Abstract
Conductive hearing loss (CHL) is known to produce hearing deficits, including deficits in sound localization ability. The differences in sound intensities and timing experienced between the two tympanic membranes are important cues to sound localization (ILD and ITD, respectively). Although much is known about the effect of CHL on hearing levels, little investigation has been conducted into the actual impact of CHL on sound location cues. This study investigated effects of CHL induced by earplugs on cochlear microphonic (CM) amplitude and timing and their corresponding effect on the ILD and ITD location cues. Acoustic and CM measurements were made in 5 chinchillas before and after earplug insertion, and again after earplug removal using pure tones (500 Hz to 24 kHz). ILDs in the unoccluded condition demonstrated position and frequency dependence where peak far-lateral ILDs approached 30 dB for high frequencies. Unoccluded ear ITD cues demonstrated positional and frequency dependence with increased ITD cue for both decreasing frequency (± 420 µs at 500 Hz, ± 310 µs for 1–4 kHz ) and increasingly lateral sound source locations. Occlusion of the ear canal with foam plugs resulted in a mild, frequency-dependent conductive hearing loss of 10–38 dB (mean 31 ± 3.9 dB) leading to a concomitant frequency dependent increase in ILDs at all source locations. The effective ITDs increased in a frequency dependent manner with ear occlusion as a direct result of the acoustic properties of the plugging material, the latter confirmed via acoustical measurements using a model ear canal with varying volumes of acoustic foam. Upon ear plugging with acoustic foam, a mild CHL is induced. Furthermore, the CHL induced by acoustic foam results in substantial changes in the magnitudes of both the ITD and ILD cues to sound location.
Keywords: Conductive hearing loss, Interaural level differences (ILD), Interaural timing differences (ITD), Otitis media with effusion
1. Introduction
Conductive hearing loss (CHL) arises from a mechanical disruption of sound wave transmission through the external ear and middle ear structures to the inner ear. There are a number of etiologies in CHL including cerumen impaction, tympanosclerosis (fibrosis of the tympanic membrane), otosclerosis (stapes fixation) and fluid within the middle ear space (Torres and Backous, 2010). Fluid within the middle ear space is characteristic of otitis media with effusion (OME), a common cause of childhood hearing loss. Between the ages of 2 months and 2 years, 91.1% of young children will have one episode of middle ear effusion, and 52.2% will have bilateral involvement (Paradise et al., 1997). The hearing loss associated with OME is often a mild to moderate hearing loss across all frequencies (mean 26 dB, range 0–50 dB, SD 9.9; Bluestone et al., 1973). Furthermore, OME is highly prevalent with 2.2 million cases per year (Shekelle et al., 2003), underscoring the need for continued research on the effects of CHL on auditory system structure and function.
Early deprivation of hearing experience due to OME has been implicated in decreased communicative skills, attention deficits, reading ability, depression and educational achievement (Welsh et al., 1983; Silva et al., 1986; Teele et al., 1990; Gravel and Wallace, 1992; Asbjørnsen, 2000; Bennett et al., 2001; Winskel, 2006; Gouma et al., 2010). To gain understanding of the underlying anatomical, physiological and behavioral consequences of OME, research in animal models of CHL and hearing deprivation (reviewed in Tollin, 2010) have demonstrated concomitant changes to auditory system brainstem anatomy (Webster and Webster, 1977; Webster, 1988; Coleman and O’Connor, 1979; Brugge, et al., 1985; Reale et al., 1987; Walger et al., 1993; Laska et al., 1992; Tucci et al., 2001), physiology (Clopton and Silverman, 1978; McAlpine et al., 1997; Knudsen, 1999; Xu et al., 2007; Popescu and Polley, 2010) and behavior (Clements and Kelly, 1978; Knudsen et al., 1984a,b; Moore et al., 1999; King et al., 2000). Hearing and auditory processing tasks that involve binaural hearing (i.e., the use of acoustic information from both ears), such as sound localization, are particularly negatively impacted by CHL (Fisher and Freedman, 1968; Häusler et al., 1983; Slattery and Middlebrooks, 1994; Van Wanrooij and Van Opstal, 2007) and often persist after the effusion has resolved (Pillsbury et al., 1991; Gravel and Wallace, 2000; Moore et al., 2003). Additionally, children with history of chronic OME perform poorly in studies replicating the ‘cocktail party effect’ (Moore et al., 1991) indicating an inability to spatially filter and process sounds of interest. To fully understand the experience-dependent changes to the neural sound localization circuitry in the auditory system and its impact on behavior revealed in animal studies such as those cited above, it is first necessary to accurately characterize the effects of commonly used laboratory-induced CHL techniques on the input experience to the auditory system and specifically the acoustic cues to sound source location.
CHL has been shown in animal studies to affect two important binaural cues for sound localization, the interaural level difference (ILD) and interaural timing difference (ITD). Inducing a unilateral CHL (i.e., at one ear) increases the magnitude of the ILD presented to the auditory system simply because the inputs to one ear are attenuated to a greater extent than normal (Knudsen et al., 1984a; Hartley and Moore, 2003). Timing changes in the input to the inner ear as a result of CHL have been suggested in human psychophysical studies by Hall et al. (1995, 1998) where subjects achieved higher (i.e., worse) binaural masking level difference thresholds than expected when the phase of the masking noise was varied relative to the tone to be detected; the decrease in psychophysical performance in these cases was consistent with the addition of a temporal delay in the stimulus on the side of the CHL. Similar changes in the temporal transmission of sound to the inner ear resulting from experimentally-induced CHL have been measured directly in animal (Knudsen et al., 1984a; Hartley and Moore, 2003), human cadaveric temporal bones (Ravicz et al., 2004), and human behavioral studies (Hogan et al., 1995; McPartland et al., 1997; Kumpik et al., 2010). In these studies, CHL-induced temporal changes to stimuli were inferred based primarily on the assessment of sound sources presented directly in front of the subject. None of these studies, however, have investigated directly the effect of experimentally-induced CHL on the specific cues to sound localization nor as how they are affected by sound sources presented across locations in the horizontal plane
The purpose of this paper was to further characterize the effect of an experimentally-induced CHL on both ILD and ITD for stimuli presented from multiple positions across azimuth from left (−90°) to right (+90°) for a single elevation. In this study, using methods pioneered by Knudsen et al. (1984a) and Hartley and Moore (2003), we used foam earplugs to implement a CHL in the chinchilla and measured the effect on the intensity and timing of cochlear microphonic signals across azimuth for a single elevation. Although the use of earplugs is a common method to implement a CHL in experimental animals, little is actually known about how earplugs alter the spectral and temporal transmission of sound to the inner ear in animals. In particular, while past investigations have added to our understanding of the spectral affects of earplugs in animal models (see Knudsen et al., 1984a; Moore et al., 1989; Hartley and Moore, 2003), the effect on temporal cues is less well understood or appreciated. Given that low-frequency ITDs are the dominant cue for sound source localization (Wightman and Kistler, 1992), a re-examination of the effect of a common experimental method to induce a CHL on temporal transmission of sound to the inner ear is warranted. The specific hypothesis tested in this experiment was that CHL by ear plugging leads to the formation of an azimuth shift of auditory space secondary to sound localization cue disruption correlating with the subjective findings of a shift of lateralization to the open ear (Oldfield and Parker, 1986; Butler and Humanski, 1990; Slattery et al., 1994; Kumpik et al., 2010).
2. Materials and Methods
2.1 Animal Preparation
Five chinchillas (chinchilla lanigera, 2 female 3 male) weighing 0.5–0.62 kg (mean 0.58, S.D. 0.04 kg) were used for experimentation. The animal experiments and methods were approved by the University of Colorado Denver Institutional Animal Care and Use Committee. The study subjects were anesthetized with an initial intramuscular (IM) dose of ketamine hydrochloride (KetaVed, 30 mg/kg IM) and xylazine hydrochloride (TranquiVed, 5 mg/kg IM). Anesthesia was maintained throughout the experiment with supplemental doses of ketamine (15 mg/kg IM) and xylazine (2.5 mg/kg IM) based on reflex response to a paw pinch and changes in physiological parameters (heart rate and respiration rate). Each subject underwent tracheotomy with tracheostomy tube placement. Heart rate, blood-oxygen levels (Sp02), respiratory rate, and end-tidal CO2 were measured continuously via a capnograph (Surgivet V90040, Waukesha, WI) and displayed graphically on a PC monitor. Body temperature was continuously monitored with a rectal probe and maintained with a heating pad at 37°C.
Two incisions were made to access the surface of the posterior bullae bilaterally. A small hole (2–3 mm diameter) was bored through the bony wall of each bulla to gain access to the middle ear cavity with a 2-mm diameter diamond burr attached to a microdrill (RAM Microtorque II, East Brunswick, NJ). Two Teflon-insulated, silver electrodes (bare wire diameter: 0.0050”) were stripped of insulation at both ends, and one end was bent into a small loop. The electrode wire was advanced through the hole by hand under microscopic visualization and placed on the round window. The electrode was fixed into place and the hole was partly closed by the application of dental acrylic leaving a small opening to vent the bullae. After fixing the electrode into place, the CM was differentially amplified (x1000, DAM ISO-50 or DAM ISO-80, WPI, Sarasota, FL) and filtered (10 – 20,000 Hz) and presence of CM activity verified by oscilloscope. This process was repeated for the other ear. The differential electrode was placed on the posterior musculature of the neck and the ground placed on one paw.
The external auditory canal (EAC) was otoscopically visualized to the tympanic membrane (TM) and all subjects had EACs free of debris and without evidence of pathology. An incision was made over the skin and connective tissue overlaying the lateral wall of the bony EAC. After exposure of the bone, a small hole was bored through the bone 5 mm inferior to the opening of the external auditory canal. A probe tube of 50 mm in length (Brüel and Kjær flexible probe tube part no. AF-0555, Norcross, GA) was advanced under otoscopic visualization towards the TM and fixed into place with cyanoacrolate adhesive. Using this approach, post-mortem examination revealed that the tip of the probe tube was located ~1.8 mm from the TM, a distance sufficient to provide a flat frequency response up to the maximum frequency (24 kHz) used for experimentation (for 1.8 mm, the ¼ wavelength corresponds to a frequency of 47.2 kHz). After placement of the electrodes and probe tubes, the subject was then placed onto a custom foam holder which was then placed in the sound-attenuating chamber for recordings. The posterior aspect of the pinnae were supported and positioned with wire (0.55 mm diameter) to approximate the erect, forward facing position of the pinnae of the alert chinchilla.
2.2 Acoustic stimulation
Experiments were performed in a ~3 × 3 × 3 m double-walled, sound-attenuating room (Industrial Acoustics Company, Bronx, NY). All walls and equipment were lined with 4” and 2”, respectively, acoustic foam (Sonex Classic, Minneapolis, MN). Stimuli were presented from speakers (Morel MDT-20, Elmont, NY) attached to a custom-built horizontally-oriented (i.e., poles at the sides) semicircular boom (1 m radius); there were 25 speakers spaced at 7.5° from – 90° (left) to +90° (right). The 25 loudspeakers were selected from a larger set of 100 on the basis of best matching frequency responses. A stepper motor under computer control positioned the boom in elevation with a precision of < 1°. In these experiments, the boom was positioned at 0° elevation so that the speakers were all in the horizontal plane. The interaural axis of each subject was aligned in the center of the sphere using three lasers attached to the two poles and the 0° azimuth position of the boom. Short broadband stimuli [11th order maximum length sequences (MLS), Rife and Vanderkooy, 1989] were presented from all speaker positions. In addition to the three lasers, the interaural time delays (see below) were computed to acoustically verify correct placement of the animal at the center of the loudspeaker array before proceeding with the experiment (i.e., the ITD for a source at the midline was confirmed to be within ± 10 µs of 0 µs).
