Abstract
Background
Our laboratory has demonstrated that the Notch1 signaling pathway acts as a tumor suppressor in carcinoids. In this study we examined hesperetin, a flavinoid, as a potential Notch1 activator and carcinoid tumor suppressor.
Methods
A high throughput drug screen revealed hesperetin as a Notch1 activator. Human GI carcinoid (BON) cell growth after hesperetin treatment was assessed with a 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Western blots were used to measure neuroendocrine tumor markers, human achaete-scute complex-like 1 (ASCL1) and chromogranin A (CgA). Notch1 expression was measured using western blot analysis and real-time PCR.
Results
Hesperetin induces cell death in a dose dependent manner and reduces ASCL1 and CgA expression with a concomitant rise in Notch1 levels. It also induces Notch1 mRNA, indicating regulation at the transcriptional level.
Conclusion
Hesperetin induces Notch1 expression in carcinoid cells, subsequently suppressing tumor cell proliferation and bioactive hormone production. This provides evidence for further study into hesperetin as a potential treatment for carcinoid cancer.
Summary
A retrospective review of patients with thin melanoma to determine factors associated with a positive sentinel lymph node from a single institution.
Keywords: carcinoid tumors, Notch1 signaling pathway, hesperetin
Introduction
Carcinoid tumors are neuroendocrine neoplasms found in the gastrointestinal tract and pulmonary system. They grow slowly when compared to adenocarcinomas, but they frequently metastasize to the liver. Once these tumors have metastasized they often secrete hormones and peptides such as 5-hydroxytryptamine (serotonin (5-HT)), chromogranin A (CgA), and neuron-specific enolase (NSE) which cause debilitating symptoms such as flushing, diarrhea, wheezing, and heart failure known as carcinoid syndrome. Somatostatin analogues, such as octreotide, provide relief from some symptoms, but in most patients their effects begin to decrease over time. Currently, few treatments are available to patients and surgery remains the only curative option, but is often not feasible due to widespread disease.
Several signaling pathways have been reported to regulate the proliferation of neuroendocrine cancers. The Notch1 signaling pathway has been found to be oncogenic in colon cancers, renal cell carcinomas, and non-small cell lung cancers, but is minimally active or absent in neuroendocrine tumors such as carcinoids, medullary thyroid cancers, and pheochromocytomas (1, 2). Activation of Notch1 has been shown to suppress neuroendocrine tumor marker expression and inhibit proliferation of both gastrointestinal and pulmonary carcinoids as well as medullary thyroid cancers (3, 4, 5).
Hesperetin is a flavanone found in citrus fruits such as oranges and grapefruits. It has previously been reported to decrease lipid levels and to decrease TNF-alpha stimulated secretion of free fatty acids (6, 7). In 2007, researchers found that treatment of mice inoculated with melanoma cells reduced their metastatic potential (8). Later, dietary flavinoids, including hesperetin were shown to induce apoptosis in human pancreatic cells (9). We developed a quantitative high throughput drug screen to screen for Notch1 activating agents and identified hesperetin as a potential Notch1 activator (10). In this study we sought to validate hesperetin as a Notch1 activator and gastrointestinal carcinoid tumor suppressor.
Materials and Methods
Cell Culture
Human gastrointestinal carcinoid (BON) cells were obtained from Drs. B. Mark Evers and Courtney M. Townsend, Jr. (University of Texas Medical Branch, Galveston, TX). The cells were maintained in Dulbeccos’s modified Eagle medium-nutrient mixture Ham’s F-12K (DMEM/F12 K, 1:1, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO), and a combination of 100 IU/mL penicillin and 100 μg/mL streptomycin (Invitrogen) in a humidified atmosphere of 5% CO2 in air at 37°C. Hesperetin (MP Biomedicals, Solon, OH) was dissolved in dimethyl sulfoxide (DMSO) (Sigma) to prepare a 100 mM stock solution.
High-Throughput Screen
BON cells were initially transfected with a centromere-binding factor 1 (CBF1)/luciferase plasmid to assess functional Notch1 activity as previously described (10). To perform the high-throughput screen, BON-CBF1-Luc cells were plated onto 384-well microtiter dishes. The compounds were added to each dish at a dose of 0.5 μL. Assays were then read on a Safire 2 micoplate reader (Tecan, Mannedorf, Switzerland).
