, and increased oxidative stress75–77.
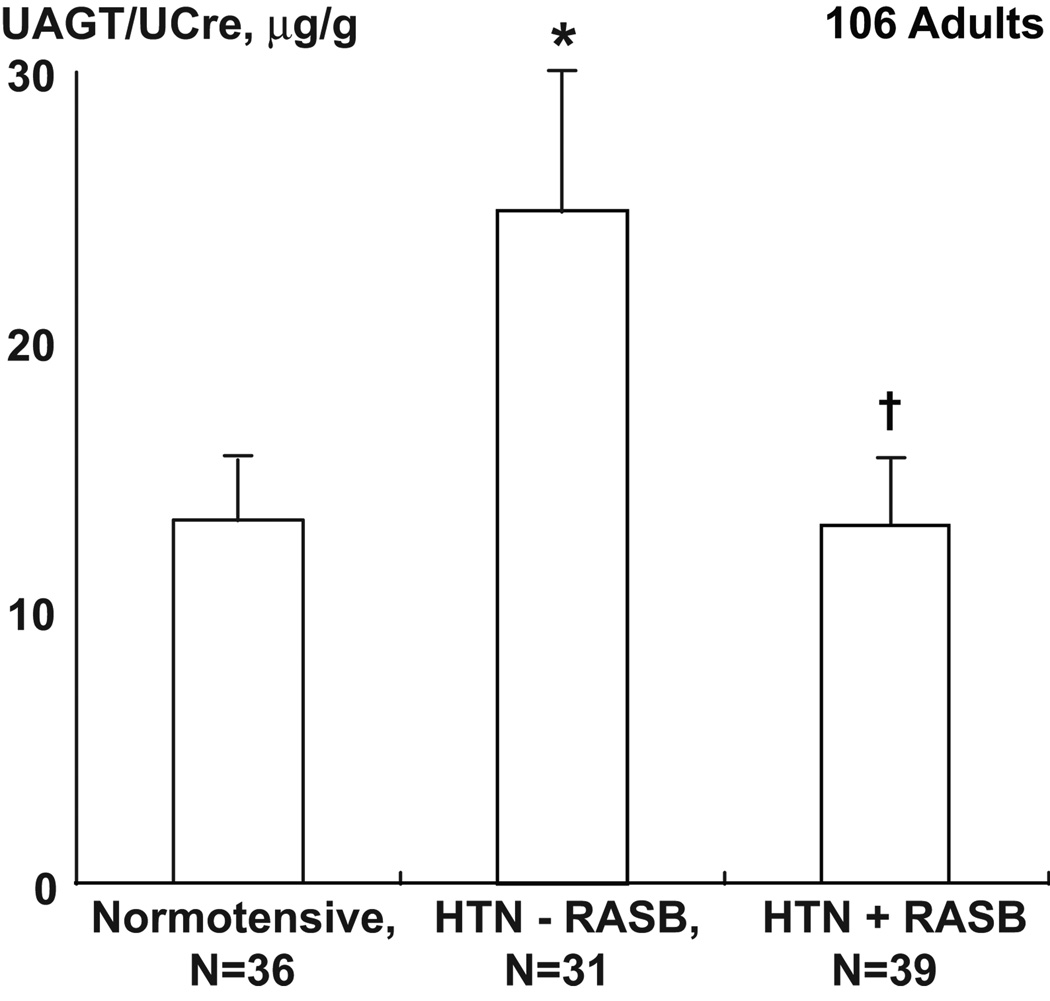
Urinary excretion rates of AGT provide an index of intratubular RAS status and are correlated with kidney Ang II levels in Ang II-dependent hypertensive rats78,79. Furthermore, mice overexpressing AGT only in proximal tubules have increased urinary Ang II excretion77. Because of its potential importance in identifying Ang II dependent hypertension in human subjects, direct quantitative methods to measure urinary AGT using human/mouse/rat AGT ELISA were recently developed80,81. Using this system, urinary excretion rates of AGT have been used as an index of intrarenal RAS status in patients with chronic kidney disease74,82,83, diabetes mellitus84,85, and hypertension86–88. In a cross-sectional study, we reported that urinary AGT levels are significantly greater in hypertensive patients not treated with RAS blockers compared with normotensive subjects (Figure 2). Moreover, patients treated with RAS blockers showed reduced urinary AGT levels87. In a population study, we showed that urinary AGT levels are correlated with high blood pressure in humans88. Urinary AGT levels were significantly correlated with systolic and diastolic blood pressures and high correlations between urinary AGT and blood pressure were shown in male subjects, especially in male African-American subjects88. These recent translational studies strengthen the hypothesis that intratubular AGT exerts a crucial role in the development and progression of hypertension and kidney disease. The augmentation of proximal tubule AGT leads to spillover into the distal nephron segments providing substrate for additional generation of Ang I and subsequent formation of Ang II (Figure 3).