Short bursts of sinusoidal stimuli (sine phase) were presented for CM calibration, ITD and ILD measurements. Sinusoids (in octave steps from 0. 5–16 kHz, plus additional frequencies of 12,16, 20, 24 Hz) spanning the behavioral audiogram of the chinchilla (Heffner and Heffner, 1991) were designed with a total duration of 10 ms, consisting of a linear 2.5 ms rise/fall and a 5 ms plateau, with a 40 ms interstimulus period. Each stimulus was presented in phase at least 25 times from each source location. The tones and MLS broadband stimuli were presented again once the ear plug was placed. Finally, as a control to demonstrate that spectral and temporal modifications to the sounds were due to the foam earplug, this measurement protocol was repeated once more immediately after the earplug was removed to confirm that the TM was not damaged. Stimuli were generated in MATLAB® (v7.1, The MathWorks Inc, Natick, MA) and presented using Tucker Davis Technologies (TDT, Gainesville, FL, USA) System III hardware (TDT RP 2.1) with a sampling rate of 97656.25 Hz at full 24-bit resolution. After digital-to-analog conversion, stimuli were then passed through an attenuator (TDT PA5), appropriately amplified (TDT SA1) and then delivered to a multiplexer (TDT PM2R) that selected the single speaker on the boom on which to play the stimulus. All stimulus presentation, acquisition, and processing was done using custom software written in MATLAB®.
2.3 Measurement System
The cochlear microphonic (CM) recording procedure followed closely that described by Knudsen et al. (1984a), Moiseff (1989) and Hartley et al. (2003). However, in addition to recording the CM, we simultaneously recorded the resultant stimuli near the TM using probe tube microphones, predominantly in the unoccluded ear condition [see Fig. 1 of Koka et al., 2010 in press Hearing Research]. The CM and probe tube microphone data were compared in the unoccluded ear conditions to verify that the spatial cues derived from the CM waveforms were correlated with those computed directly from the probe tube microphone waveforms. The rationale for using CM waveforms in this study was that it could not be assumed that the probe tube microphones remained unoccluded in the ear canal once the earplugs were placed there. Probe tubes were attached to a microphone (Brüel and Kjær, type 4182). For each ear, the output of the microphone was pre-amplified by 20 dB (Brüel and Kjær, dual microphone supply 5935L) and then fed to a TDT MA2 microphone amplifier for an additional 30 dB amplification. The CM electrode inputs from each ear were fed into two differential amplifiers (DAM ISO-50 or DAM ISO-80, WPI, Sarasota, FL). The CM and microphone signals for both ears were simultaneously captured with two analog-to-digital converters (TDT RP 2.1) at a sampling rate of 97656.25 Hz, high pass filtered with a cutoff of 10 Hz and stored on a PC hard drive for later processing. The acoustic and CM signals were averaged in the time domain based on the number of presented repetitions. Both the time and frequency domain representations of these signals were displayed in real time on a PC monitor for inspection during the experiment.
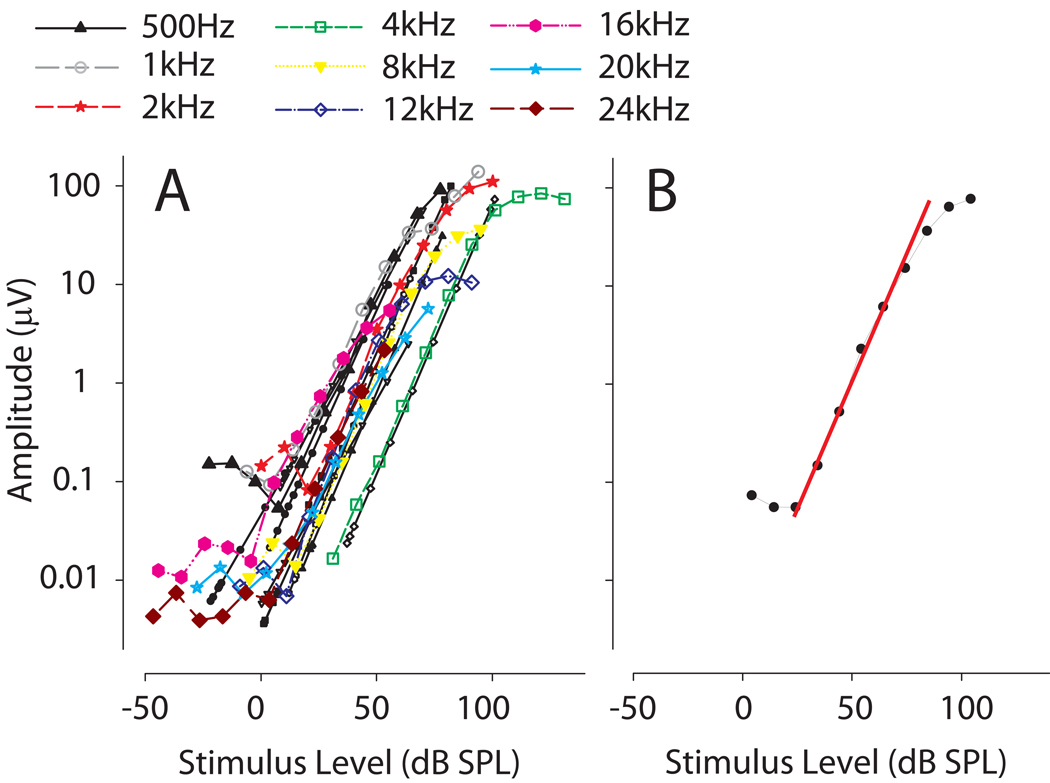
Figure 1.
A: Cochlear microphonic (CM) amplitude recorded from the left ear of one study subject as a function of tympanic membrane stimulus level (dB SPL) for 9 frequencies. The CM amplitude increased linearly over a wide range of SPLs. The average (n=6) CM amplitude dynamic range across frequencies was found to be 56.1 ± 3.1 dB (SD) with a range of 53.3 to 59.2 dB B: Linear regression curves (RED line) were fit to the linear portion of the CM response. The regression curve was used to convert measured CM amplitudes at a given frequency to the equivalent SPL value at the tympanic membrane. This technique was employed to circumvent unknown patency status of the probe tube microphone after plugging.
2.4 Cochlear Microphonic Signal Calibration
At the beginning of each experiment, CM recordings were made from the tones presented from the speaker directly ahead, at 0° azimuth. The amplitude of the CM (in µV) was measured over a wide range of frequencies and attenuation values (at least 100 dB). Acoustic signal amplitudes as recorded from the probe tube microphone were measured simultaneously for each frequency and attenuation and converted to sound pressure level (SPL) via a previously measured reference of a 94 dB SPL 1 kHz tone produced by a sound level calibrator (Brüel and Kjær, model 4231) and appropriately adjusted by the frequency response of the 50 mm length of the probe tube. The amplitudes of both the CM and the acoustic recordings were computed from the Fourier transform at the stimulus frequency. Plotting the CM amplitude as a function of the calibrated sound pressure level (dB SPL) measured in the ear canal on semi-logarithmic coordinates (Fig. 1A) generates the CM amplitude curves for each ear and each frequency. These functions then allowed us to determine the attenuation level necessary to ensure CM recordings from the linear range. These curves also allowed us to determine directly the amount of attenuation that the earplugs produced based on the reduction on CM amplitude.
2.5 Ear plugging
Once calibration was complete, an attenuation level for each frequency was selected to ensure operation within the linear portion of the CM (e.g., Fig. 1B) and tones were played for each azimuth position from +90° to −90°. The ITDs and ILDs were calculated from the CM waveform data as described below. After baseline recordings with both ears unoccluded, a 1 cm segment (volume = 0.5 cc) of compressible acoustic foam (E.A.R. Inc. Boulder, CO, USA) was introduced into the external ear canal and secured in place with a thin layer of audiological ear impression compound (Siliclone, Westone, Colorado Springs, CO, USA). This method of CHL induction was adapted directly from the method used to study the anatomical, physiological, and behavioral consequences of CHL in the ferret (Moore et al., 1999; Moore et al., 1989; Kacelnik et al., 2006) to secure the foam segment. Measurements of the CM amplitude as a function of stimulus level were repeated. The stimulus intensity was selected to operate in the linear range for both the occluded and unoccluded ears. Based on the baseline CM amplitude vs. calibrated sound pressure level (dB SPL) measurements (section 2.4), the CM-amplitude linear dynamic range could accommodate, on average, 56 dB of additional attenuation due to both plug and level differences across frequencies studied here. Measurements to sinusoidal tone stimuli from across azimuth were then repeated in the state of experimentally induced hearing loss. At the end of the experiment, the impression compound and the acoustic foam were carefully removed and measurement of the CM amplitude as a function of stimulus intensity was again repeated. Comparing these functions to the baseline measurements served as a critical control experiment necessary to show that the external and/or middle ear was not damaged by the ear plug. Thus, the observed modifications to the spectral and temporal aspects of sounds were due to occlusion and not physical damage (i.e., TM puncture, etc.) to the periphery.
2.6 Control experiments
2.6.1 Effect of the volume of foam earplug material on signal timing and intensity
The acoustic properties of foam ear plugs were measured in an artificial ear using the method and apparatus described by Kolpe and Oliviera (2003) who studied the attenuation characteristics of different otoplastic materials. Here an aluminum tube with one end closed was fabricated with a length of 3 cm an inside diameter of 5 mm, dimensions similar to that of the chinchilla external ear canal (Vrettakos et al., 1988). Two 1.6 mm holes were bored into the side of the tube, 3 mm from the entrance and 3 mm from the end, for placement of probe tube microphone tips. The metal tube was secured in the center of our acoustic test chamber and acoustic recordings were made from both probe tubes simultaneously as described above for tone stimuli presented at 0° azimuth. The microphones themselves were further shielded with plastic and acoustic foam. Three cylindrical pieces of earplug foam (E.A.R. Inc. Boulder, CO, USA; 8 mm dia. × 4 mm height, Volume = 0.2 cc) were cut and were sequentially placed into the tube with recordings made after each placement. The acoustic responses from the proximal and distal probe tube microphones were subsequently analyzed to compute the resulting change in signal timing and attenuation due to the foam plug.
2.6.2 Influence of room reflections upon ILD and ITD measurements
In order to rule out any role of standing acoustic waves in our recording chamber as a source of the large ITDs that were measured, a control experiment was performed with two probe tube microphone tips placed 20.0 mm apart. Acoustic recordings were made for sounds of different frequencies and different spatial locations in azimuth. The temporal waveform delay between the two microphones did not show any dependence on stimulus frequency for all stimulus frequencies ≥ 250 Hz. The empirical temporal delays were constant across all frequencies (≥ 250 Hz) at 51 µs, which is consistent with the predicted delay (within the 10.24 µs resolution of our measurement system). Moreover the SPLs measured at the two microphones did not differ for frequencies >250 Hz, thus ensuring that our ILD measurements were not confounded by standing waves. These results confirm the experimental sound chamber or standing waves themselves were not a confounding parameter to our measurements over the frequencies of interest in the present study (≥ 250 Hz).
