Abstract
Since the Fontan/Kreutzer procedure was introduced, evolutionary clinical advances via a staged surgical reconstructive approach have markedly improved outcomes for patients with functional single ventricle. However, significant challenges remain. Early stage mortality risk seems impenetrable. Serious morbidities - construed as immutable consequences of palliation - have hardly been addressed. Late functional status is increasingly linked to pathophysiologic consequences of prior staged procedures. As more single ventricle patients survive into adulthood, Fontan failure is emerging as an intractable problem for which there is no targeted therapy. Incremental solutions to address these ongoing problems have not had a measurable impact. Therefore, a fundamental reconsideration of the overall approach is reasonable and warranted. The ability to provide a modest pressure boost (2-6 mmHg) to existing blood flow at the total cavopulmonary connection can effectively restore more stable biventricular status. This would impact not only treatment of late Fontan failure, but also facilitate early surgical repair. A realistic means to provide such a pressure boost has never been apparent. Recent advances are beginning to unravel the unique challenges which must be addressed to realize this goal, with promise to open single ventricle palliation to new therapeutic vistas.
Keywords: univentricular Fontan circulation, cavopulmonary assist, mechanical circulatory support
Perspective
The idealistic notion of powering the Fontan circulation is not necessarily a new one [1]. Early reports of experimental and clinical attempts to bypass the right heart laid the foundation for successful staged surgical palliation of functional single ventricle [2]. It was known then that a pressure gradient of only 6 mmHg was needed to propel blood from the pulmonary artery through the lungs [3]. From these reports, it can be inferred that complete bypass of the right heart as a primary, direct intervention was nearly accomplished. With incorporation of the right atrium as a low energy right-sided power source, it ultimately was. Using a right-sided power source to supplement a univentricular Fontan circulation is highly compelling: it would biventricularize it toward more stable 2-ventricle physiology. This could serve not only to stabilize an existing Fontan circulation, but also serve in direct Fontan conversion as a facilitator for stable systemic transition to an unsupported Fontan.
Because a safe and reliable method to provide right-sided circulatory support in the Fontan total cavopulmonary connection (TCPC) has never been apparent, this concept has been largely relegated to the realm of wistful speculation. However, this may be about to change. Concepts are emerging which address in earnest how right-sided circulatory support may be reasonably applied to power the Fontan. The ability to provide a modest pressure boost within the Fontan venous pathway – temporarily or permanently – is a tremendous opportunity to resolve many of the serious problems in the current staged palliative approach and to improve treatment of late Fontan failure.
Intractable problems in the current approach
Unlike most congenital heart defects, contemporary outcomes for repair of functional single ventricle remain benchmarked in terms of survival. While reported early operative mortality may provide a sense that outcomes are acceptable, the reality of one year survival (62-72% in a recent NIH-sponsored prospective trial) makes it abundantly clear they are not [4]. And this statistic does not account for the morbidity and pathophysiology inherent in the palliative process; these insidious problems have really not begun to be addressed. Survival of single ventricle repair is not necessarily synonymous with excellent outcome.
Once Fontan completion is achieved, mortality risk plateaus – with a slow attrition decades thereafter. Thus, it is logical to implicate the highly unstable interim pathophysiologic state, rather than the underlying anatomic substrate, as the factor primarily responsible for adverse outcomes. In addition, poor late functional status is increasingly linked to prior palliative procedures in which a shunt source of pulmonary blood flow was utilized. Therefore, by extension, if interim-stage pathophysiology is the crux of both early and late problems, then perhaps the overall staged approach ought to be reassessed [5].
This is difficult because there is no readily foreseeable alternative: An unforeseen alternative may be cavopulmonary assist. It permits a palliative strategy based on normal two ventricle physiology. Viewed through this lens, the problems and paradoxes inherent in the existing staged approach are more strikingly apparent.
Incremental modifications have failed to make an impact
Amid ongoing problems, substantial surgical innovations have been proposed (Table 1). Principal among these are modification of the source of pulmonary blood flow in the neonatal period (i.e. attempts to address the instability of stage-I Norwood physiology). Examples include the right ventricle-to-pulmonary artery shunt [6] and the “hybrid” stage-I procedure with comprehensive stage-II approach [7]. The fact that these have been readily assimilated into clinical practice, ostensibly for high-risk candidates, signals a substantial need for better solutions. Although these pragmatic modifications address, and perhaps improve, specific facets of the existing staged palliative approach, none provide a comprehensive solution to this enigmatic problem.
Table 1.
Efforts to improve various components of the existing staged surgical paradigm for repair of functional single ventricle.
|
In essence, all single ventricle patients are high risk - not necessarily because of their anatomic substrate - but because of the interceding palliative physiologic states necessary to achieve the end goal of a series single ventricle circulation: It is the ‘means’, not the ‘end’. The survival curve after staged repair of hypoplastic left heart syndrome reflects this: a dramatic mortality drop-off in the first 2 years is followed by a plateau. The staged approach offers hope for survival, but is it the best path? Significant research and clinical resources are expended to improve upon the existing approach, which has evolved on a subjective clinical basis. In our view, this strategy is inherently unstable, has pathophysiologic consequences, and cannot ever be perfected - no matter how well it is refined. There may be a safer and more sensible approach.
Interim staged palliation - pathophysiology and paradox
In staged single ventricle palliation, are we doing more harm done than good? The pathophysiologies associated with early stages undermine the goals of later stages and late functional status (For purposes of clarity, discussion of single ventricle in this review relates generally to Hypoplastic left Heart Syndrome).