Figure 2.
Uriinary AGT (uAGT), expressed as ratio of uAGT/uCreatine, in normotensive, and in hypertensive patients (HTN) treated with renin-angiotensin system blockers (RASB) and compared with those treated with other drugs. *P<0.05 vs normotensive; P<0.05 vs HTN–RASB. Data from Kobori et al. Hypertension 53[Part 2]:344–350, 200987.
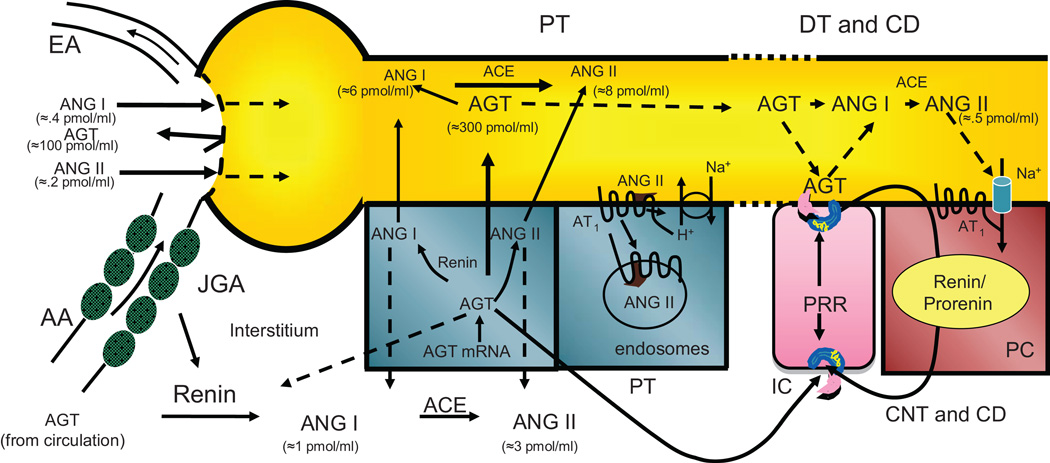
Figure 3. Cascade of Intratubular RAS in Ang II Dependent Hypertension.
In Ang II dependent hypertension, the kidney maintains de novo intrarenal Ang II formation enhanced proximal tubule AGT formation and spillover into distal nephron segments coupled with enhancement of CD renin and stimulation of tubular ACE. (Refer to text for relevant references).
Renin and (Pro)renin Receptor in the Collecting Duct During Ang II-dependent Hypertension
Renin is also produced by the principal cells of CNT and cortical and medullary CD of mouse, rat, and human kidneys89–91. Renin co-localizes with aquaporin 291. In response to chronic Ang II infusions, renin mRNA and protein levels increase in CNT and CD91. This effect contrasts with the effect of Ang II to suppress JG renin92, but is also an AT1 receptor-mediated process93. As shown in Figure 4, the stimulation of CD renin during Ang II-dependent hypertension occurs independently of blood pressure since both non-clipped and clipped kidneys of Goldblatt hypertensive rats exhibit augmentation of renin synthesis and renin activity in the renal medulla, which is devoid of JG cells94. Thus, CD renin is increased by Ang II in association with increased AGT spillover from the proximal tubules95. In hypertensive models, the increased renin is primarily active renin 94 while in diabetic models, the increased CD renin is primarily (pro)renin90. There is also an enhancement of ACE and inhibition of ACE2 gene expression associated with decreases in intrarenal Ang 1–7 levels 96,97 suggesting that suppression of ACE2 activity contributes to augmentation of intrarenal Ang II.