2.7 Data analysis
Two binaural and 3 single occluded ears provided data for analysis (n = 7 ears). In the monaural case, perfect symmetry of the head was assumed in order to compute the interaural cues (Shaw, 1974; Mehrgardt and Mellert, 1977). All data presented here assume that the left ear was occluded; thus, if the right was occluded here the data were simply adjusted to conform to a left ear occlusion convention. Once all recordings were made, ILDs were determined by equating the measured CM amplitude to calibrated SPL at the TM and computing the difference between ears. In order to equate the measured CM amplitude to the corresponding calibrated SPL at the TM (measured with probe tube microphones), linear regression curves were fit to the linear portion of the CM amplitude curves by selecting at least 5–6 (corresponding to a 50–60 Db dynamic range or more) contiguous data points in the linear region resulting in a correlation coefficient > 0.95 via the MATLAB curve fitting toolbox (see Fig. 1B). For each stimulus frequency, regression equations for each curve were generated to translate the measured CM voltage to equivalent ear canal SPL.
Temporal delays were computed using a reference sinusoid (i.e., the raw stimulus) which was cross-correlated with the filtered CM recording (5th order low pass butterworth with cutoff 1.5 octaves above stimulus frequency) from each ear via MATLAB to determine the absolute acoustic time delays from each loudspeaker. This computational method of computing delay is analogous to the use of a lock-in amplifier as employed in the study of Hartley and Moore (2003). The compound action potential, a frequency specific set of synchronous electrical responses from spiral ganglion cells (Schoonhoven, 2007) recorded concurrently with the CM, was not found to be a confounder in analysis of timing delays at the stimulus levels used here. The absolute time delays were then used to compute the ITD by simply differencing the left and right ear delays. The computed delays were verified visually via a custom MATLAB analysis program. Once time delays were computed, corresponding phase delays were calculated by the formula θ=2πft where f is stimulus frequency in Hz and t is the time delay in seconds. CM measurements, as opposed to the acoustic recordings from the probe tube microphones were used in the current study for calculating ILD, ITD, attenuations and phase delays as we could not be sure whether the earplug occluded the tip of the probe microphones. In the unoccluded conditions, however, the correlation between the baseline acoustic and baseline CM recording measurements across 5 ears resulted in an average correlation coefficient (R) of 0.88 for the ILD cues and 0.96 for the ITD cues demonstrating that the CM recordings are a suitable proxy for the acoustic recordings.
3. Results
3.1 CM waveform recordings – general observations
As expected for tonal stimuli presented with increasing intensity, the CM amplitude for all frequencies (0.25–24 kHz) increased linearly with increasing intensity over a wide range of SPLs (Fig. 1A). The average (n = 5 animals) CM amplitude dynamic range across frequencies was found to be 56.1 ± 3.1 dB (SD) with a range of 53.3 to 59.2 dB
Additionally, the CM waveform was generally a well-defined representation of the presented sinusoidal stimuli across frequencies (0.5–24 kHz) and azimuth. Figure 2A illustrates this finding for 500 and 4000 Hz. CM amplitude, particularly for higher frequencies, varied systematically as the sound source was moved from left (−90°) to right (+90°). As expected, the ITD and ILD varied across azimuth with the ipsilateral ear leading in time and also exhibiting higher CM amplitude. Also, as expected, there was negligible ILD (i.e., near 0 dB) and ITD (i.e., near 0 µs) for sound sources at the center (0° azimuth).
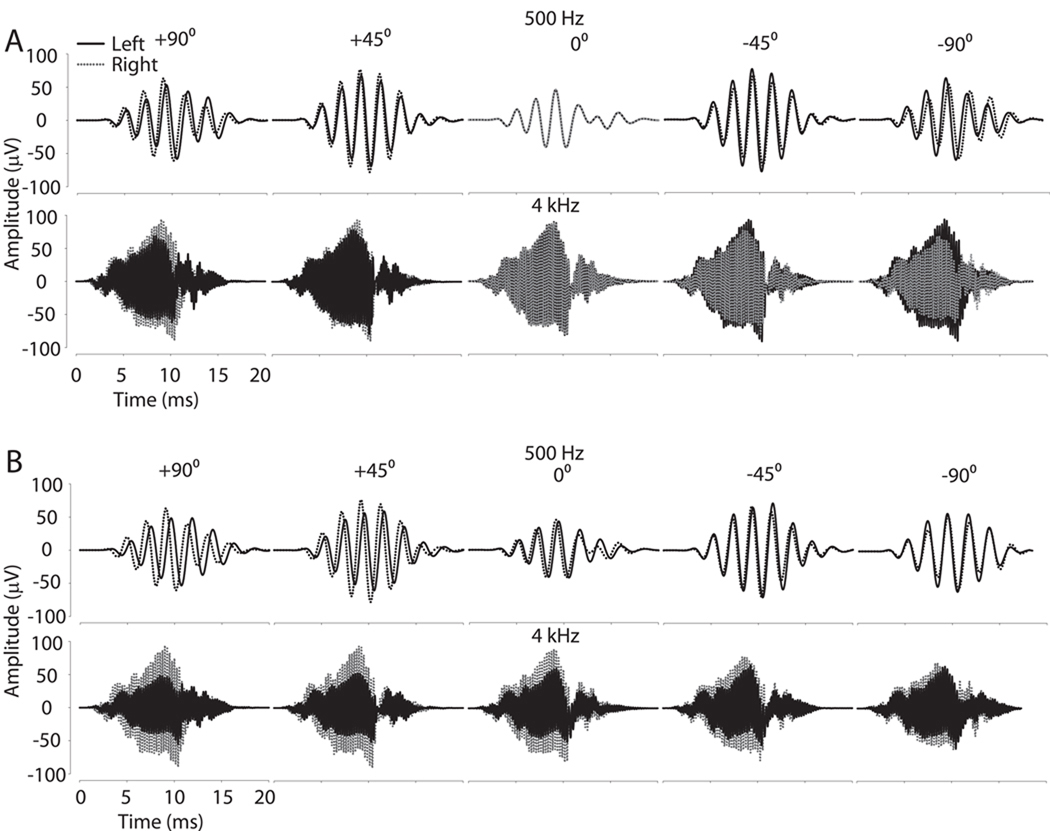
Figure 2.
Example CM waveform recordings from one representative subject for two different frequencies (500 Hz and 4 kHz) at five sound source positions in azimuth (−90°, left side; +90°, right side). A: CM amplitudes and timing delays at the left (black line) and right (dashed) ears vary as a function of sound source azimuth. B: CM waveform recordings after occlusion of the left (dashed) ear. Note that after occlusion, the left ear CM waveform is delayed and attenuated as compared to the waveform observed in panel A.
Upon left ear occlusion, the amplitude of the CM was substantially diminished for the left ear as illustrated for 500 and 4000 Hz in Fig. 2B. The data in Fig. 2B also demonstrate that the occlusion caused a delay of the left CM signals which can be clearly seen with a noticeable deviation of the ITD at 0° azimuth outside the expected 0 µs (Fig. 2B 500 Hz center panel).
3.2 CM phase is invariant with varying sound pressure level
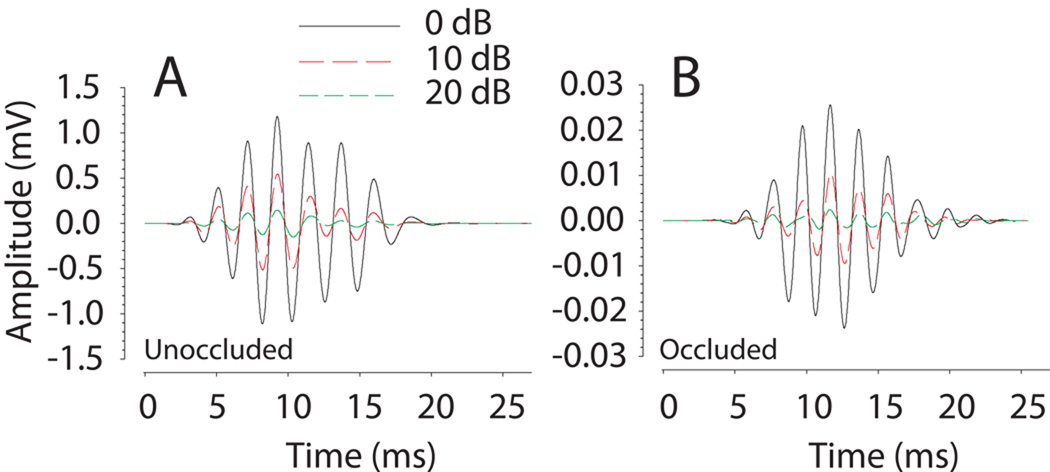
In the current experiment, we use the CM signal to infer the ITD input to the left and right inner ears. The reason that the CM can be used for this purpose is that the phase of the CM signal for tonal stimuli is invariant to overall stimulus level (Dallos and Cheatham, 1971; Knudsen et al., 1984a; Hartley and Moore, 2003). Our observations here are consistent with these reports. The CM amplitude varied linearly with SPL over a wide range of SPL for each animal (Fig. 1A). The absolute amplitude of the CM for a given SPL varied with frequency (Fig. 1A). The phase of the CM as a function of stimulus SPL, however, remained virtually constant over a wide range of stimulus levels. Figure 3A shows representative CM signals for a 500 Hz stimulus and three sound levels from 0 to 20 dB attenuation. Although only three levels are shown here, combinations of frequency and intensity within the linear range of the CM demonstrated phase invariance down to intensities approaching the noise floor. In fact, even occlusion of the ear, which generates a large attenuation of the CM amplitude, did not introduce an amplitude-dependent phase shift secondary to the occlusion material itself which can be seen in Figure 3B for a 500 Hz stimulus at three different attenuation levels (0, 10, and 20 dB). After removal of the foam ear plug, subsequent CM measurements demonstrated CM dynamic range and phase characteristics comparable to the baseline condition indicating the plug did not irreversibly affect the auditory periphery. These results (i.e., Fig. 3) indicate that the CM signal can be used to estimate the ITD as presented to the inner ears.
Figure 3.
A: Representative cochlear microphonic (CM) recordings in response to a 500 Hz sinusoidal stimulus presented at systematically decreasing intensities (0–20 dB attenuation) from a single unoccluded ear. B: CM recordings as function of decreasing stimulus intensity upon introduction of ear plug. Though not all stimulus levels are demonstrated, for both the unoccluded (A) and occluded (B) conditions, the phase of the CM waveform as a function of stimulus SPL was constant over a wide range of stimulus levels and occlusion of the ear did not introduce amplitude-dependent phase shifts in the CM waveforms even though the amplitude of the CM changed considerably. Note the change in the scale of the ordinate from A to B as a result of occlusion.