1) Stage-I
Shunt-dependent single ventricle physiology is highly unstable with an exceedingly narrow margin of stability. At the expense of providing a reliable source of neonatal pulmonary blood flow, the systemic-to-pulmonary arterial shunt creates 4 potentially lethal problems: 1) it places the pulmonary and systemic circulations in an inherently unstable parallel arrangement. Positive physiologic feedback loops escalate instability; overperfusion of the lungs or body occurs at the expense of the other vascular bed. Supplemental oxygen is destabilizing. Maintenance of circulatory balance may require paradoxical management strategies. 2) the single ventricle must pump twice normal volume, leading to myocardial hypertrophy and atrioventricular valve insufficiency. 3) myocardial perfusion is impaired due to: a) reduced diastolic blood pressure from shunt run-off; and b) increased myocardial wall tension due to volume overload. 4) the ventricle must perform this doubled workload under conditions of severe hypoxemia (PAO2 30-40 mmHg), required to maintain circulatory balance. By comparison, PAO2 for the fetus, and for the few who are able to summit Mt. Everest at 29,029 ft without supplemental oxygen, is in a similar range at ~30 mmHg. In this thin air, cellular and end organ function (brain, kidney, heart) are compromised with little margin for stability. How might these patients fare if they could be managed with normoxia?
In the interstage interval, with a shunt source of pulmonary blood flow, the following problems are pervasive: severe hypoxemia, ventricular volume overload with pathologic remodeling, neurocognitive dysfunction, prolonged complex hospital course, sudden instability, and death. The shunt preserves conditions best for prenatal life, secondarily creating a lasting impediment to subsequent Fontan conversion and late functional status. Additionally, synthetic shunts have high risk of thrombosis and lack growth potential. In the clinical mindset, these problems are construed as immutable consequences of palliative repair. All are integral to poor outcomes; all are attributable to the systemic-to-pulmonary arterial shunt. Yet, implication of the shunt as the common denominator is seldom heard - because there is no apparent means to avoid it. If a systemic venous source of neonatal pulmonary blood flow were possible, these problems would be addressed.
The right ventricle to pulmonary artery conduit addresses only the issue of impaired diastolic coronary perfusion, at the expense of a ventriculotomy in the sole functional ventricle. It does not alleviate hypoxemia, parallel circulations, and ventricular volume overload; a survival benefit has not been demonstrated. In the “hybrid” approach, patients are still subjected to shunt-equivalent physiology – just without use of a synthetic shunt. The hypoxemia and ventricular volume overload imposed in the hybrid approach are fundamentally the same pathophysiology as incurred in other shunted single ventricle states.
Paradoxically, the shunt induces and exacerbates the very conditions which mandate its use in the first place. PAO2 near fetal levels and anaerobic threshold is a potent and sustained stimulus of pulmonary vasoconstriction. Transitional pulmonary maturation is key to health of the pulmonary vascular bed; ultimate health of the pulmonary vascular bed is, in turn, a keystone to late Fontan functional status. Shunt physiology impairs transitional pulmonary maturation [8]. By comparison, it is worth considering normal newborns, in whom pulmonary vascular resistance falls to near adult levels within the first 1-2 days of life, and is maintained at an extremely low level absent noxious stimuli (hypoxemia, high-pressure source of pulmonary blood flow (i.e. shunt)) which may induce a hypertensive response. Excluding extenuating factors on the fetus such as a restrictive foramen ovale, the single ventricle neonate is able to maintain low pulmonary resistance under ideal physiologic conditions. The risk of pulmonary vasoreactivity recedes with involution of the fetal smooth muscle arteriolar layer over 6-8 weeks, the majority of which occurs in the first 2 weeks of life [9]. Clearly, a knowledge gap exists between maintenance of low pulmonary resistance in normal newborns versus that of shunted single ventricle physiology where pulmonary resistance sufficiently low for Fontan conversion is not assured until 3-4 months of age. Does pulmonary resistance materially improve beyond this certain age, or does the infant eventually outgrow the shunt to arrive at a low pulmonary arterial pressure? Shunt physiology may be the principal factor responsible for prolonging this gap - by prolonging the interval of increased pulmonary vascular tone.
Neurocognitive dysfunction is common in this group, yet interestingly, severe hypoxemia is not clearly implicated as a primary cause. It is a reasonable assumption that sustained severe hypoxemia, near anaerobic threshold and after major reconstructive surgery requiring cardiopulmonary bypass and regional low (or no) flow perfusion to the brain, is a major determinant of neurocognitive dysfunction. Consider what the neurocognitive outcome might be if single ventricle infants could be managed post-palliation with a PAO2 of 80mmHg or higher: Would it be improved? Would it be normal?
2) Stage-II
Stage-II palliation involves take-down of the shunt, and commits the superior vena cava (SVC) distribution to serve as the sole source of pulmonary blood flow into a vascular bed which has – unfortunately – previously acclimated in defense of hypoxemia and high-pressure flow. In infants, SVC flow represents ~ 60% total systemic venous return, of which ~ 90% is cerebral in origin. As a result, SVC pressure is increased 2-3-fold, the extent to which depends upon basal resistance of the pulmonary vascular bed. Less pulmonary resistance equates to less SVC pressure required to sustain adequate preload.
Historically, the rationale for the intermediate stage-II procedure has been to protect the ventricle from a longer period of sustained volume overload. Because the shunt will not grow to provide adequate pulmonary blood flow before an age when a Fontan might be considered, stage-II conversion eventually becomes mandatory. How to safely transition to Fontan completion at this point has not been established. Although stage-II volume unloading of the single ventricle provides early partial ventricular volume reduction and improvement in hypoxemia, counter to assumptions, it has not been associated with improved late ventricular function or exercise performance [10]. Thus, the majority benefit of interim staging likely exists early from take down of the shunt, rather than from a benefit of partial ventricular volume reduction and partially improved oxygenation.