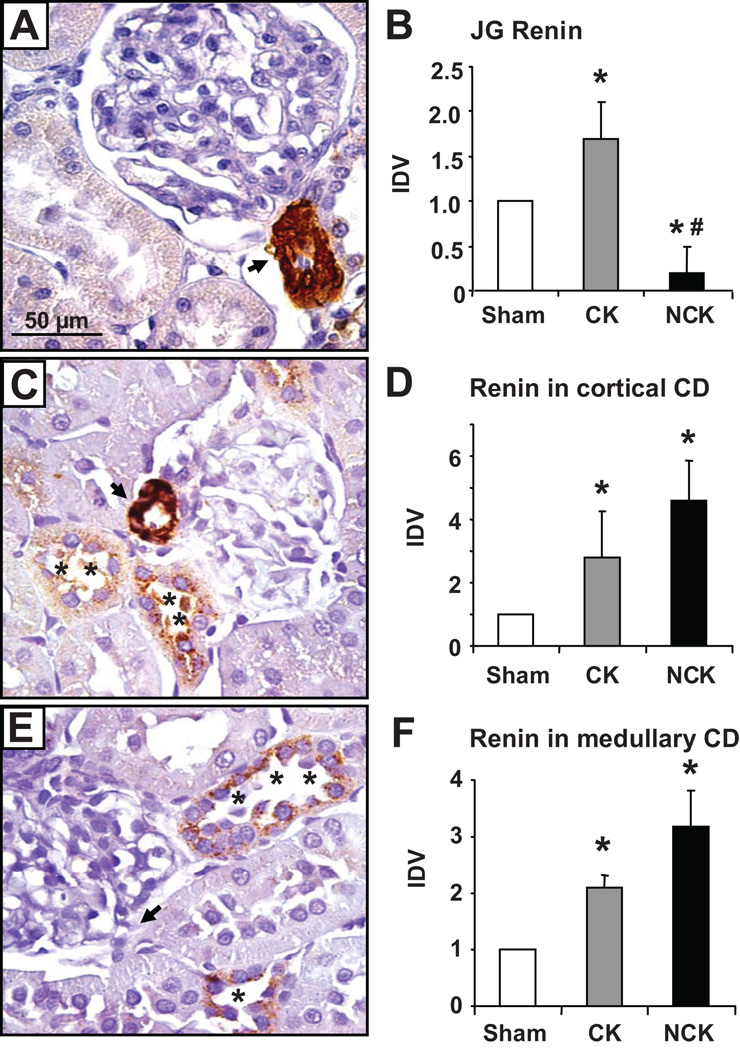
Figure 4. Renin immunoreactivity in juxtaglomerular (JG) cells and cortical collecting duct cells.
Renin immunoreactivity by immunoperoxidase technique (A,C, and E) in paraffin embedded kidney sections (3 µm) from sham rats (A), and clipped (C) and non-clipped kidney (E) of Goldblatt rats. Specific JG renin immunoreactivity (arrows; DAB chromogen) in a sham (A) and in the clipped kidney (C) of a Goldblatt rat. Higher renin immunoreactivity (asterisks) are shown in the collecting ducts of the renal cortexes of both, clipped (C) and non-clipped (E) kidneys relative to the sham kidney (A). Densitometry the renin immunoreactivity in JG cells (B) and cortical (D) and medullary (F) collecting duct cells of sham, and clipped (CK) and non-clipped (NCK) kidneys of Goldblatt rats were performed using four kidney sections/animal (10 microscopic fields/kidney sections at the renal cortex and medulla regions) and compared to sham kidneys. Sham rats (n= 5). Goldblatt rats (n= 6). Glom: Glomerulus. Values are mean ± S.E. *P<0.0001 versus sham. Renin antibody dilution 1:4,000. *P<0.05 versus sham. #P< 0.05 clipped kidney versus non-clipped. CD: collecting duct; JG: juxtaglomerular; CK: clipped kidney; NCK: non-clipped kidney; IDV: integrated densitometric values. Modified from Prieto-Carrasquero et al. Hypertension 51:1590–1596, 200894.