Cell proliferation assay
Carcinoid cell proliferation was measured using a 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay according to manufacturer instructions. Cells were plated in quadruplicate in 24-well plates and incubated overnight. The following day (day 0) cells were treated with hesperetin (0 – 125 μM) and incubated for up to 6 days with medium being changed every two days. Cell growth was assessed on day 2, 4, and 6.
Western blot analysis
Cells were treated with 0 – 100 μM doses of hesperetin and harvested on day 2 or 4, with 3 mM EDTA (Sigma) in PBS (Invitrogen). The cells were centrifuged at 2000rpm for 5 minutes at 4°C. Pellets were then lysed according to a previously described protocol (3). Total cellular protein concentrations were determined with bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Denatured cellular extracts were resolved by SDS-PAGE (Invitrogen), transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), blocked in milk (5% dry skim milk and 0.05% Tween-20 in PBS) for 1 hour, and then primary antibodies were applied. The antibody dilutions used were as follows: 1:1000 for mammalian ASCL1 (BD Pharmingen, San Diego, CA), β-actin (Cell Signaling Technology, Beverly, MA), Notch1 (Santa Cruz Biotechnology, Santa Cruz, CA), and chromogranin A (Zymed Laboratories, San Francisco, CA). Membranes were incubated overnight at 4°C and were washed with wash buffer (0.05% Tween-20 in PBS) the following day according to protocol (3). HRP-conjugated goat anti-rabbit IgG secondary antibody was then applied in a 1:3000 dilution for CgA, and β-actin and in a 1:6000 dilution for Notch1. A dilution of 1:3000 Goat anti-mouse IgG secondary antibody was used for ASCL1. After incubating for 1 hour on a shaker at room temperature, membranes were washed 3–5 times with wash buffer. The proteins were then visualized using SuperSignal West Femto (Pierce) or Immunstar (Bio-Rad) kits according to manufacturer instructions.
Quantitative Real-time PCR
Following treatment with hesperetin as described above, RNA was isolated using an RNeasy Mini-kit (Qiagen, Valencia, CA) according to the manufacturer’s directions. RNA concentrations were determined using a spectrophotometer (Thermo Scientific, Waltham, MA). cDNA was synthesized with the iScript cDNA Synthesis Kit (Bio-Rad) according to manufacturer’s instructions. The following PCR primer pairs were used: Notch1 (5′-GTCAACGCCGTAGATGACCT -3′ and 5′ – TTGTTAGCCCCGTTCTTCAG – 3′) and GAPDH (5′-ACCTGCCAAATATGATGAC-3′ and 5′-ACCTGGTGCTCAGTGTAG-3′) as a loading control. The PCR reactions were performed in triplicate according to a previously published protocol (11). Cycle numbers obtained at the log-linear phase of the reaction were plotted against a standard curve prepared with serially diluted control samples. Expression of Notch1 was normalized by GAPDH mRNA levels and plotted as an average ± standard error of the mean (SEM).
Results
Hesperetin suppresses carcinoid cell growth
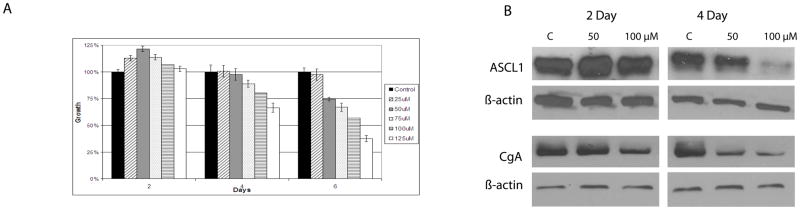
To determine whether treatment of BON cells with hesperetin affected cellular proliferation, MTT assays were performed. As demonstrated in Figure 1A, there was suppression of cellular growth in a dose-dependent manner beginning at Day 4. The most considerable inhibition in growth was seen beginning with a dose of 50 μM of hesperetin. After 6 days of treatment, cells exposed to 50 μM of hesperetin or greater were all inhibited by >25% when compared to DMSO treated control cells.
Figure 1.