To shorten the high risk interstage interval, early stage-II conversion has been proposed. However, the safety and rationale for this has not been established. Intensive home monitoring programs have had an impact, but the very need for high-acuity home monitoring suggests that interstage physiology is unacceptably perilous. Younger patients are susceptible to poor outcomes after stage-II conversion, especially less than 3 months of age. At this age, given sustained hypoxemia from inferior vena cava (IVC) flow into the single ventricle, pulmonary resistance is unlikely to decrease to an extent that would make early stage-II conversion feasible. In other words, partial Fontan conversion in the setting of ongoing hypoxemia likely precludes any chance of feasibility in younger patients. Alternatively, if IVC flow was also diverted (i.e. total Fontan conversion), hypoxemia would no longer exist: Would pulmonary resistance decrease sufficiently to permit adequate transpulmonary flow driven by a systemic venous source alone?
3) Stage-III
By diverting IVC flow to the pulmonary arteries, stage-III Fontan conversion completes the separation of the pulmonary and systemic circulations to the end goal of a series arrangement. Hypoxemia and circulatory imbalance are no longer problems and survival is much improved. The circulation is now equivalent to a 2-ventricle circulation, with the notable exception that it lacks of a subpulmonary power source. The result is the “Fontan paradox”: elevated systemic venous pressure concurrent and interdependent with relative pulmonary arterial hypotension [11]. With additional pressure loss across the lungs, ventricular preload is chronically subnormal. Because IVC pressure is now increased 2-3 fold, subsequent morbidities may arise from the IVC distribution, including hepatic and gut dysfunction secondary to elevated splanchnic venous pressure.
Stage-III Fontan conversion is performed anywhere from 1 to 4 years after stage-II. This timing is highly variable and subjective. Timing decisions are typically driven by the constraints of preferred technique and choice of conduit material (i.e. minimum size extracardiac conduit which will serve into adulthood), rather than by what is physiologically best with respect to late functional status. Earlier Fontan conversion is increasingly associated with improved long-term ventricular function, incidence of normal sinus rhythm, and freedom from atrioventricular valve insufficiency – all of which are presumably secondary to a shortened interval of ventricular volume overload and hypoxemia [12]. This is consistent with trends toward earlier Fontan repair. However, it counters clinical assumptions that younger children (infants) are less durable candidates for Fontan. From a purely systemic venous standpoint, this may not be the case. There are no data which examine the relationship of age and tolerance to elevated systemic venous pressure. Herein lies another paradox: subjective clinical concern for intolerance to Fontan physiology at an early age does is at odds with clinical outcomes data that patients undergoing earlier Fontan conversion fare better long term. By mining this issue, important clues to improve outcomes of Fontan palliation are likely to be found.
4) Late Fontan
The problems of elevated systemic venous pressure and subnormal ventricular filling underlie subsequent Fontan-related disease for life. Risk for decompensation is high. Fontan failure is a somewhat misleading term; although patients with failing Fontan physiology exhibit classic features of congestive heart failure, the underlying cause is typically not primary myocardial dysfunction. In a reversal of assumptions, it may be more accurately stated that the rest of the circulation is failing the Fontan heart. Therefore, the target for support is not the systemic ventricle – it is the cavopulmonary segment of the circulation. In late follow up, systolic dysfunction is preserved in 70% [10]. The ventricular dysfunction observed is characteristically diastolic, likely secondary to chronic preload deprivation superimposed on a noncompliant ventricle [13]. Studies are also finding no benefit of intermediate staging with regard to late ventricular function and functional status [10], casting doubt on clinical assumptions that interim staging is beneficial with respect to late ventricular function by an earlier transition to a less volume loaded state. Late functional status has also been linked in part to prior staging procedures in which a shunt was utilized [14]. Thus, the sequelae of early palliative decision-making are now negatively reflected in late functional status. If shunt pathophysiology could be avoided early, might the lungs and myocardium be better prepared to serve late in Fontan? Currently, there is no good exit strategy for late Fontan problems.
The “optimal” Fontan
An optimal Fontan circulation rests on 2 implicit physiologic objectives: 1) least possible basal pulmonary vascular resistance - for best possible transpulmonary blood flow; 2) best possible ventricular compliance - for best possible ventricular filling. Prior shunt physiology diametrically opposes these objectives. Hypoxemia and high-pressure flow impair ultimate health of the pulmonary capillary bed. Ventricular volume overload (in the setting of severe hypoxemia) induces cardiomyopathy. This is poor preparation for a ventricle whose ultimate task is to perform optimally when filled by upstream systemic venous pressure alone. Subnormal filling pressure, superimposed on a hypertrophied and non-compliant ventricle, equates to poor function and poor output.
As more single ventricle patients survive to adulthood and late outcomes are further assessed, causality between palliative decision-making in infancy and late functional status will become increasingly clear. Interim staged palliation may represent a tradeoff between improved early outcomes - at the expense of late functional status. Best long-term outcome may derive from a strategy which exposes the single ventricle to the shortest possible staging interval (or no staging at all), limiting or avoiding the consequences of shunt pathophysiology (severe hypoxemia, ventricular volume overload). In other words: the sooner to Fontan, the better.
Of the preceding multitude of reasons why the existing staged approach is problematic, one defining statement trumps them all: a direct approach to Fontan palliation is possible based on normal two-ventricle physiology. This path has been paved by millions of years; a way should be found to take it. With implications from the molecular to whole organ level, it is simple, powerful, and hard to refute. Precisely how it can be accomplished is a complex and provocative bioengineering challenge.
The approach to Fontan: what is best path?