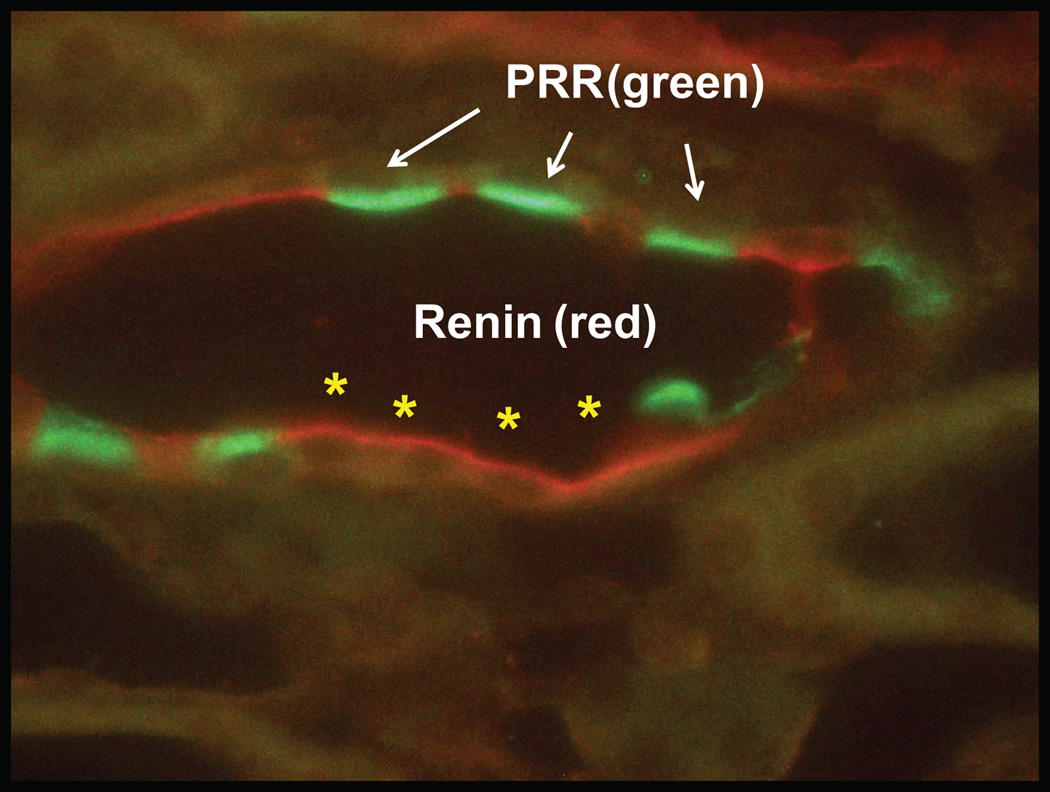
The (pro)renin receptor, (P)RR, a 350-amino acid protein with a single transmembrane domain which binds renin or (pro)renin, increases the catalytic activity of renin and fully activates (pro)renin98. (P)RR activation also elicits intracellular signals via extracellular signal-regulated kinase (ERK)1 and ERK2 mitogen-activated protein (MAP) kinase. (P)RR has been localized in glomerular mesangial cells, subendothelium of renal arteries, podocytes, macula densa cells, distal tubules and collecting ducts98,99. (P)RR is predominantly expressed at the apex of the intercalated cells100. An example of this localization is depicted in Figure 5. Recent findings have also shown that the full length form of (P)RR can be processed intracellularly by cleavage leading to a soluble form (s(P)RR) that can be secreted into the plasma and consequently bind renin101. While the function of (P)RR or s(P)RR in hypertensive conditions has not been established102, (P)RR data from various models suggest its contribution to hypertension, diabetes and associated cardiovascular and renal diseases90,103,104. These observations are of relevance in light of CD renin upregulation in Ang II-dependent hypertensive rats91,93,94, and renin and/or (pro)renin secretion by CD cells89,90,94.
Figure 5. Double immunolabeling for renin and (pro)renin receptor ((P)RR) in the chronic Ang II-infused rat kidney.