3.3 Interaural level difference (ILD)
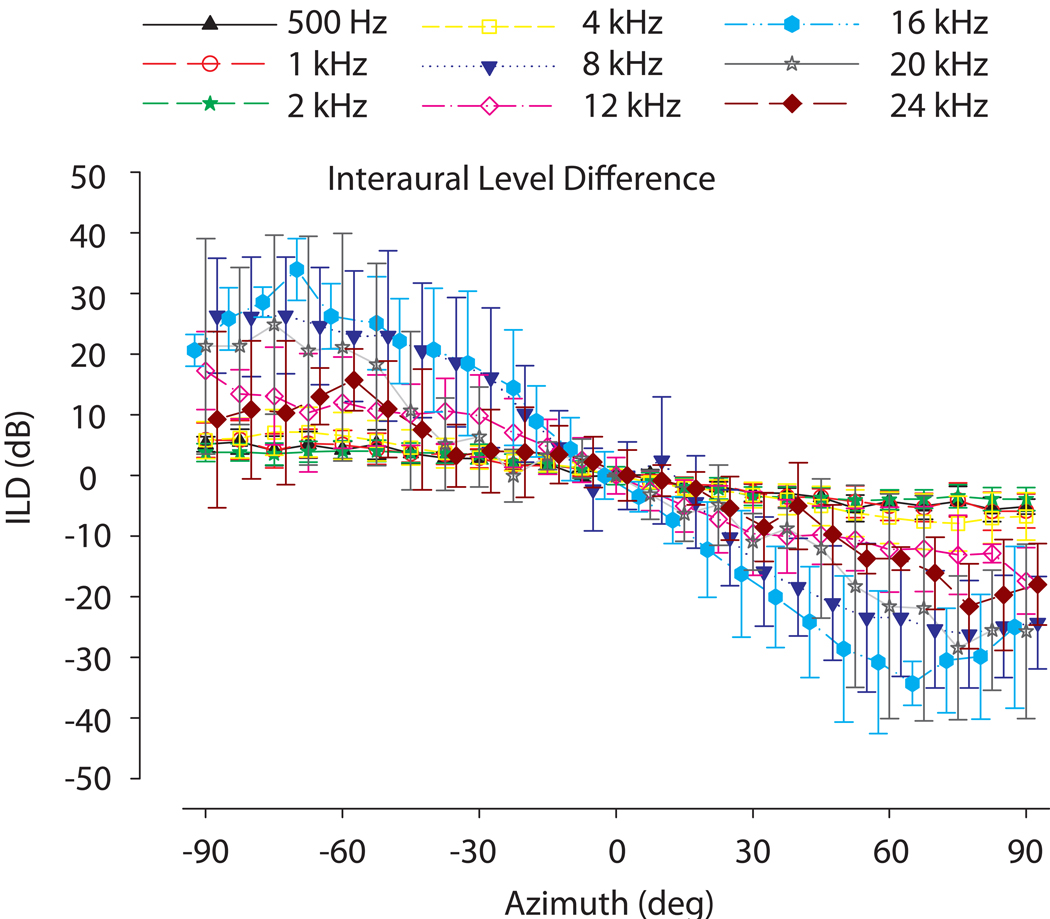
Because CM amplitude varies linearly with SPL over a wide range of SPLs (e.g., Fig. 1), the CM amplitude in this linear range can be used to compute the ILD as presented to the inner ears. ILDs were calculated from the recorded CM amplitudes, by first converting CM amplitudes to corresponding SPL via the initial calibration and linear function fit (see methods above). Our results show, as expected, that ILD cues demonstrate sound source positional as well as stimulus frequency dependence. The average ILDs (± 1SD) as measured in all study subjects (n = 7) from 25 positions across azimuth (−90° to +90°) at 0° elevation are demonstrated in Fig 4. At 0° azimuth, amplitudes between the two ears were largely equivalent, indicating ILD magnitudes of 0 dB. As a function of frequency, ILDs were small, on the order of a few dB for lower frequencies (500Hz – 4 kHz), and became substantially larger for higher frequencies. For frequencies from 8–20 kHz, very large ILDs approaching 30 dB are apparent. The magnitude of the maximum ILDs at the poles (±90°) increased with increasing frequency with a peak around 8 kHz (27 dB) and then dropped at 12 kHz (17 dB) with discrete peaks again at 16 and 20 kHz (25 dB). In these unoccluded conditions, the correlation between the ILDs computed via probe tube microphone measurements near the TM and CM measurements across 5 ears was 0.88 (p < 0.0001).
Figure 4.
Average interaural level differences (ILDs) computed from the CM amplitudes (±1 SD) as a function of sound source azimuth (+90°, right side; −90°, left side) for stimulus frequencies from 500 Hz – 24 kHz without ear occlusion as measured by the cochlear microphonic (n=7). Positive ILDs indicate higher intensity in the left ear. ILDs change substantially with frequency and source azimuth along the horizontal plane
3.4 Interaural time difference (ITD)
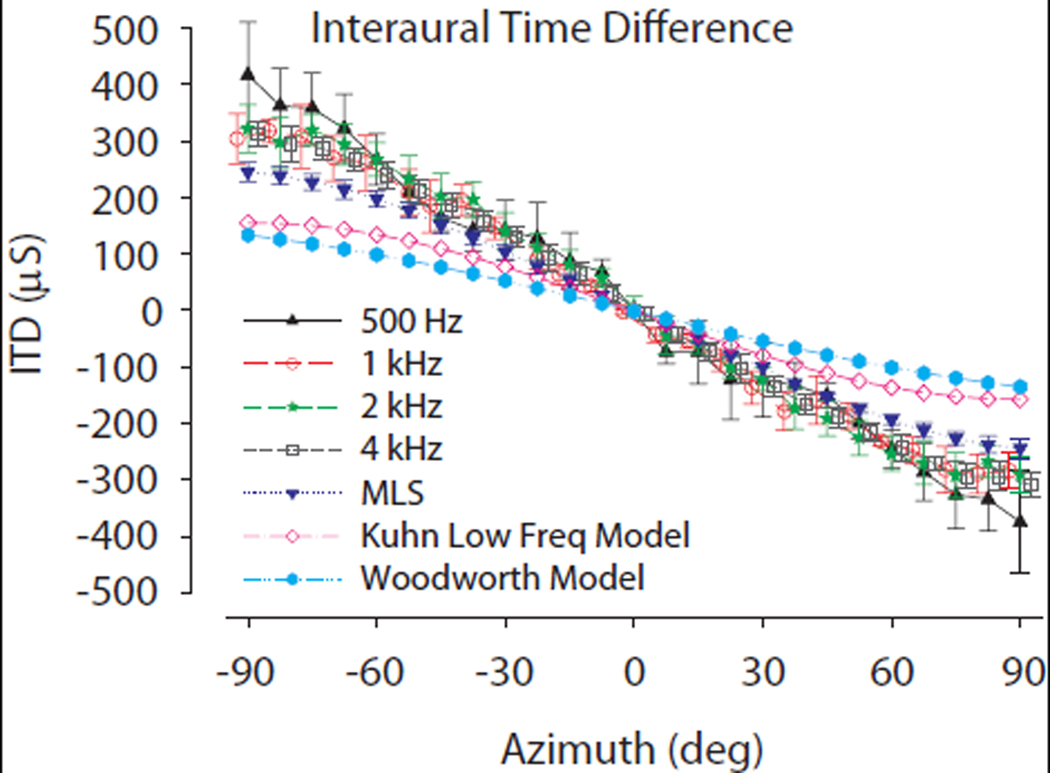
The low frequency ITDs (500 Hz – 4 kHz) were calculated by correlating the CM signals with a reference signal to determine the absolute time delay as described above in the Methods. The absolute delays to the two ears were then used to determine the ITD. Across-animal mean ITD (± 1SD) measurements across azimuth (−90° to 90°) at 0° elevation are demonstrated in Fig. 5. Additionally, Figure 5 demonstrates ITDs as measured from broadband stimuli (the MLS stimuli, see Methods), and ITDs predicted from the low-frequency spherical head model of Kuhn (1977) as well as the classical model of Woodworth (1938) using the adult chinchilla head diameter (36 mm). Similar to ILDs, ITDs demonstrated positional and frequency dependence. Positive and negative ITD values indicate that sound was leading at the left ear and right ear, respectively. With increasing frequency, the across-animal mean ITDs tended to decrease from 387 µs at 500 Hz to 336 µs at 1–4 kHz at positions of −90° azimuth. Kuhn’s model [3rsin(α)/c where r is head radius (chinchilla = 18 mm), c is the speed of sound (343 m/s) and α is azimuth position] predicts ITDs in the range of ±158.8 µs while the Woodworth model [r/c*(sin(α) + α), with variable definitions given above] ±136.1 µsec. ITD magnitudes were symmetrical around the midline (azimuth of 0°), largest at the poles (±90°) and larger than the spherical head model predicted ITDs at all frequencies. In these unoccluded conditions, the correlation between the ITDs computed via probe tube microphone measurements near the TM and CM measurements across 5 ears was 0.96 (p < 0.0001).
Figure 5.
Average low frequency interaural time differences (ITDs) computed from the CM waveforms (n=5, ±1 SD) as a function of azimuth along the horizontal plane without ear occlusion. Individual stimulus frequencies are indicated. Also demonstrated are ITD vs. azimuth computed from broadband noise stimuli (triangles). The predicted ITDs based on two spherical head models (closed circles, Woodworth (1938) model; open circles, Kuhn (1977) low–frequency ITD model) using an adult chinchilla head diameter (d=36mm) are also shown. Positive ITDs indicate stimulus arrival at the left ear preceding right.
3.5 Effects on ILD as a result of unilateral occlusion
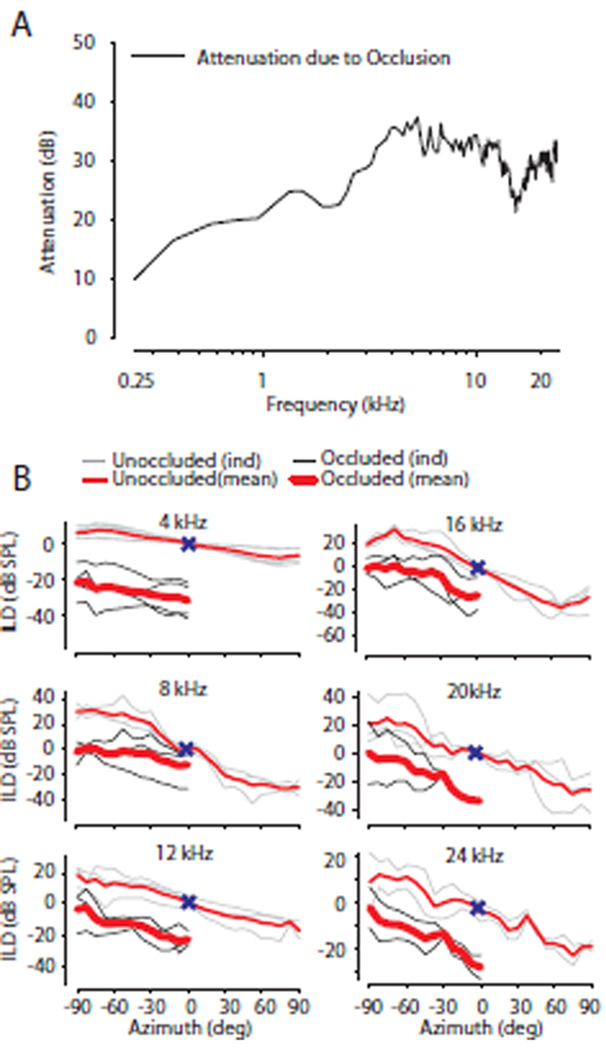
As in the baseline condition without occlusion, ILDs were calculated from the recorded CM amplitudes which were converted to corresponding SPL via the initial calibration as described in the methods section. Occluding the ear with acoustic foam produced a variable mild hearing loss. On average across animals and frequencies, the occlusion of the ear canal attenuated stimuli levels by 10–38 dB (mean 31 ± 3.9 dB). However, the attenuation was dependent on stimulus frequency (500 Hz – 24 kHz), with larger attenuations (30–40 dB) for higher frequencies (> 4 kHz) than those (10–30 dB) for lower frequencies (< 4 kHz) as shown in Figure 6A for the left ear. As expected from a unilateral ear occlusion, the resultant ILDs with the ear occluded demonstrated a downward (or, equivalently, a leftward) shift of the ILD curve (Fig. 6B) indicating a greater intensity difference favoring the unoccluded ear. Figure 6B shows the ILDs measured across the mid- to high-frequencies (4–24 kHz) for the normal and occluded ear conditions. As observed in the baseline condition, with occlusion of the ear canal, the ILD increased with increasing frequency. The difference in ILDs from the baseline condition to the occluded state generally remained consistent for stimuli presented across azimuth in the hemifield ipsilateral to the occlusion. Results contralateral to the occlusion are not reported secondary to the attenuation from the foam which were sometimes attenuated to the limit of signal-to-noise ratio of the CM signal (~56 dB), particularly for the highest frequency stimuli where the ILDs were largest. Thus, the effect of the ear occlusion on the spatial and frequency dependence of the ILD cue to location is probably best summarized for sound sources from around the midline and extending towards sources ipsilateral to the occlusion (i.e., negative azimuths in a left ear plugged convention, as in Fig 6B). This can be extended to the contralateral side assuming the occlusion produces attenuation independent of space.