It is clear that humans with a series single ventricle circulation can be quite functional. What is not clear is the best path to reach this state [15]. A direct approach to Fontan is rooted in the sensibility of normal 2-ventricle physiology, conditions under which biologic and circulatory function are teleologically best. In considering cavopulmonary assist as a bridge to unsupported Fontan, the physiologic paradigm for Fontan conversion would shift to focus on 2 new core issues. First, is the time and determinants for the highly compliant systemic venous circulation (including capillary, interstitial, and oncotic compartments) to stably adapt to the pressure required (10-15 mmHg) to independently perfuse the lungs and maintain ventricular filling and cardiac output: How long does it take for adaptation to occur?; by what mechanisms does it occur?; what are the age-dependent variables for it to occur? Second, is strategies to minimize/optimize early and late basal pulmonary vascular tone and preserve ventricular function as the pivotal factors for best late functional status (of which, shunt physiology is not one). In the single ventricle neonate, ventricular compliance is normal; it becomes abnormal in subsequent staging. Excluding factors such as shunt hypoxemia, when is neonate/infant pulmonary resistance low enough to support a systemic venous source of pulmonary blood flow? The scientific underpinnings for transition to Fontan are essentially unknown.
Why is Fontan conversion staged?
It is instructive to consider this question. When staged as 2 procedures, Fontan conversion effectively divides the physiologic stress imposed on the SVC and IVC territories as separate events in time. This improves the safety margin by allowing partial, rather than global, increase in systemic venous pressure. Surely, some time-limited component must be integral to compensatory adaptation to this state, especially within the interstitial and oncotic compartments. Fontan conversion in one-stage is possible in select patients with excellent results, although this has fallen out of favor due to improved safety margins and outcomes obtained with staged Fontan conversion [16]. If a means existed to support a gradual transition of the entire systemic venous circulation (SVC + IVC) to Fontan-level venous pressure (i.e. cavopulmonary assist), Fontan conversion might be more readily accomplished in a single stage with excellent results.
Systemic venous adaptation to Fontan
The systemic venous change which occurs subsequent to Fontan conversion has been largely overlooked for several reasons. First, timing decisions regarding staged palliative repair have historically focused on more pressing downstream physiologic issues (i.e. pulmonary resistance) rather than upstream physiologic issues (the systemic venous compartment). Second, short of minimization or avoidance, there are few, if any, direct means to therapeutically manage the sequelae of systemic venous hypertension: The best medicine has been minimization or avoidance. Third, chronic animal models of Fontan do not exist, making it impossible to investigate the physiologic response to Fontan conversion and identify the determinants of ideal Fontan status. For example, what is the impact of venous collateral flow between the SVC and IVC distributions at each staging interval? With stage-II conversion and exposure of the SVC territory to higher venous pressure, how much venous effluent is rerouted via collaterals to the lower-pressure IVC distribution? What is the impact of age, from infancy to adulthood, on the relative contribution of the SVC and IVC territories on venous return profiles in Fontan? Which territory is best to transition first, and why? Support for basic and clinical investigation in this area is critically important if we hope to improve clinical approaches to Fontan [17].
In animal studies, we have sought to investigate the physiologic adaptive process and time frame for the systemic venous circulation to adapt to Fontan-level systemic venous pressure [18]. To be clear, this model has limitations: It is a model of systemic venous hypertension (bi-caval restriction), and the right heart is maintained in the circulation. Juvenile sheep were instrumented with adjustable SVC and IVC occluders. During 1 week recovery, normal hemodynamics were maintained. At intervention, both SVC and IVC upstream pressures were simultaneously increased, by tightening the vena caval occluders, to 15 mmHg as a physiologic mimic of one-stage Fontan conversion. The acute 3-fold elevation in systemic venous pressure was surprisingly well-tolerated in awake, conscious animals. After an initial decrease, cardiac output recovered coincident with neurohormonally mediated salt and water retention. Circulating blood volume increased, and remained elevated, as a persistent adaptive response. Subsequently, neurohormonal status (antidiuretic hormone, aldosterone, angiotensin II, basic natriuretic peptide, epinephrine, norepinephrine) normalized to baseline. This process, which presumably includes interstitial and oncotic adaptation, occurred naturally over a 2 week period. Thus, in a strategy of primary Fontan repair using temporary cavopulmonary assist as a bridge from supported (cavopulmonary assist + systemic ventricle (≈2-ventricle) to unsupported Fontan, partial assist of Glenn/Fontan flow may be required no longer than 2 weeks in order for full systemic adaptation to take place.
Re-engineering the approach to single ventricle repair
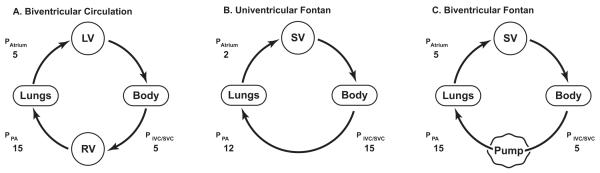
Cavopulmonary assist can convert the univentricular Fontan circulation to a “biventricular Fontan”: single ventricle anatomy with the attributes of biventricular physiology (Figure 1) [19, 20]. Thus, with an appropriate device, a patient with single ventricle and a surgical Fontan construction, at any stage of life, can be managed with: normal oxygenation; balanced QP:QS; normal cerebral perfusion and oxygenation; normal systemic venous pressure; normal atrial filling pressure; normal cardiac output. Surprisingly low energy input (2-6 mmHg) will reverse the Fontan paradox. In clinical terms, this makes sense: a simultaneous 2-6 mmHg decrease in systemic venous pressure and 2-6 mmHg increase in ventricular filling will substantially improve hemodynamic status. More energy input may be undesirable - or even deleterious. Partial support can then be weaned gradually beyond the stabilization period (or perioperative period after third spacing and fluid mobilization have occurred and lung function is optimal), at a time when the entire circulation is better prepared to stably adapt to the systemic venous pressure required to independently perfuse the lungs and maintain cardiac output. Fulfillment of this therapeutic opportunity hinges entirely upon successful development of a device.
Figure 1.