Double immunolabeling for renin (red) and (P)RR (green) was performed to confirm that renin is localized in principal cells (asterisks) while (P)RR is expressed in intercalated cells (arrows). A rabbit polyclonal anti-renin antibody (T. Inagami, Vanderbilt University) at a 1:4,000 dilution detected by a fluorescent secondary antibody (Alexa Fluor 594, red; Invitrogen) chicken anti-rabbit, was followed by a goat anti-(P)RR antibody (Abcam 5959, Cambridge, MA) at 1:400 dilution detected with a fluorescent secondary antibody donkey anti-goat (Alexa Fluor 488, green; Invitrogen). (Unpublished data)
Intrarenal ACE-derived Ang II formation in hypertension
ACE is responsible for most conversion of Ang I to Ang II and is expressed on endothelial cells of the vasculature, on brush border of proximal tubule cells, glomeruli and distal nephron segments including inner medullary CD6,23,105–107. ACE knockout mice display very low arterial pressures coupled with an impaired capacity to generate Ang II, that is reflected as low levels of circulating and intrarenal Ang II, high levels of circulating Ang I108, and failure to show increases in blood pressure in response to Ang I infusions109.
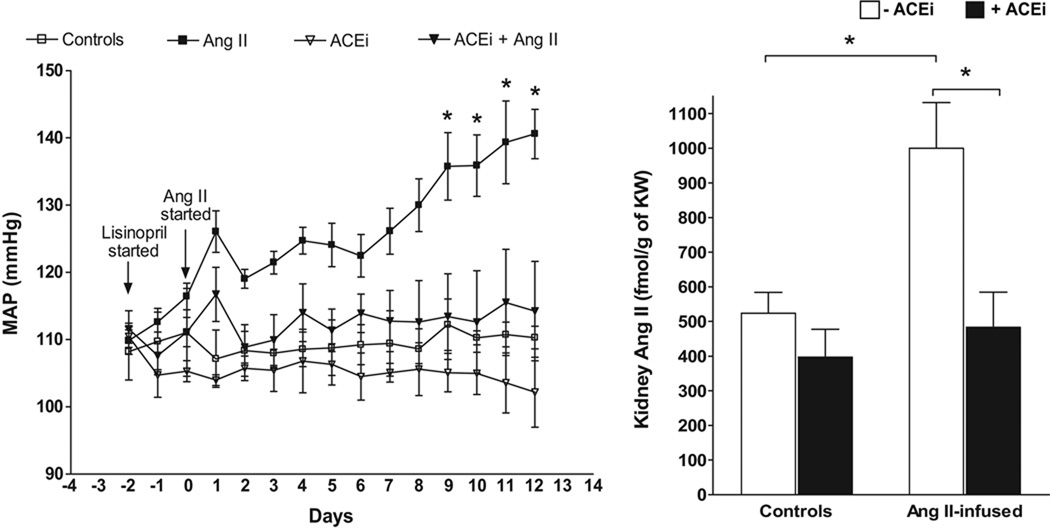
As shown in Figure 6, mice treated chronically with an ACE inhibitor show markedly attenuated responses in arterial pressure and lower intrarenal Ang II levels with low dose infusions of Ang II htat elicit a slow pressor response64. Thus, endogenous ACE-derived Ang II formation contributes to the development of high local Ang II levels and hypertension induced by chronic Ang II infusions. To further determine the ability of kidney-specific ACE to augment intrarenal Ang II content and blood pressure, mice expressing ACE exclusively in the kidneys were infused chronically with Ang I110. Kidney specific ACE-derived Ang II formation increased Ang II content and led to the progressive development of hypertension, indicating that intrarenal ACE is a major contributor to the development of hypertension and increased intrarenal Ang II levels. Indeed, ACE expression is sustained or even augmented during Ang II-dependent hypertension 6,106 and other models of kidney injury111.
Figure 6. Changes in mean arterial blood pressure and intrarenal Ang II in Ang II-infused mice with or without an ACE inhibitor.
Blood pressure and Ang II concentrations were determined by telemetry and radioimmunoanalysis respectively. Ang II = Angiotensin II (400 ng/kg/min), ACEi = Lisinopril (100 mg/L in the drinking water). *p < 0.05 vs. controls by TWO-WAY ANOVA for MAP changes and ONE-WAY ANOVA for Ang II changes. (From Gonzalez-Villalobos et al. Hypertension 53:351–355, 2009)64.