A. Cell proliferation analysis of BON cells treated with DMSO (control) or 25 to 125 μM hesperetin for up to 6 days. BON cell growth was inhibited compared to the control beginning with a 50 μM dose. B. Western blot analysis of neuroendocrine tumor markers was performed on BON cells treated with DMSO (control) or 50 to 100 μM hesperetin for 2 and 4 days. ASCL1 expression was inhibited by both 50 μM and 100 μM doses after 4 days of treatment. CgA expression was inhibited by both treatments after 2 days of treatment when compared to controls. All samples were loaded equally as shown by β-actin.
Hesperetin decreases expression of neuroendocrine tumor markers
BON cells are known to express high levels of neuroendocrine tumor markers such as ASCL1 and CgA. ASCL1 is a basic helix-loop-helix transcription factor that plays a role in neuronal differentiation (12). CgA is a secretory glycoprotein found in neuroendocrine cells that is secreted with other bioactive hormones (12). It has been previously shown that Notch1 activation decreases levels of ASCL1 and CgA in neuroendocrine tumors (3, 5, 13).
To demonstrate suppression of these neuroendocrine tumor markers by hesperetin, western blot analysis was performed (Figure 1B). As seen in Figure 1B, BON cells express a high amount of baseline ASCL1. The addition of hesperetin did not affect ASCL1 levels on day 2, but led to a dose-dependent decrease in ASCL1 levels on day 4. Similarly, the BON control cells have high levels of CgA present. Treatment with hesperetin reduced CgA levels in a dose-dependent manner after 2 days.
Hesperetin induces Notch1 expression
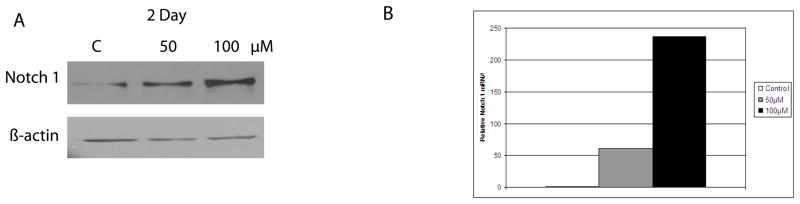
After demonstrating that carcinoid cell growth and tumor marker expression were inhibited by treatment with hesperetin, we wished to validate that the Notch1 signaling pathway was responsible for this action. The Notch1 signaling pathway has been shown to be absent or near absent in neuroendocrine tumors, including carcinoids (1, 2). Previous studies have established that over expression of the Notch1 signaling pathway decreases carcinoid tumor cell proliferation and expression of neuroendocrine tumor markers (2, 3). To determine whether hesperetin was acting on carcinoid cells via this pathway Notch1 protein expression was assessed by western blot analysis. BON cells were once again treated with 50 μM and 100 μM of hesperetin for 2 days. There was minimal Notch1 protein expressed at baseline and treatment led to an increase in Notch1 levels in a dose-dependent manner (Figure 2A).
Figure 2.
A. Western blot analysis of hesperetin’s effects on Notch1 protein level demonstrates minimal Notch1 in DMSO (control) treated cells. After a 2 day treatment of 50 μM and 100 μM hesperetin, Notch1 protein expression is increased. B. Expression of Notch1 relative mRNA levels was measured with qPCR after treatment of BON cells with 50 μM and 100 μM hesperetin. At baseline, BON cells exhibit minimal Notch1 mRNA. After treatment, Notch1 relative mRNA levels are increased 1000 fold with a 50 μM treatment of hesperetin and 4000 fold with a 100 μM dose.
To establish whether hesperetin affected Notch1 transcription real-time PCR was performed on BON cells to evaluate levels of Notch1 mRNA. After treatment with 50 μM and 100 μM of hesperetin for 2 days, Notch1 mRNA levels increased 1000 and 4000 fold respectively when compared to the control (Figure 2B).
Discussion
Carcinoid tumors, although rare, are the most common neuroendocrine tumor to affect the gastrointestinal tract and are the second most common source of isolated hepatic metastases (3). Surgical resection remains the only curative treatment option, but most patients are not surgical candidates due to the advanced stage of their disease at diagnosis. In recent years, research has focused on signaling pathways that play a role in neuroendocrine tumor development, specifically the Notch1 signaling pathway which acts as a tumor suppressor in these cancers (1). Our data have previously shown that induction of Notch1 signaling in carcinoid cells leads to cellular growth arrest and reduction of neuroendocrine tumor markers (2, 3). Therefore, there is a need to identify Notch1 activators in carcinoid cells.