Cavopulmonary assist: pump placement at the level of the TCPC reduces systemic venous pressure, improves single ventricle filling, thereby reproducing normal 2-ventricle physiology. SV: single ventricle; SVC: superior vena cava; IVC: inferior vena cava; PA: pulmonary artery.
Optimization of passive TCPC flow
In a Fontan circulation, any potential for pressure loss in the low-pressure right-sided circulation is of critical importance. It may exist as anatomic pressure loss across the TCPC (2-5 mmHg), or physiologic pressure loss across the pulmonary capillary bed (5-8 mmHg). Pressure loss at each level is potentially modifiable. A goal of minimizing both is tantamount to best achievable Fontan circulatory status. Seemingly minor improvement in dissipative energy loss can translate to major improvement in hemodynamic status. This is supported clinically by Fontan conversion from an atriopulmonary to a TCPC connection, where 2-6 mmHg gain in right-sided hydraulic energy improves functional status considerably.
The TCPC has been long recognized as a target for flow optimization strategies to minimize pressure loss. It is a 4-way flow path with bidirectional and orthogonally related inflow and outflow channels. Relatively significant power loss (2-6 mmHg) occurs in this low-pressure environment due to colliding and recirculating flow. Computational fluid dynamic studies have been utilized to investigate anatomic optimization of the Fontan venous pathway. Various optimizations have been proposed. In summary, these consist of either a) lateral or b) antero-posterior offset of the vena caval axes (optimal ~ 20% diameter) where they connect to the TCPC junction [21,22]. This basically averts a head-on collision of inflow streams, and reduces turbulent energy loss. Excessive vena caval offset (> 50% diameter) negates any hydraulic benefit of partial offset and induces disparate pulmonary blood flow distribution and risk of arteriovenous malformation due to maldistribution of hepatic venous efflux.
Recently, more aggressive modifications of the Fontan venous pathway have been proposed. These include vena caval splitting surgeries (principally IVC), branched synthetic conduits, and flow splitting devices sutured into the midst of the TCPC junction [23,24]. The IVC is a principal target because it comprises the majority of systemic venous return in the adult (70%), and because the majority of late Fontan morbidities arise from the IVC distribution. Although the hydraulic benefit of streamlining TCPC flow is intuitive, the magnitude of pressure relief and the practicalities of surgical reconstruction in the low-pressure TCPC pathways remain to be resolved in clinical practice.
Powering the Fontan
We have focused on adding hydraulic energy to existing TCPC flow early or late after Fontan repair [19,20]. A cavopulmonary assist pump would serve as a low energy input “primer” for the primary pump (single ventricle), rather than as a primary pump per se. This is identical to the essential function of the right ventricle in a normal biventricular circulation. The biomechanical requirements for a pump to augment flow in the complex 4-way geometry and unique physiologic environment of a TCPC are unlike any other circulatory support application [Table 2]: no such pump currently exists. Extravascular devices are not a good consideration. The inflow/outflow cannulation issues are complex, and recirculation in the open-channel Fontan venous pathway cannot be reasonably prevented short of taking down the Fontan connection.
Table 2.
Bioengineering considerations for the ideal cavopulmonary assist device.
|
A catheter-based intravascular device which utilizes the existing Fontan venous pathway is a much more attractive option. The TCPC is favorable for intravascular support due to its relatively straight longitudinal axis and non-compliant, noncontractile walls. The ideal device must support flow in a 3-way or 4-way geometry with a double inlet, double outlet flow pattern. It should not be obstructive under any circumstance – rotating or stationary. As little as 2-6 mmHg obstruction in the venous pathway has grave consequences. It must deliver low-pressure, high-volume flow similar to normal right ventricular hemodynamics. Confirmed in animal studies, optimum pressure is 6 mmHg in mature and 8 mmHg in newborn (healthy) animals [19,20,25,26]. The device should also be capable of higher performance range, up to 30 mmHg, in the event of increased pressure head (pulmonary hypertension). Conversely, it is just as vitally important that it perform well and with minimal risk of thrombogenicity at very low pressure and flow rates prior to withdrawal. It must be weaned to zero net contribution to flow, without compromise of existing Fontan flow, prior to safe withdrawal.
A Fontan power source - evolving concepts
We first demonstrated the concept of cavopulmonary assist using two opposed unidirectional axial flow pumps: one positioned in the SVC, the other in the IVC [19]. Other groups have followed with similar concepts of venous assist for Glenn/Fontan, focusing for the most part on intravascular microaxial devices modified to perform in the low-pressure venous circulation [27-30]. Models of single device support in the IVC have predominated for 3 principal reasons: 1) the majority of systemic venous return in adults arises from the IVC distribution (70% total systemic venous return); 2) the majority of late Fontan pathophysiology arises in the IVC distribution (gut/hepatic dysfunction, protein losing enteropathy, ascites, lower extremity edema); 3) it is hoped that the use of a single pump only, rather than the complexity of 2 separate pumps, might suffice. A single unidirectional pump is problematic, however, in that it does not account for all 4 limbs of TCPC flow and will produce undesirable back-pressure elevation in the opposing venous territory (SVC). In reports espousing single pump support in the IVC, offset of the vena caval axis by > 1 diameter skirts this problem.