Figure 6.
A: Occluding an ear with acoustic foam produced a variable mild hearing loss with attenuation levels ranging from 20–38 dB (across-frequency mean = 31 ± 3.9 dB; n = 5 ears) which depended on stimulus frequency (500 Hz – 24 kHz). B Mean and individual occluded (thick RED = across animal mean, thin BLACK = individual animals) and unoccluded (thin RED = across animal mean, thin GRAY = individual animals) ILD vs. stimulus azimuth (−90° left) for different frequencies (4–24 kHz) for a left ear occlusion convention. Occlusion measurements are presented from the side ipsilateral to the ear occlusion secondary to attenuation from head shadow effects in the contralateral field. Positive ILD values indicate intensity at the left ear is greater than the right ear. 0° azimuth and 0 dB ILD indicated by X.
3.6 Effects on ITD as a result of occlusion
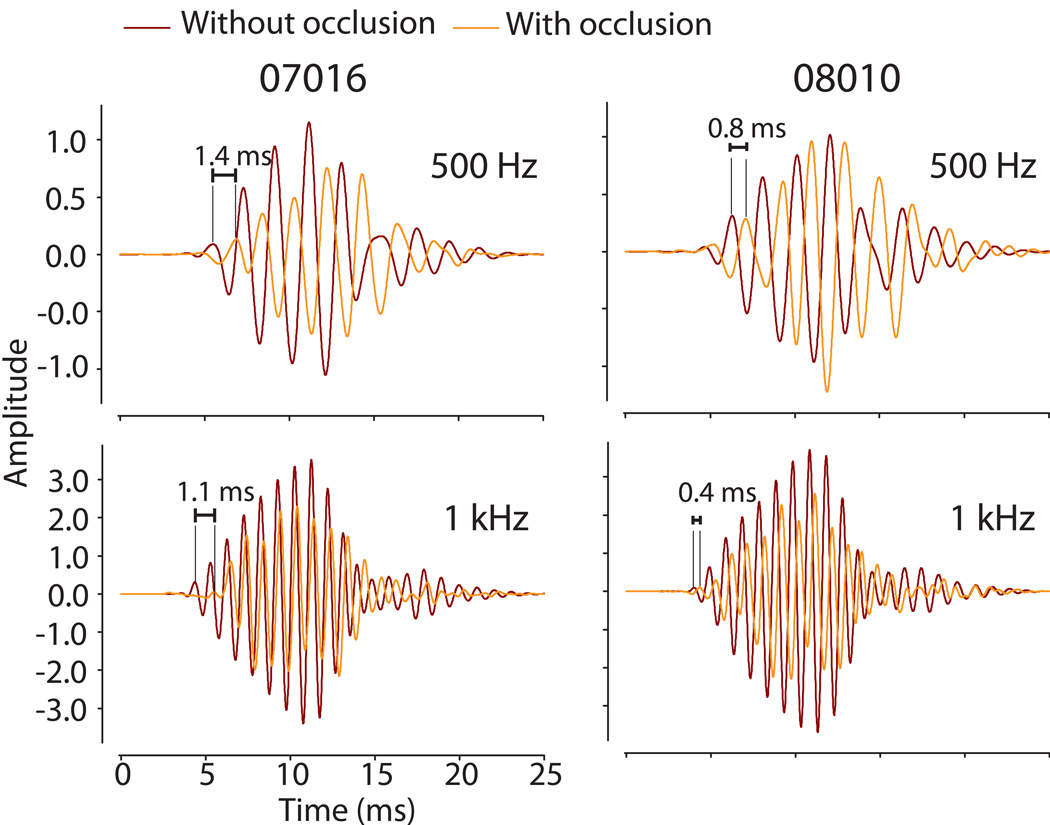
The low frequency ITDs (500 Hz – 4 kHz) were calculated as in the baseline condition. In addition to the expected attenuation, unilateral occlusion of the ear with a foam plug produced time delays and thus subsequent shifts in the ITD cue. Figure 2 illustrates this finding, particularly for the 500 Hz stimulus (i.e., compare the ITD for a source at 0° azimuth between Figure 2 A and B). Figure 7 also demonstrates for two different animals and stimulus frequencies the time delay between CM signals with and without occlusion. With the ear plug in place, the resulting CM waveforms were delayed by ~0.4–1.4 ms which can be seen most clearly at the onset of the CM (i.e., the delay intervals marked in Figure 7). Recall, as documented in Figure 3, CM phase (and thus delay) is independent of acoustic stimulus level and the resulting CM amplitude. Thus, we conclude that the ear plug itself, an interaction effect of the earplug on TM and middle ear function, or a mixed mode of inner ear stimulation (i.e., joint air and bone conduction) effectively alters the temporal propagation of sound energy to the inner ear.
Figure 7.
CM waveform recordings from two individual animals before (BLACK) and after (GRAY) introduction of the occlusion material into the left ear at two frequencies (500 Hz and 1 kHz) from a single position at 0° azimuth. The delay induced by the acoustic foam between the two recorded signals was computed via a cross-correlation algorithm with results noted.
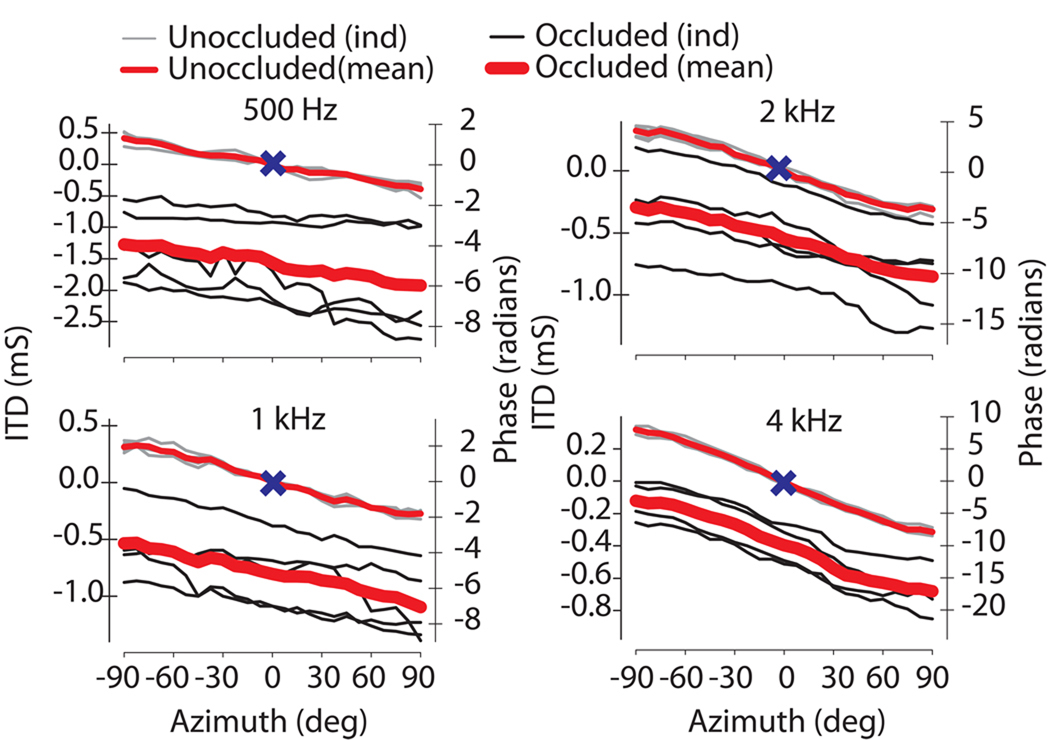
As a result of the additional delay upon introduction of an ear plug, the ITD vs. azimuth functions demonstrated a downward (or, equivalently, a leftward) shift of the ITD curve from the normal state indicating an increased delay between the two ears. As expected, the computed ITD in the normal, unplugged state varied systematically from left to right showing a shift towards an increasingly negative phase delay as the source was moved towards 90° azimuth (Fig. 8). Corresponding phase delays (Fig. 8, right ordinates) are indicated by the right y-axis and are related to the ITD by θ=2πft. The data demonstrate that the timing delays in the occluded condition decreased with increasing frequency. Thus, after plugging the greatest magnitude ITD was demonstrated at 500 Hz with an across-animal range of −1.9 to −1.25 ms while ITDs at 4 kHz ranged across animals from −0.68 to −0.12 ms. When computed across animals and frequencies, at a source location of 0°, unilateral occlusion with acoustic foam introduced additional mean timing delays of 0.8 ±0.5 ms (n = 5 ears).
Figure 8.
Mean and individual occluded (thick RED = across animal mean, thin BLACK = individual animals) and unoccluded (thin RED = across animal mean, thin GRAY = individual animals) interaural time (left ordinate) and phase differences (right ordinate) as a function of stimulus azimuth (+90° 12 = right) for four frequencies (500 Hz – 4 kHz) for a left ear occluded convention (n=5). Positive time and phase differences indicate stimulus arrival at left ear before the right ear (left ear leading). Occlusion introduced a downward shift of the frequency dependent unoccluded ITD curve. 0° azimuth and 0 ms ITD indicated by X.