Perspective
The results obtained to date indicate that increases in circulating or local Ang II concentrations elicit a positive augmentation of intrarenal AGT mRNA and protein leading to increased secretion of AGT into the tubular fluid. Together with the sustained or increased tubular ACE levels, the augmented AGT increases intratubular Ang II which further augments sodium transport via stimulation of AT1 receptors. The augmented AGT production and secretion increase AGT delivered to the distal nephron segments which can interact with renin and ACE produced by principal cells of CNT and CD cells to form more Ang II and stimulate distal transport activity. Nevertheless, in a pathophysiologic environment, inappropriate stimulation of the intratubular RAS may be an important contributor to the development and maintenance of hypertension and associated renal injury112. While this positive augmentation of intrarenal angiotensin by Ang II appears to be counter-intuitive to normal feedback regulation, the process is primarily a local amplification mechanism to increase intratubular Ang II thus effecting rapid homeostatic regulation of sodium reabsorption without equivalent increases in circulating Ang II. Furthermroe, there are “brakes” in the system as described earlier to prevent uncontrolled positive feedback51.
Acknowledgements
The authors thank Debbie Olavarrieta for preparing the manuscript and figures.
Sources of Funding:
Research support to the authors include grants from NIH (RO1HL-26371, RO1DK-072408, 2K99DK-083455 and P20RR-017659) and from the American Heart Association (09BGIA2280440 and 10GRNT3020018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navar LG, Prieto-Carrasquero MC, Kobori H. Molecular aspects of the renal renin-angiotensin system. In: Re R, DiPette DJ, Schiffrin EL, Sowers JR, editors. Molecular Mechanisms in Hypertension. Taylor & Francis Group; 2006. pp. 3–14. [Google Scholar]
- 4.Douglas JG, Hopfer U. Novel aspects of angiotensin receptors and signal transduction in the kidney. Annu Rev Physiol. 1994;56:649–669. doi: 10.1146/annurev.ph.56.030194.003245. [DOI] [PubMed] [Google Scholar]
- 5.Navar LG, Harrison-Bernard LM, Imig JD, Mitchell KD. Renal actions of angiotensin II at AT1 receptor blockers. In: Epstein M, Brunner HR, editors. Angiotensin II Receptor Antagonists. Philadelphia: Hanley & Belfus, Inc.; 2000. pp. 189–214. [Google Scholar]
- 6.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 Receptor and ACE Binding in Angiotensin II-Induced Hypertensive Rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navar LG, Harrison-Bernard LM, Wang C-T, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 8.Schuster VL. Effects of angiotensin on proximal tubular reabsorption. Federation Proceedings. 1986;45:1444–1447. [PubMed] [Google Scholar]
- 9.Yanagawa N. Potential role for local luminal angiotensin II in proximal tubule sodium transport. Kidney Int Suppl. 1991;32:S33–S36. [PubMed] [Google Scholar]
- 10.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol-Renal Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 12.Navar LG, Imig JD, Zou L, Wang C-T. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 13.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J PhysioL Endocrinol Metab. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. [Review] [92 refs] Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension. 2008;52:666–671. doi: 10.1161/HYPERTENSIONAHA.108.114058. [DOI] [PubMed] [Google Scholar]
- 17.Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension. 2005;45:133–137. doi: 10.1161/01.HYP.0000149105.75125.2a. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo J, Ohishi M, Mendelsohn FAO. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene trasngenic rat kidney. Hypertension. 1999;33:347–353. doi: 10.1161/01.hyp.33.1.347. [DOI] [PubMed] [Google Scholar]
- 19.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 20.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 21.Harrison-Bernard LM, El-Dahr SS, O'Leary DF, Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension. 1999;33:340–346. doi: 10.1161/01.hyp.33.1.340. [DOI] [PubMed] [Google Scholar]
- 22.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 23.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 24.Hall JE. Regulation of glomerular filtration rate and sodium excretion by angiotensin II. Fed. Proc. 1986;45:1431–1437. [PubMed] [Google Scholar]