Limitations of existing microaxial devices
At first thought, the idea of modifying a microaxial flow device to function in the low-pressure venous circulation is appealing. We have learned, however, that this has serious inherent limitations. First, they provide one-way flow, necessitating the use of 2 pumps to address all limbs of 4-way TCPC flow. Some groups have responded to this concern by proposing to surgically modify the Fontan venous pathway to a 3-way ‘Y’-configuration using synthetic graft material in order to accommodate one pump in a common outflow limb [28]. Second, the smaller the diameter of a percutaneous microaxial pump, the more a barrier to recirculation is required. A small diameter, percutaneous axial flow pump positioned in a relatively much larger diameter central vein requires a barrier to recirculation to ensure effective flow. Barriers to recirculation are problematic with respect to thrombus formation, especially in the low pressure Fontan venous pathway. Third, fixed diameter microaxial devices function at high rotational speed to generate equivalent large vessel flow, increasing risk of hemolysis and negative pressure at the inlet and suction collapse of the low-pressure upstream vena cavae. Fourth, axial flow devices are obstructive if they are slowed, stopped, or fail within the primary flow path. Depending on their diameter, and the presence of a barrier to recirculation, the pump cannot be shut off without obstruction.
A single-pump, 4-way flow solution
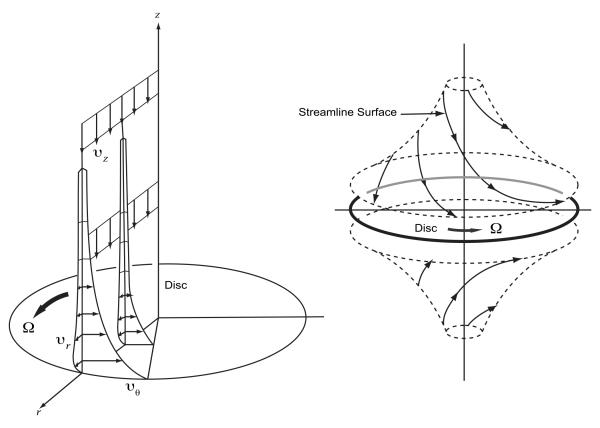
To overcome the limitations of existing microaxial devices, we are developing a pump solution which is expandable and multidirectional. Our current investigative focus is on a catheter-based, percutaneous, expandable viscous impeller pump [31]. Based on the von Karman viscous pump principal, a flat disk rotated in fluid will induce rotation of the fluid adjacent to the disk based on the no-slip condition (Figure 2). Viscous effects diffuse away from the disk and induce rotation. As the fluid rotates, the centrifugal force generates a radial outflow. By continuity, the outward moving fluid is replaced by axial flow toward the disk from quiescent fluid far away from the disk - on both sides. Therefore, a relatively simple, single, bi-conical impeller with surface vanes positioned in the midst of the TCPC junction will draw venous inflow from 2 opposing directions, and propel outflow radially in the diametrically opposed pulmonary arteries, simultaneously augmenting flow in all 4 axes of the TCPC.
Figure 2.
Von Karman viscous pump. Left: Fluid is induced to rotate by disc rotation, resulting in radial outflow. The outgoing fluid is replaced by inflow from the axial field. Right: On both sides of the disk, this results in opposed axial (vena caval) inflow and orthogonally opposed (pulmonary arterial) outflow.
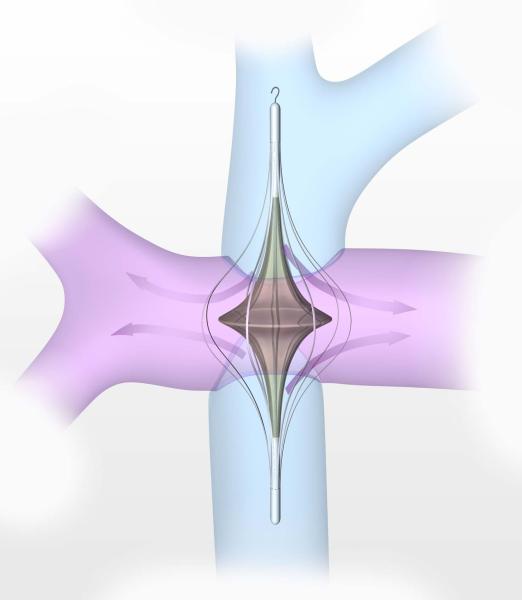
This unobvious solution is an exciting conceptual breakthrough which is uniquely suited to Fontan. The solution is improved from a complex 2-pump application with competing, colliding flows to a much simpler, flow facilitative single impeller design. The impeller poses no risk of venous pathway obstruction (Figure 3). As a static implant, it decreases turbulent energy loss and optimizes fluid transfer through the TCPC. As a pump, if it fails for any reason, its default function as a flow optimizer counteracts any obstructive potential. It provides low-pressure, high-volume flow similar to normal right ventricular hemodynamics. There is no back-pressure elevation in the opposing systemic venous territory. By expanding the impeller within the central vessels, rotational rates are lowered substantially, reducing fluid shear, hemolysis potential, and thrombogenicity risk. A high degree of fluid slip will reduce risk of vein collapse and cavitation due to inlet suction, and avoid excessive downstream pressure and perfusion lung injury. The impeller can be modified (geometric asymmetry or differential surface vane expression) to accommodate split differential venous inflow rates. Cavopulmonary assist is fully compatible with the existing staged paradigm. It does not require any surgical modification of existing Fontan pathways. It applies to a 3-way “T” (stage-II) and a 4-way “t” (stage-III) cavopulmonary connection. Placed similar to a central venous catheter, a percutaneous support option for patients with failing Fontan circulation will have extraordinary therapeutic value not only to surgeons, but also to cardiologists and critical care physicians who care for these patients.
Figure 3.
Viscous impeller pump in the 4-way TCPC junction. As a rotary pump, a catheter-based biconical impeller, with a cage to center the impeller and protect the vessel wall, will augment TCPC inflow and outflow in all 4 limbs at ideal pressure. As a static implant it will reduce turbulent kinetic energy loss.
Forward looking considerations
The availability of a right-sided mechanical circulatory support device specific to univentricular Fontan circulations will improve the approach to Fontan conversion in the short-term, and Fontan maintenance in the long-term (Table 3). Research in progress is making this a more realistic possibility.
Table 3.
Therapeutic potential of cavopulmonary assist.