3.7 Effect of sound attenuating foam and impression material on signal timing and intensity
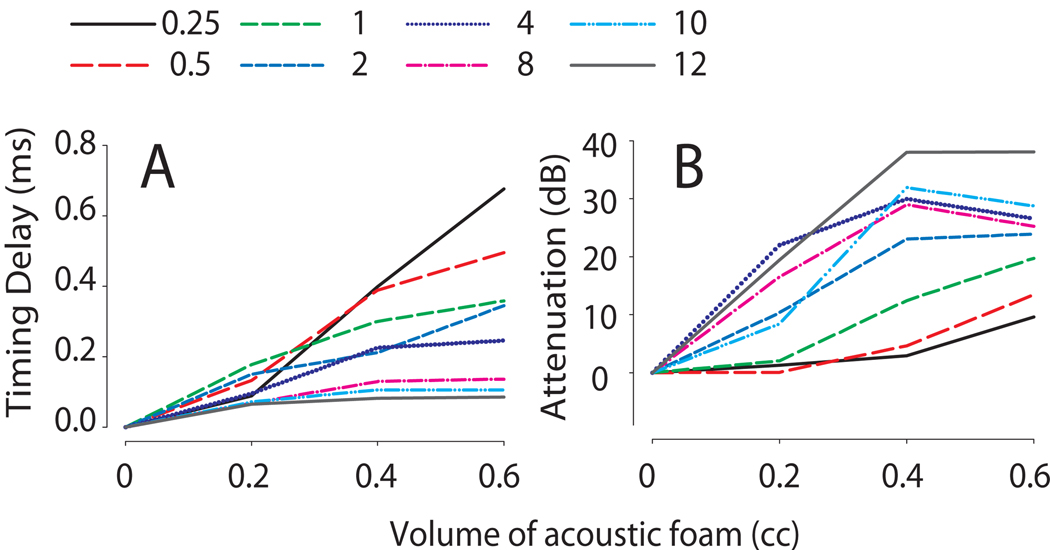
While the attenuation properties of foam and silicone earplugs are well documented, the magnitudes of the temporal modifications demonstrated here and elsewhere (Knudsen et al., 1984a; McPartland et al., 1997; Harley and Moore, 2003; Kumpik et al., 2010) were somewhat unexpected. In order to investigate this quantitatively, the acoustic properties of foam plugs were measured directly in an artificial ear using the method based on Kolpe and Oliviera (2003) who studied the attenuation characteristics of different otoplastic materials (see Methods). We found that sequential introduction of 8 × 4 mm cylindrical blocks (V = 0.2 cc) of sound attenuating foam into the model ear canal introduced both signal attenuation and timing delays. Figure 9A demonstrates a frequency and volume dependent delay in the temporal aspects of the presented stimuli. For the low frequencies, delays were observed on the order of 400–700 µs at volumes of 0.4 and 0.6cc of foam while the higher frequencies produced smaller delays of ~100 µs at both 0.4 and 0.6 cc of foam. The attenuation of the stimulus varied in a frequency and volume dependent manner as seen in Figure 9B. With increasing amounts of foam there was an increase in attenuation up to a volume of 0.4 cc, but which then tended to saturate. Stimulus attenuation increased steadily to ~25–35 dB at 4–12 kHz at foam volumes of 0.4cc. For low frequencies of 250–500 Hz, a slow increase in attenuation was observed to 10 dB with increasing amounts of acoustic foam. To more accurately simulate the in-vivo measurements in this paper, measurements in the model ear canal were also measured before and after placing a thin layer of silicone ear impression compound material over the ear foam (see methods). This procedure introduced an additional ~0.2 ms delay (unplotted data). Thus, as can be seen for low frequencies ( ≤500 Hz), the combination of foam and silicone compound may produce physical delays on the order of ~1 ms which is wholly consistent with the delays generated in-vivo as seen in Fig 7 and 8.
Figure 9.
The effects of acoustic foam on signal intensity and timing measured in a model ear canal. Increasing the volume of acoustic foam increased signal delay (A) and attenuation (B) in a frequency dependent manner.
4. Discussion
4.1 On the use of cochlear microphonic measurements to assess conductive hearing loss
The cochlear microphonic (CM) is a potential generated by the peripheral auditory system representing the ensemble activity of the outer hair cells which reproduces acoustic waves over a wide range of intensities (Dallos, 1973). Our CM recordings under unoccluded and occluded ear canal conditions demonstrate that the CM is indeed representative of the acoustic signal at the tympanic membrane (TM). There is a long history of using CMs to infer the cues to location (chick: Hyson, 1994; owl: Knudsen et al., 1984a & Moiseff, 1989; cat: Calford et al., 1986; bat: Fuzessery and Pollak, 1985). Furthermore, confirming earlier observations by Wever and Lawrence (1954), Knusden et al. (1984a) and Hartley and Moore (2003), the CM recordings demonstrate linearity over a wide range of intensities enabling accurate calibration to relate CM amplitude to equivalent sound pressure level (SPL). Through analysis of the CM, a representative recording of the acoustical sound wave, the effects of manipulations of the outer or middle ear on the transformation of sound waves are therefore quantifiable.
Here we utilized the CM as a means to characterize the effective input to the inner ear of a subject in the presence of an experimental conductive hearing loss (CHL) induced by acoustic attenuating foam. Multiple means of achieving an experimentally-induced CHL in a laboratory setting have been devised, such as a simulated effusion with injection of fluids into the middle ear space (Weiderhold et al., 1980; Marsh et al., 1985; Hartley and Moore, 2003). Other common methods for inducing CHL include malleus extirpation (Xu et al., 2007) and ear canal ligation (Silverman and Clopton, 1977; Brugge et al., 1985; Popescu and Polley, 2010). Utilization of foam plugs, as performed in this paper, is another such means to induce CHL (Knudsen et al., 1984a; Hyson et al., 1994; Moore et al., 1989, 1999; Hartley and Moore 2003).
The spectral and temporal aspects of stimuli at the two ears define two important cues to sound location in azimuth, the interaural level and timing difference (ILD and ITD respectively) (Blauert, 1997). ILD and ITD cues have recently been characterized in the chinchilla (Koka et al. 2010, in press Hearing Research). Koka et al. (2010) report ILDs of < 10 dB for frequencies < 5 kHz with a range of 10- 30 dB for frequencies >5 kHz and maximum ITDs of ~250 µs for low –frequency (< 3500) broadband noise. Our results here based on CM measurements are in agreement. Behaviorally the chinchilla has been shown to utilize both ITD and ILD cues for sound source localization (Heffner et al., 1994).
In previous studies, CM measurements have been analyzed as a function of azimuth to directly compute ILDs and ITDs in different animal models (owl- Volman and Konishi,1989 & Moiseff, 1989; chick –Hyson et al., 1994; cat - Calford et al., 1986). The resulting ILD and ITDs in an animal model of conductive hearing loss, CHL, however, have not been directly measured. Thus, although the spectral and temporal modifications of sounds to the inner ear due to experimental CHL, such as earplugs, and the concomitant affect of this on the acoustical cues to location have been widely speculated to be responsible for experience-dependent modifications of the anatomy, physiology, and psychophysics of sound localization (see Introduction), the exact effect of CHL on the cues to location have not been measured. For example, while Knudsen et al. (1984a) and Hartley and Moore (2003) measured the effects of CHL on CM amplitude and phase, they did not directly measure the effect of CHL on the cues to sound location themselves. Through their measurements, however, the resultant cues to sound location can be inferred. In the present study we have extended these past studies to investigate the influence of CHL on the spatial dependence of the ILD and ITD cues to location across azimuth in the frontal hemisphere through CM measurements.
4.2 ITD and ILD without ear plugging
The low-frequency ITDs were measured without ear plugging. The maximum ITD of the chinchilla without ear occlusion was 379–410 µs for a 500 Hz tone pip (Fig. 5). The observed trend of decreasing ITD with increasing frequency has been documented in a number of investigations into acoustical cues from human (Kuhn, 1977) and also in animal species (rat- Koka et al., 2008b; cat- Roth et al., 1980 & Tollin and Koka, 2009; gerbil- Maki and Furukawa, 2005; guinea pig- Sterbing et al., 2003). In computing the acoustic ITDs across 4 frequencies (500 Hz and 1, 2 and 4 kHz) and comparing them to the predicted ITD based on chinchilla head size (i.e., diameter), the chinchilla ITDs are 58–171% larger than expected. For example, the classical spherical head model of ITDs by Woodworth (1938) predicts maximum ITDs of ±136.1 µs and the low-frequency ITD model of Kuhn predicts ±158.8 µs for a head diameter of an adult chinchilla of 36 mm. These models do not take into account the effects of the pinnae or other anatomic features on ITD. The pinnae have been shown, however, to have an effect on the maximum ITD in other species (cat- Roth et al., 1980 & Tollin and Koka, 2009; rat- Koka et al., 2008b; rhesus monkey-Slee and Young, 2010). We have recently shown that the pinnae increase the maximum ITD by ~25% in the chinchilla (Koka et al., 2010 in press Hearing Research).
As expected, ILDs were highly dependent on both frequency and source position across azimuth. As a function of frequency, ILDs were on the order of a few dB for lower frequencies (500Hz – 4 kHz), and become larger for higher frequencies with increasing contribution from the head shadow. For frequencies from 8–20 kHz, very large ILDs approaching 30 dB were apparent. The magnitude of the maximum ILDs at the poles (±90°) demonstrated a peak around 8 kHz (27 dB) and then dropped at 12 kHz (17 dB) with discrete peaks again at 16 and 20 kHz (25 dB). The ILDs observed in the study are larger than the ILDs predicted (not shown) by the spherical head model of ILDs of Duda and Martens (1998). The chinchilla pinnae have been shown to be responsible in part for the larger than predicted ILDs (Koka et al., 2010 in press Hearing Research), an effect that has also been shown in other mammalian species (Tammar wallaby- Coles and Guppy, 1986; guinea pig- Carlile and Pettigrew, 1987; ferrets- Carlile and King, 1994, Schnupp et al., 1998; Parsons et al., 1999). The aspects of pinnae effects in the chinchilla acoustic spectral cues (i.e. directional gain, ILD, and ITD) are not directly germane to the current topic of CHL, and are investigated more thoroughly and appropriately in a separate paper (see Koka et al., 2010 in press Hearing Research).
4.3 The effect of experimentally-induced CHL on the binaural cues to sound location
Occluding the ear canal with a plug to introduce a CHL produces both an attenuation of the incoming stimulus as well as a delay. These spectral and temporal modifications of the stimuli are apparent at the input to the inner ear (i.e., the CM waveform). Occluding the ear with acoustic foam produced a variable mild hearing loss with attenuation of stimuli levels by 20–38 dB (mean = 31 ±3.9 dB), the overall magnitude depending on the stimulus frequency (500 Hz – 24 kHz). Generally, our attenuation findings in the chinchilla are consistent with the frequency dependent 24 – 48.5 dB attenuation after occlusion by ear foam measured previously by Knudsen et al. (1984a) in the barn owl. In the ferret, occlusion of an ear with foam caused frequency-dependent attenuations of 10–40 dB (Moore et al., 1989). Our findings are also directly consistent with the behaviorally-determined (i.e., audiogram) attenuation levels of ~30–50 dB in chinchillas due to foam earplugs of comparable dimensions (V = 0.55 cc) as reported by Patterson et al. (1991). These latter attenuation levels, in addition to our own data here, are somewhat higher than those of Hartley and Moore (2003) who observed an across-frequency average of 13 dB attenuation in the gerbil. We believe that the large variation in earplug induced attenuation between studies (and species) may be due to the amount of (i.e., volume) or physical properties of the acoustic foam used in the individual studies. The volume of foam was found in the present experiment to be an important variable in total level of attenuation (see below).