- 25.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 26.Wang C-T, Navar LG, Mitchell KD. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J Hypertens. 2003;21:353–360. doi: 10.1097/00004872-200302000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Cervenka L, Wang C-T, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 29.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 30.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervenka L, Vaneckova I, Huskova Z, Vanourkova Z, Erbanova M, Thumova M, Skaroupkova P, Opocensky M, Maly J, Chabova VC, Tesar V, Burgelova M, Viklicky O, Teplan V, Zelizko M, Kramer HJ, Navar LG. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. J Hypertens. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 33.Zhao D, Seth DM, Navar LG. Enhanced Distal Nephron Sodium Reabsorption in Chronic Angiotensin II-Infused Mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao W, Seth DM, Navar LG. Angiotensin II Type 1 Receptor-Mediated Augmentation of Urinary Excretion of Endogenous Angiotensin II in Val5-Angiotensin II-Infused Rats. Hypertension. 2010;56:378–383. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren Y, D'Ambrosio MA, Garvin JL, Carretero OA. Angiotensin II Enhances Connecting Tubule Glomerular Feedback. Hypertension. 2010;56:636–642. doi: 10.1161/HYPERTENSIONAHA.110.153692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovacs G, Peti-Peterdi J, Rosivall L, Bell PD. Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol. 2002;282:F301–F306. doi: 10.1152/ajprenal.00129.2001. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Villalobos R, Navar LG. Intrarenal Angiotensin II Augmentation in Hypertension. In: Frohlich ED, Re RN, editors. The Local Cardiac Renin-Angiotensin Aldosterone System. New York: Springer Science+Business Media, LLC; 2009. pp. 121–131. [Google Scholar]
- 39.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular Angiotensin II Induces Cytosolic Ca2+ Mobilization by Stimulating Intracellular AT1 Receptors in Proximal Tubule Cells. Am J Physiol Renal Physiol. 2005;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol. 2010;298:H1807–H1818. doi: 10.1152/ajpheart.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 42.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung. 2002;89:427–438. doi: 10.1556/APhysiol.89.2002.4.3. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Mukhin YV, Garnovskaya MN, Thielen TE, Iijima Y, Huang C, Raymond JR, Ullian ME, Paul RV. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- 44.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–941. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 45.Ingelfinger J, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 47.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang S-S. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol Renal Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 51.Satou R, Miyata K, Katsurada A, Navar LG, Kobori H. Tumor necrosis factor-{alpha} suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am J Physiol Cell Physiol. 2010;299:C750–C759. doi: 10.1152/ajpcell.00078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaguri A, Eguchi S. Tumor necrosis factor-{alpha}: a reno-protective cytokine? Focus on "Tumor necrosis factor-{alpha} suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells". Am J Physiol Cell Physiol. 2010;299:C729–C730. doi: 10.1152/ajpcell.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 54.Bohlender J, Menard J, Ganten D, Luft FC. Angiotensinogen concentrations and renin clearance : implications for blood pressure regulation. Hypertension. 2000;35:780–786. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 55.Smithies O. A mouse view of hypertension. Hypertension. 1997;30:1318–1324. doi: 10.1161/01.hyp.30.6.1318. [DOI] [PubMed] [Google Scholar]
- 56.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, Hamet P, Chan JS. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 58.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286:F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 59.Kim H-S, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 61.Zhao YY, Zhou J, Narayanan CS, Cui Y, Kumar A. Role of C/A polymorphism at −20 on the expression of human angiotensinogen gene. Hypertension. 1999;33:108–115. doi: 10.1161/01.hyp.33.1.108. [DOI] [PubMed] [Google Scholar]
- 62.Lalouel JM, Rohrwasser A. Genetic susceptibility to essential hypertension: insight from angiotensinogen. Hypertension. 2007;49:597–603. doi: 10.1161/01.HYP.0000257145.20363.9c. [DOI] [PubMed] [Google Scholar]
- 63.Pereira TV, Nunes AC, Rudnicki M, Yamada Y, Pereira AC, Krieger JE. Meta-analysis of the association of 4 angiotensinogen polymorphisms with essential hypertension: a role beyond M235T? Hypertension. 2008;51:778–783. doi: 10.1161/HYPERTENSIONAHA.107.100370. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension. 2009;53:351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, Morikawa T, Okumura M, Meda I, Kiyomoto H, Hosomi N, Mori T, Ito S, Imanishi M. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, Harrison-Bernard LM. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–F48. doi: 10.1152/ajprenal.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–927. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takamatsu M, Urushihara M, Kondo S, Shimizu M, Morioka T, Oite T, Kobori H, Kagami S. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008;23:1257–1267. doi: 10.1007/s00467-008-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Activation of reactive oxygen species and the renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:509–515. doi: 10.1111/j.1440-1681.2008.05107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, Cohen EP, Navar LG. Young Scholars Award Lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–550. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 75.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008;295:F283–F289. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, Navar LG, Kobori H. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009;311:24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Godin N, Liu F, Lau GJ, Brezniceanu ML, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int. 2010;77:1086–1097. doi: 10.1038/ki.2010.63. [DOI] [PubMed] [Google Scholar]
- 78.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. HypertensioN. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG. Determination of Plasma and Urinary Angiotensinogen Levels in Rodents by Newly Developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal Renin-Angiotensin system activity. Am J Nephrol. 2010;31:318–325. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogawa S, Kobori H, Ohashi N, Urushihara M, Nishiyama A, Mori T, Ishizuka T, Nako K, Ito S. Angiotensin II Type 1 Receptor Blockers Reduce Urinary Angiotensinogen Excretion and the Levels of Urinary Markers of Oxidative Stress and Inflammation in Patients with Type 2 Diabetic Nephropathy. Biomark Insights. 2009;4:97–102. doi: 10.4137/bmi.s2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lantelme P, Rohrwasser A, Vincent M, Cheng T, Gardier S, Legedz L, Bricca G, Lalouel JM, Milon H. Significance of urinary angiotensinogen in essential hypertension as a function of plasma renin and aldosterone status. J Hypertens. 2005;23:785–792. doi: 10.1097/01.hjh.0000163147.20330.f5. [DOI] [PubMed] [Google Scholar]
- 87.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study) J Hypertens. 2010;28:1422–1428. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 90.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 93.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prieto MC, Navar LG. Collecting Duct Renin: A Critical Link in Angiotensin II-Dependent Hypertension. In: Frohlich ED, Re RN, editors. The Local Cardiac Renin-Angiotensin Aldosterone System. New York: Springer Science+Business Media, LLC; 2009. pp. 133–141. [Google Scholar]
- 96.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. Journal of the American Society of Hypertension. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H. Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med. 2008;86:629–635. doi: 10.1007/s00109-008-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 101.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53:1077–1082. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 102.Reudelhuber TL. Prorenin, Renin, and their receptor: moving targets. Hypertension. 2010;55:1071–1074. doi: 10.1161/HYPERTENSIONAHA.108.120279. [DOI] [PubMed] [Google Scholar]
- 103.Burckle CA, Jan Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 104.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly Progressive, Angiotensin II-Independent Glomerulosclerosis in Human (Pro)renin Receptor-Transgenic Rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 105.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 106.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, Alves Correa SA, Shimuta SI, Pesquero JB, Mortara RA, Casarini DE. Expression of angiotensin I-converting enzymes and bradykinin B2 receptors in mouse inner medullary-collecting duct cells. Int Immunopharmacol. 2008;8:254–260. doi: 10.1016/j.intimp.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 108.Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P, Bernstein KE. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension. 2004;43:854–859. doi: 10.1161/01.HYP.0000119190.06968.f1. [DOI] [PubMed] [Google Scholar]
- 109.Tian B, Meng QC, Chen Y-F, Krege JH, Smithies O, Oparil S. Blood pressures and cardiovascular homeostasis in mice having reduced or absent angiotensin-converting enzyme gene function. Hypertension. 1997;30:128–133. doi: 10.1161/01.hyp.30.1.128. (part 1) [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, Navar LG. Kidney ACE is sufficient to induce hypertension in response to angiotensin I infusion. Journal of the American Society of Nephrology. 2010 doi: 10.1681/ASN.2010060624. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vio CP, Jeanneret VA. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int Suppl. 2003;86:S57–S63. doi: 10.1046/j.1523-1755.64.s86.11.x. [DOI] [PubMed] [Google Scholar]
- 112.Polichnowski AJ, Jin C, Yang C, Cowley AW., Jr Role of renal perfusion pressure versus angiotensin II on renal oxidative stress in angiotensin II-induced hypertensive rats. Hypertension. 2010;55:1425–1430. doi: 10.1161/HYPERTENSIONAHA.110.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]