Existing paradigm:
|
New paradigm:
|
1) Support of Failing Fontan
First clinical application of cavopulmonary assist is most likely to occur in adult patients with failing Fontan physiology. The ability to percutaneously temporize their circulatory insufficiency by simultaneously off-loading systemic venous pressure, and improving preload and cardiac output will, for the first time ever, provide a targeted therapy for failing Fontan. This mode of therapy may also be useful in patients listed for transplant, by improving their status for transplant once a donor organ becomes available.
In patients undergoing surgical repair in the existing paradigm, cavopulmonary assist may help to bridge them through early Fontan failure in the perioperative period to directly address the sequelae of elevated systemic venous pressure and low cardiac output. These marginal patients are difficult to manage, often with the only option being to take down the Fontan connection. Cavopulmonary assist may provide targeted stabilization to bridge them through the critical perioperative period.
2) Compression of staged palliation
Cavopulmonary assist may be applied to compress surgical staging, while, at the same time, vastly improve physiology.
a. One-stage (combined stage-II and III) Fontan
Fontan conversion can be performed as one-stage repair, with a period of temporary support to bridge the systemic transition to unsupported Fontan. In cases where a fenestration might be considered, cavopulmonary assist can provide the same benefit of increased cardiac output without the expense of oxygen desaturation and possibly with less risk of systemic thromboemboli.
b. Combined stage-I and II
By performing an aortic arch reconstruction and providing an SVC source of pulmonary blood flow, shunt-related pathophysiology can be avoided. This would require cavopulmonary support of SVC flow for a period of time. With ongoing hypoxemia secondary to continued IVC flow into the systemic ventricle, a concern is whether pulmonary resistance would remain sufficiently low to permit transpulmonary blood flow from an SVC source alone. Counter intuitively, the best physiology may be obtained from total rather than partial cavopulmonary diversion (no hypoxemia).
c. Bridge to Neonatal Fontan repair
Highly speculative and likely last to be implemented clinically, it may be possible to palliate functional single ventricle using cavopulmonary assist in newborns in whom a total cavopulmonary connection is constructed. Further studies are warranted to determine if the newborn will tolerate the elevated venous pressure and maintain low pulmonary resistance sufficient to perfuse the lungs and maintain preload from a systemic venous source alone. Contemporary therapies to mitigate pulmonary vascular tone (nitric oxide, phosphodiesterse-5 inhibitors), and hasten transitional pulmonary maturation may play a facilitative role.
Using mechanical cavopulmonary support as a bridge to neonatal Fontan repair of single ventricle would obviate the problems of single ventricle shunt physiology. In such an approach, single ventricle neonates could be effectively managed with a 2-ventricle circulation until subsequently weaned from cavopulmonary assist at a later point in time. Supplemental oxygen could be safely administered without inducing circulatory instability. Normal ventricular volume, coronary perfusion pressure, and PAO2 would be maintained [20].
3) The “biventricular Fontan”
A percutaneously implanted static flow diverter or rotary pump may be developed as a chronically implanted right-sided cavopulmonary assist device. If accomplished, a univentricular Fontan patient could be effectively managed as a biventricular Fontan - with normal 2-ventricle physiology - for the duration of their lives. As the ultimate exit strategy for ongoing Fontan problems, it may extend quality and duration of life comparable to biventricular patients and offer hope of relieving the problems that currently plague patients with late Fontan failure. For a static device, percutaneous deployment would avoid the need for extensive surgical reconstruction. For an active device, the level of chronic support which would be beneficial or necessary remains to be determined (i.e. 25-100% normal right ventricular support). Like any chronically implanted device, this must also overcome issues common to other cardiovascular implants (tissue ingrowth, thrombus formation). Much work remains to be done.
Despite advances, staged surgical repair of functional single ventricle remains a significant challenge. The intractable problems in the current approach should not be accepted as inevitable consequences of palliation. A radically different approach may be necessary to overcome them, and solutions will not come easily. Currently in development, a cavopulmonary assist device which is designed to provide a modest pressure boost to existing blood flow at the total cavopulmonary connection can effectively restore more stable biventricular status. To paraphrase Voltaire’s Candide, while it may at first seem to be the best of all possible worlds to support a single ventricle Fontan on the basis of biventricular physiology, the development a safe and reliable cavopulmonary assist device is an important step toward this goal. It may revolutionize the approach to single ventricle management.