In concordance with the Knudsen et al. (1984a) and the Hartley and Moore (2003) studies, we also found that the timing (or phase) of the CM waveform for a given stimulus frequency was not affected by changing stimulus level within the linear range of operation of the CM (Fig. 3). This fact is critical because it means that the amplitude of the CM does not present as a confounding factor in the analysis of timing differences based on the CM waveforms. We found a mean 6.6 rad phase delay across all frequencies at 0° azimuth. In terms of stimulus frequency dependence, the across animal low frequency plug induced delays ranged from 400 µs at 4 kHz to 1.5 ms at 500 Hz. Although these delays are large, their magnitudes are not without precedent. For comparison, Hartley and Moore (2003) found delays due to ear occlusion in gerbils with foam that averaged 104 µs for low frequencies (1–6 kHz) and 20 µs at high frequencies (8–16 kHz), but in some animals reached nearly 250 µs at low frequencies. Knudsen et al (1984a) in the barn owl found phase delays exceeding 100 µs in many cases. Knudsen also measured a phase advance at 2 kHz postulated to occur secondary to the presence of the interaural canal in the barn owl. Moreover, in the Hartley and Moore study (2003), measurement of ITD in the case of simulated middle ear effusions (i.e., fluid in the middle ear) produced frequency dependent delays of up to 230 µs. In a cadaveric human temporal bone model of middle ear effusion, Ravicz et al. (2004) demonstrated that fluid in the middle ear can cause signal delays of up to 500 µs or more (~0.25 cycles) for frequencies around 500 Hz (Fig. 4A of Ravicz et al., 2004). Finally, Hogan et al. (1995) reported that foam earplugs in humans can produce delays of up to 500 µs or more at 500 Hz. Similar earplug induced delays of comparable magnitude were reported recently in humans by Kumpik et al. (2010).
The large ITDs measured in the present study relative to those reported previously may be best explained by the introduction of larger volumes of the acoustic foam into the external auditory canal (EAC) in the chinchilla. For example, relative to the barn owl (Knudsen et al., 1984a) and gerbil (Hartley and Moore, 2003), in which foam occlusion produced delays of up to 100 µs and 230 µs, respectively, these two species have comparably smaller EAC volumes [barn owl EAC volume ~ 0.8–0.9 cc (Keller et al., 1998)]. On the other hand, the human, in which foam occlusion produced upwards of ~500 µs delays (Hogan et al., 1995), exhibits ear canal volumes (1.75 cc; Gerhardt et al., 1987) that more closely approximate that of the chinchilla (1.6 cc; Kanick et al., 2006). Assessment of the physical properties of the acoustic foam that actually causes such delays is beyond the scope of the current investigation.
To test the hypothesis that the amount of signal delay and attenuation is related to the quantity, or volume, of sound attenuating acoustic foam material in the ear canal, a metal model ear canal was constructed with dimensions similar to the chinchilla external ear. Figure 9 demonstrates that the absolute delay and attenuation was dependent on stimulus frequency, and acoustic foam volume, with larger delays occurring for lower frequencies and larger volumes, and larger attenuations occurring for higher frequencies and larger volumes. Additionally, we found an additional increase time delay from the application of silicone ear impression material on top of the acoustic foam in the model ear canal of ~0.2 ms, which produces a total delay of ~1 ms at 500 Hz.
The delays induced by the foam used for in-vivo studies of CHL correlate well with and can generally be predicted from the findings from the metal ear canal model. For example, Hartley and Moore (2003) induced CHL with 4 mm3 (0.064 cc) acoustic foam cubes from the same manufacturer utilized in the present experiment. With 0.064 cc foam plugs, a 0.125 ms delay at 1 kHz was observed. Examination of the data from the model ear canal in Figure 9 shows that for foam volumes approximating that used by Hartley and Moore (2003), the induced delays at 1 kHz are ~80 µs. Moreover, the across-frequency attenuation values predicted for this small volume of foam are in the range of 15 dB or less, again consistent with the 13 dB attenuation levels reported by Hartley and Moore (2003) empirically. In the ferret, Moore et al. (1989; see their Figure 1A) used a combination of 0.064 cc of acoustic foam covered by a layer of silicone impression compound (similar to that used in the present experiment) and found attenuation levels of < 10 dB for frequencies < 5 kHz and attenuations of ~20 dB for frequencies from 5–16 kHz. Figure 9B shows that 0.064 cc of foam will result in attenuations of ~10 dB for frequencies above 8 kHz; the additional attenuation noted by Moore et al. (1989) likely result from the additional silicone impression compound. Knudsen et al. (1984a) reported using acoustic foam volumes in the barn owl of approximately 0.8cc (Eric Knudsen, personal communication) and observed a frequency dependent 24 – 48.5 dB attenuation. Although we did not measure attenuation in the canal model to levels of 0.8 cc of acoustic foam, the observed attenuation is generally expected based on the model ear canal data. Finally, the attenuation levels of ~30–50 dB due to foam earplugs of ~0.55 cc in chinchillas as reported by Patterson et al. (1991) is also generally predicted by our model ear canal data in Figure 9B.
The variability seen in the timing data across different studies is likely a result of the species-dependent ear canal anatomy requiring placement of a larger quantity of sound attenuating foam in the ear canal for adequate occlusion. In our study here, the entire volume of foam was placed completely into the ear canal of the chinchilla. In the human studies reported above, the actual volumes of foam residing in the ear canal itself (i.e., that volume of foam actually in the canal) were not reported, so direct predictions based on our model canal cannot be made. Assuming, however, a standard ear plug insertion depth of 50% of the EAC (Berger 2003) in an average human EAC (Gerhardt et al., 1987) an effective ear canal occlusion volume would be equivalent to 0.87 cc. Although model measurements did not exceed 0.6 cc, the upper limit of our model, frequencies > 1 kHz were attenuated from 20–40 dB and delayed up to 600 µs for low-frequency sounds corroborating findings by Hogan et al. (1995) who observed delays up to 500 µs at 500 Hz. The magnitudes of the spectral and temporal modifications of sounds due to foam earplugs measured in-vivo are consistent with the hypothesis that foam introduces a volume-dependent delay in the propagation of sound energy to the inner ear.
4.4 Implications of CHL-induced effects on the binaural cues to sound location
Earlier studies of the effect of acute ear occlusion on sound localization performance in humans reported a shift of the apparent location of sound sources to the side of the unplugged ear (Musicant and Butler, 1984; Slattery and Middlebrooks, 1994). This effect is supported by the data obtained here. Induction of CHL by ear plug disrupts the normal presentation of the ILD and ITD cues to sound location to the inner ear. As can be seen in Fig. 6 and 8, the location cue shifts introduced by ear occlusion lead to cues biasing the unplugged ear (the right ear in the convention of the current experiment). Although these experiments did not correlate behavior with localization cue shifts, it demonstrates the ability of acute ear occlusion via acoustic foam to disrupt normal binaural localization ability.
It is well-accepted that CHL during human development can change auditory system structure and function (Yoshinaga-Itano et al., 1998). Early life exposure to CHL, particularly unilateral, leads to impairments in binaural hearing tasks (Hall and Derlacki, 1986; Pillsbury et al., 1991; Moore et al., 1991; Hogan and Moore, 2003; Hall et al., 1998) even after resolution of the CHL and bilateral hearing sensitivity returned to normal. The persistently-impaired binaural hearing abilities that result from CHL often recovers, but this can take months or even years (Hall et al., 1995; Hogan and Moore, 2003). During recovery, a child may present as audiologically normal, yet speech perception in noisy, reverberant environments may continue to be compromised. Because language is learned in such complex environments these binaural impairments may contribute to the long term deficits in language, reading, overall behavior and attentional tasks (Bennett et al., 2001).
In addition to the prior research cited above, our observations here that a common form of experimentally-induced CHL using acoustic foam induces a substantial temporal delay of sound transmission to the inner ear has profound implications for future studies. For example, there is a well-known dominant role of low-frequency ITDs for binaural hearing tasks such as sound source localization (Wightman and Kistler, 1992). Moreover, children with history of chronic OME perform poorly in studies replicating the ‘cocktail party effect’ (Moore 1991) indicating an inability to spatially filter and process sounds of interest. Finally, timing changes in the input to the inner ear as a result of CHL due to OME have been directly suggested in human psychophysical studies by Hall et al. (1995, 1998). Thus, in addition to the spectral modifications of sounds by CHL, the temporal modifications and the concomitant alteration of the ITD cue to sound location may have profound implications for auditory processing in individuals with chronic CHL, such as children with OME.
Conclusions
The levels of attenuation produced by a unilaterally-placed foam earplug were found here to approximate that seen in human populations in cases of middle ear effusion. Timing delays in conductive hearing loss (CHL) are less well characterized in the literature and have been shown here to be dependent on the acoustic foam itself. The timing delays induced with foam plugging produce ITD magnitudes that fall outside the range of useful ITD cues, a finding with implications for future studies utilizing plugging to study the effects of CHL on neuroanatomy, neurophysiology, and psychophysics. Given the CHL induced by plugging the ear with foam, the ILD and ITD cues change in predictable ways leading to a hemispheric bias of the ILD cue to the contralateral side of ear plugging. The alteration of binaural localization cues in CHL, as demonstrated in the present study, likely play a factor in deficits such as localizing sound and processing sounds within a noisy environment from a CHL such as OME. Future work into the neurophysiological effects of CHL during development will provide further insight into understanding pathophysiology of CHL and guide studies into appropriate clinical interventions.
Acknowledgments
This work was supported by a National Institutes of Deafness and Other Communicative Disorders Grant (R01DC006865) and the Evie & Ron Krancer Grant in Auditory Science from the National Organization of Hearing Research to DJT. Support was also provided by an American Academy of Otolaryngology-Head and Neck Surgery Foundation (AAO-HNSF) resident research grant to JEL and a Neuroscience Training Grant to JLT (National Institute of Child Health and Human Development grant 5T32HD041697-08). We thank Dr. Michael Hall for preparing custom laboratory hardware (supported by NIH grant P30 NS041854-05).