A high-throughput drug screen developed by our laboratory identified hesperetin as a Notch1 activating agent. Naturally occurring compounds, such as hesperetin, are an attractive source of potential cancer therapeutics as they are readily available and are already consumed in the human diet. To validate these results we evaluated the effect of hesperetin on levels of Notch1 in carcinoid cells. Hesperetin treatment increased the levels of Notch1 mRNA significantly, indicating that Notch1 transcription was up regulated. This also translated into an increase in Notch1 protein levels as demonstrated by western blot analysis.
In this study we also show that hesperetin decreased the proliferation of carcinoid cells in a dose-dependent manner. We also demonstrate decreased expression of the neuroendocrine tumor markers, ASCL1 and CgA with hesperetin treatment. Furthermore, although hesperetin modestly increased Notch1 protein levels when compared with mRNA levels, this still translated into an inhibition of cellular growth as well as tumor marker expression.
Conclusion
Treatment of carcinoid cells with hesperetin inhibits not only cell growth, but also leads to a decrease in the expression of tumor markers. Hesperetin up regulates the expression of Notch1 at both the mRNA and protein level in carcinoid cells, providing evidence that hesperetin acts as a Notch1 transcription activator. Further studies, including in vivo experiments, are warranted to establish hesperetin as a possible carcinoid treatment.
Acknowledgments
This study was funded by a research scholarship from the American College of Surgeons (BZ) and a NIH grants RO1 CA109053 (HC), RO1 CA121115 (HC), and T32 Training Grant-CA090217 (BZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G636–642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 2.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 3.Greenblatt D, Vaccaro A, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 4.Greenblatt D, Cayo M, Adler J, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247:1036–1040. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platta C, Greenblatt D, Kunnimalaiyaan M, Chen H. Valproic acid induces Notch1 signaling in small cell lung cancer cells. J Surg Res. 2008;148:31–37. doi: 10.1016/j.jss.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H, Takamura N, Shuto T, Ogata K, Tokunaga J, Kawai K, Kai H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-alpha in mouse adipocytes. Biochem Biophys Res Commun. 2010;394:728–732. doi: 10.1016/j.bbrc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Jeong T, Lee M, Park Y, Choi M. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin Chim Acta. 2003;327:129–137. doi: 10.1016/s0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 8.Lentini A, Forni C, Provenzano B, Beninati S. Enhancement of transglutaminase activity and polyamine depletion in B16-F10 melanoma cells by flavonoids naringenin and hesperitin correlate to reduction of the in vivo metastatic potential. Amino Acids. 2007;32:95–100. doi: 10.1007/s00726-006-0304-3. [DOI] [PubMed] [Google Scholar]
- 9.Patil J, Chidambara Murthy K, Jayaprakasha G, Chetti M, Patil B. Bioactive compounds from Mexican lime ( Citrus aurantifolia ) juice induce apoptosis in human pancreatic cells. J Agric Food Chem. 2009;57:10933–10942. doi: 10.1021/jf901718u. [DOI] [PubMed] [Google Scholar]
- 10.Pinchot SN, Jaskula-Sztul R, Ning L, Peters NR, Cook MR, Kunnimalaiyaan M, Chen H. Identification and Validation of Notch Pathway Activating Compounds through a Novel High-Throughput Screening Method. Cancer. 2010 doi: 10.1002/cncr.25652. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook M, Pinchot S, Jaskula-Sztul R, Luo J, Kunnimalaiyaan M, Chen H. Identification of a novel Raf-1 pathway activator that inhibits gastrointestinal carcinoid cell growth. Mol Cancer Ther. 2010;9:429–437. doi: 10.1158/1535-7163.MCT-09-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinchot S, Adler J, Luo Y, Ju J, Li W, Shen B, Kunnimalaiyaan M, Chen H. Tautomycin suppresses growth and neuroendocrine hormone markers in carcinoid cells through activation of the Raf-1 pathway. Am J Surg. 2009;197:313–319. doi: 10.1016/j.amjsurg.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler J, Hottinger D, Kunnimalaiyaan M, Chen H. Inhibition of Growth in Medullary Thyroid Cancer Cells with Histone Deacetylase Inhibitors and Lithium Chloride. J Surg Res. 2008 doi: 10.1016/j.jss.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]