Acknowledgments
Supported in part by National Institutes of Health grants HL080089, HL098353.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at STS/AATS Congenital Heart Disease Symposium, American Association for Thoracic Surgery, 90th Annual Meeting, Toronto, ON, May 2, 2010
References
- 1.de Leval MR. The Fontan circulation: what have we learned? What to expect? Pediatric Cardiol. 1998;19:316–20. doi: 10.1007/s002469900315. [DOI] [PubMed] [Google Scholar]
- 2.Rodbard S, Wagner D. By-passing the right ventricle. Proc Soc Exp Biol Med. 1949;71:69–70. doi: 10.3181/00379727-71-17082. [DOI] [PubMed] [Google Scholar]
- 3.Nuland SB, Glenn WWL, Guilfoil PH. Circulatory bypass of the right heart. III. Some observations on long-term survivors. Surgery. 1958;43:184–201. [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Eng J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rychik J, Goldberg D, Dodds K. Long-term results and consequences of single ventricle palliation. Prog Ped Cardiol. 2010;29:19–23. [Google Scholar]
- 6.Sano S, Huang SC, Kasahura S, et al. Risk factors for mortality after the Norwood procedure using the right ventricle to pulmonary artery shunt. Ann Thorac Surg. 2009;87:178–85. doi: 10.1016/j.athoracsur.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Akintuerk H, Michel-Behnke I, et al. Stenting of the Arterial Duct and Banding of the Pulmonary Arteries. Basis for Norwood Stage I and II Repair in Hypoplastic Left Heart. Circulation. 2002;105:1099–1103. doi: 10.1161/hc0902.104709. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph AM, Auld PA, Galinko RJ, et al. Pulmonary vascular adjustments in the neonatal period. Pediatrics. 1961;55:28–34. [PubMed] [Google Scholar]
- 9.Michel R, Gordon JB, Chu K. Development of the pulmonary vasculature in newborn lambs: structure-function relationships. J Appl Physiol. 1991;70:1255–64. doi: 10.1152/jappl.1991.70.3.1255. [DOI] [PubMed] [Google Scholar]
- 10.Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leval M. The Fontan circulation: a challenge to William Harvey? Nature Clin Pract Cardiovascular Med. 2005;2:202–8. doi: 10.1038/ncpcardio0157. [DOI] [PubMed] [Google Scholar]
- 12.Backer CL. The Fontan procedure. Our odyssey continues. J Am Coll Cardiol. 2008;52:114–6. doi: 10.1016/j.jacc.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Eicken A, Petzuch K, Marek J, et al. Characteristics of Doppler myocardial echocardiography in patients with tricuspid atresia after total cavopulmonary connection with preserved systolic ventricular function. Int J Cardiol. 2007;116:212–8. doi: 10.1016/j.ijcard.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Khambadkone S, Li J, de Leval MR, et al. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 15.Rychik J. Forty years of the Fontan operation: a failed strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:96–100. doi: 10.1053/j.pcsu.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Hsu DT, Quaegebeur JM, Ing FF, et al. Outcome after the single-stage, nonfenestrated Fontan procedure. Circulation. 1997;96(suppl II):II-335–40. [PubMed] [Google Scholar]
- 17.Kaltman JR, Andropoulos DB, Checchia PA, et al. Report of the pediatric heart network and national heart, lung, and blood institute working group on the perioperative management of congenital heart disease. Circulation. 2010;121:2766–72. doi: 10.1161/CIRCULATIONAHA.109.913129. [DOI] [PubMed] [Google Scholar]
- 18.Myers CD, Ballman K, Riegle LE, et al. Mechanisms of systemic adaptation to univentricular Fontan conversion. J Thorac Cardiovasc Surg. 2010;140:850–6. doi: 10.1016/j.jtcvs.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodefeld MD, Boyd JH, LaLone BJ, et al. Cavopulmonary assist: circulatory support for the univentricular Fontan circulation. Ann Thorac Surg. 2003;76:1911–6. doi: 10.1016/s0003-4975(03)01014-2. [DOI] [PubMed] [Google Scholar]
- 20.Rodefeld MD, Boyd JH, Myers CD, et al. Cavopulmonary assist in the neonate: an alternative strategy for single-ventricle palliation. J Thorac Cardiovasc Surg. 2004;127:705–11. doi: 10.1016/j.jtcvs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Bove EL, de Leval MR, Migliavacca F, et al. Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the Norwood procedure for Hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:1040–7. doi: 10.1016/s0022-5223(03)00698-6. [DOI] [PubMed] [Google Scholar]
- 22.Migliavacca F, Dubini G, Bove EL, et al. Computational Fluid Dynamics Simulations in Realistic 3-D Geometries of the Total Cavopulmonary Anastomosis: The Influence of the Inferior Caval Anastomosis. J Biomech Eng. 2003;125:805–13. doi: 10.1115/1.1632523. [DOI] [PubMed] [Google Scholar]
- 23.Marsden AL, Bernstein AJ, Reddy VM, et al. Evaluation of a novel Y-shaped extracardiac Fontan Baffle using computational fluid dynamics. J Thorac Cardiovasc Surg. 2009;137:394–403. doi: 10.1016/j.jtcvs.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Soerensen DD, Pekkan K, de Zelicourt DD, et al. Introduction of a new optimized total cavopulmonary connection. Ann Thorac Surg. 2007;83:2182–90. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 25.Myers CD, Boyd JH, Presson RG, et al. Neonatal cavopulmonary assist: pulsatile vs steady-flow pulmonary perfusion. Ann Thorac Surg. 2006;81:257–63. doi: 10.1016/j.athoracsur.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Myers CD, Mattix K, Presson RG, et al. Twenty-four hour cardiopulmonary stability in a model of assisted newborn Fontan circulation. Ann Thorac Surg. 2006;81:264–70. doi: 10.1016/j.athoracsur.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 27.Merklinger SL, Honjo O, Al-Radi OO, et al. Primary in-series palliation of hypoplastic left heart syndrome with mechanical assist in neonatal pigs. ASAIO J. 2009;55:620–5. doi: 10.1097/MAT.0b013e3181be00a0. [DOI] [PubMed] [Google Scholar]
- 28.Lacour-Gayet FG, Lanning CJ, Stoica S, et al. An artificial right ventricle for failing Fontan: in vitro and computational study. Ann Thorac Surg. 2009;88:170–6. doi: 10.1016/j.athoracsur.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 29.Riemer RK, Amir G, Reichenbach SH, et al. Mechanical support of total cavopulmonary connection with an axial flow pump. J Thorac Cardiovasc Surg. 2005;130:351–4. doi: 10.1016/j.jtcvs.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Throckmorton AL, Kapadia J, Madduri D. Mechanical axial flow blood pump to support cavopulmonary circulation. Int J Artif Org. 2008;31:970–82. doi: 10.1177/039139880803101107. [DOI] [PubMed] [Google Scholar]
- 31.Rodefeld MD, Coats B, Fisher T, et al. Cavopulmonary assist for the univentricular Fontan circulation: von Karman viscous impeller pump. J Thorac Cardiovasc Surg. 2010;140:529–36. doi: 10.1016/j.jtcvs.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]