List of abbreviations
- CHL
Conductive Hearing Loss
- ILD
Interaural level difference
- ITD
Interaural timing difference
- CM
Cochlear Microphonic
- OME
Otitis media with effusion
- IM
Intramuscular
- EAC
External auditory canal
- TM
Tympanic membrane
- MLS
Multiple length sequence
- SPL
Sound pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asbjørnsen A, Holmefjord A, Reisaeter S, Møller P, Klausen O, Prytz B, Boliek C, Obrzut JE. Lasting auditory attention impairment after persistent middle ear infections: a dichotic listening study. Dev Med Child Neurol. 2000;42:481–486. doi: 10.1017/s001216220000089x. [DOI] [PubMed] [Google Scholar]
- Bennett KE, Haggard MP, Silva PA, Stewart IA. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis. Child. 2001;85:91–95. doi: 10.1136/adc.85.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EH, Kieper RW. Hearing protection: Surpassing the limits to attenuation imposed by the bone-conduction pathways. J Acoust Soc Am. 2003;114((4) Pt 1):1955–1967. doi: 10.1121/1.1605415. [DOI] [PubMed] [Google Scholar]
- Blauert J. Spatial hearing: The psychophysics of human sound localization. Cambridge (MA): MIT Press; 1997. [Google Scholar]
- Bluestone CD, Beery QC, Paradise JL. Audiometry and Tympanometry in relation to middle ear effusions in children. Laryngoscope. 1973;83:594–604. doi: 10.1288/00005537-197304000-00015. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Orman SS, Coleman JR, Chan JC, Phillips DP. Binaural interactions in cortical area AI of cats reared with unilateral atresia of the external ear canal. Hear Res. 1985;20(3):275–287. doi: 10.1016/0378-5955(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Butler RA, Humanski RA, Musicant AD. Binaural and monaural localization of sound in two-dimensional space. Perception. 1990;19:241–256. doi: 10.1068/p190241. [DOI] [PubMed] [Google Scholar]
- Calford MB, Moore DR, Hutchings ME. Central and peripheral contributions to coding of acoustic space by neurons in inferior colliculus of cat. J Neurophys. 1986;55(3):587–603. doi: 10.1152/jn.1986.55.3.587. [DOI] [PubMed] [Google Scholar]
- Carlile S, King AJ. Monaural and binaural spectrum level cues in the ferret: acoustics and the neural representation of auditory space. J. Neurophysiol. 1994;71:785–801. doi: 10.1152/jn.1994.71.2.785. [DOI] [PubMed] [Google Scholar]
- Carlile S, Pettigrew AG. Directional properties of the auditory periphery in the guinea pig. Hear. Res. 1987;31:111–122. doi: 10.1016/0378-5955(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Clements M, Kelly JB. Auditory spatial responses of young guinea pigs (Cavia porcellus) during and after ear blocking. J Comp Physiol Psychol. 1978;92(1):34–44. doi: 10.1037/h0077424. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Silverman MS. Changes in latency and duration of neural responding following developmental auditory deprivation. Exp Brain Res. 1978;32(1):39–47. doi: 10.1007/BF00237388. 12. [DOI] [PubMed] [Google Scholar]
- Coleman JR, O'Connor P. Effects of monaural and binaural sound deprivation on cell development in the anteroventral cochlear nucleus of rats. Exp Neurol. 1979 Jun;64(3):553–566. doi: 10.1016/0014-4886(79)90231-0. [DOI] [PubMed] [Google Scholar]
- Coles RB, Guppy A. Biophysical aspects of directional hearing in the tammar wallaby, macropus eugenii. J Exp Biol. 1986;121:371–394. [Google Scholar]
- Dallos P, Cheatham MA. Travel time in the cochlea and its determination from cochlear-microphonic data. J Acoust Soc Am. 1971;49(4) Suppl 2:1140–1143. doi: 10.1121/1.1912475. [DOI] [PubMed] [Google Scholar]
- Dallos P. The Auditory Periphery: Biophysics and Physiology. New York: Academic Press; 1973. [Google Scholar]
- Duda RO, Martens WL. Range dependence of the response of a spherical head model. J Acoust Soc Am. 1998;104:3048–3058. [Google Scholar]
- Fisher HG, Freedman SJ. Localization of sound during simulated unilateral conductive hearing loss. Acta Otolaryngol. 1968;66(3):213–220. doi: 10.3109/00016486809126288. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM, Pollak GD. Determinants of sound location selectivity in bat inferior colliculus: A combined dichotic and free-field stimulation study. J. Neurophys. 1985;54(4):757–781. doi: 10.1152/jn.1985.54.4.757. [DOI] [PubMed] [Google Scholar]
- Gerhardt KJ, Rodriguez GP, Hepler EL, Moul ML. Ear canal volume and variability in the patterns of temporary threshold shifts. Ear Hear. 1987;8(6):316–321. doi: 10.1097/00003446-198712000-00005. [DOI] [PubMed] [Google Scholar]
- Gouma P, Mallis A, Daniilidis V, Gouveris H, Armenakis N, Naxakis S. Behavioral trends in young children with conductive hearing loss: a case–control study. Eur Arch Otorhinolaryngol. 2010 doi: 10.1007/s00405-010-1346-4. DOI 10.1007/s00405-010-1346-4. [DOI] [PubMed] [Google Scholar]
- Gravel JS, Wallace IF. Listening and language at 4 years of age: effects of early otitis media. J Speech Lang Hear Res. 1992;35(3):588–595. doi: 10.1044/jshr.3503.588. [DOI] [PubMed] [Google Scholar]
- Gravel JS, Wallace IF. Effects of otitis media with effusion on hearing in the first 3 years of life. J Speech Lang Hear Res. 2000 Jun;43(3):631–644. doi: 10.1044/jslhr.4303.631. [DOI] [PubMed] [Google Scholar]
- Hall JW, III, Grose JH, Dev MB, Ghiassi S. The Effect of Masker Interaural Time Delay on the Masking Level Difference in Children with History of Normal Hearing or History of Otitis Media with Effusion. Ear Hear. 1998;19(6):220–229. doi: 10.1097/00003446-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Hall JW, III, Grose JH, Mendoza LL. Masker interaural phase and the MLD: Effects of conductive hearing loss. Hear Res. 1995;84:91–98. doi: 10.1016/0378-5955(95)00016-w. [DOI] [PubMed] [Google Scholar]
- Hall JW, III, Derlacki EL. Effect of conductive hearing loss and middle ear surgery on binaural hearing. Ann Otol Rhinol Laryngol. 1986 Sep-Oct;95(5 Pt 1):525–530. doi: 10.1177/000348948609500516. [DOI] [PubMed] [Google Scholar]
- Hartley DEH, Moore DR. Effects of conductive hearing loss on temporal aspects of sound transmission through the ear. Hear Res. 2003;177:53–60. doi: 10.1016/s0378-5955(02)00797-9. [DOI] [PubMed] [Google Scholar]
- Häusler R, Colburn S, Marr E. Sound localization in subjects with impaired hearing. Spatial-discrimination and interaural-discrimination tests. Acta Otolaryngol Suppl. 1983;400:1–62. doi: 10.3109/00016488309105590. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Behavioral hearing range of the chinchilla. Hear Res. 1991 Mar;52(1):13–16. doi: 10.1016/0378-5955(91)90183-a. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE, Kearns D, Vogel J, Koay G. Sound localization in chinchillas. I: Left/right discriminations. Hear Res. 1994 Nov;80(2):247–257. doi: 10.1016/0378-5955(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Hogan SC, Pralong D, Moore DR. Effects of unilateral ear-plugging in humans on binaural unmasking. Br. J. Audiol. 1995;29:56–57. [Google Scholar]
- Hogan SC, Moore DR. Impaired binaural hearing in children produced by a threshold level of middle ear disease. J Assoc Res Otolaryngol. 2003 Jun;4(2):123–129. doi: 10.1007/s10162-002-3007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyson RL, Overholt EM, Lippe WR. Cochlear microphonic measurements of interaural time differences in the chick. Hear Res. 1994 Dec;81(1–2):109–118. doi: 10.1016/0378-5955(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Kanick SC, Sundeep K, Swarts JD, Banks J, Yuksel S, Doyle WJ. Accuracy of CO2 conductance predicted using a morphometric model of the middle ear mucosa. Acta Otolaryngol. 2006;126:1252–1259. doi: 10.1080/00016480600794420. [DOI] [PubMed] [Google Scholar]
- Keller CH, Hartung K, Takahashi TT. Head-related transfer functions of the barn owl: measurement and neural responses. Hear Res. 1998;118:13–34. doi: 10.1016/s0378-5955(98)00014-8. [DOI] [PubMed] [Google Scholar]
- King AJ, Parsons CH, Moore DR. Plasticity in the neural coding of auditory space in the mammalian brain. Proc Natl Acad Sci USA. 2000;97:11821–11828. doi: 10.1073/pnas.97.22.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Esterly SD, Knudsen PF. Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci. 1984a Apr;4(4):1001–1011. doi: 10.1523/JNEUROSCI.04-04-01001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Esterly SD. A critical period for the recovery of sound localization accuracy following monaural occlusion in the barn owl. J Neurosci. 1984b;4(4):1012–1020. doi: 10.1523/JNEUROSCI.04-04-01012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Mechanisms of experience-dependent plasticity in the auditory localization pathway of the barn owl. J Comp Physiol A. 1999 Oct;185(4):305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- Koka K, Read HL, Tollin DJ. The acoustical cues to sound location in the rat: Measurements of directional transfer functions. J. Acoust. Soc. Am. 2008;123:4297–4309. doi: 10.1121/1.2916587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K, Jones H, Thornton JL, Lupo JE, Tollin DJ. Sound pressure transformations by the head and pinnae of the adult Chinchilla (Chinchilla lanigera) 2010 doi: 10.1016/j.heares.2010.10.007. In press Hearing Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpe W, Oliviera RJ. Chemistry and rheology of Otoplastic materials. In: Pirzanski C, editor. Ear impressions and New Laser Shell Technology, Seminars in Hearing. 24. Vol. 4. 2003. pp. 289–298. [Google Scholar]
- Kuhn GF. Model for the interaural time differences in the azimuthal plane. J.Acoust Soc. Am. 1977;62:157–167. [Google Scholar]
- Kumpik DP, Kacelnik O, King AJ. Adaptive reweighting of auditory localization cues in response to chronic unilateral earplugging in humans. J Neurosci. 2010 Apr 7;30(14):4883–4894. doi: 10.1523/JNEUROSCI.5488-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska M, Walger M, Schneider I, von Wedel H. Maturation of binaural interaction components in auditory brainstem responses of young guinea pigs with monaural or binaural conductive hearing loss. Eur Arch Otorhinolaryngol. 1992;249(6):325–328. doi: 10.1007/BF00179382. [DOI] [PubMed] [Google Scholar]
- Marsh RR, Baranak CC, Potsic WP. Hearing loss and visco-elasticity of middle ear fluid. Int. J. Pediat. Otorhinolaryngol. 1985;9:115–120. doi: 10.1016/s0165-5876(85)80011-2. [DOI] [PubMed] [Google Scholar]
- Maki K, Furukawa S. Acoustical cues for sound localization by the Mongolian gerbil Meriones unguiculatus. J Acoust Soc Am. 2005;118:872–886. doi: 10.1121/1.1944647. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Martin RL, Mossop JE, Moore DR. Response properties of neurons in the inferior colliculus of the monaurally deafened ferret to acoustic stimulation of the intact ear. J Neurophysiol. 1997;78(2):767–779. doi: 10.1152/jn.1997.78.2.767. [DOI] [PubMed] [Google Scholar]
- McPartland JL, Culling JF, Moore DR. Changes in lateralization and loudness judgements during one week of unilateral ear plugging. Hear Res. 1997;113:165–173. doi: 10.1016/s0378-5955(97)00142-1. [DOI] [PubMed] [Google Scholar]
- Mehrgardt S, Mellert V. Transformation characteristics of the external human ear. J Acoust Soc Am. 1977 Jun;61(6):1567–1576. doi: 10.1121/1.381470. [DOI] [PubMed] [Google Scholar]
- Moiseff A. Binaural disparity cues available to the barn owl for sound localization. J Comp Physiol [A] 1989 Feb;164(5):629–636. doi: 10.1007/BF00614505. Erratum in: J Comp Physiol [A] 1989 Apr;165(1):138. [DOI] [PubMed] [Google Scholar]
- Moore DR, Hutchings ME, King AJ, Kowalchuk NE. Auditory brainstem of the ferret: Some effects of rearing with a unilateral earplug on the cochlea, cochlea nucleus, and projections to the inferior colliculus. J Neurosci. 1989;9:1213–1222. doi: 10.1523/JNEUROSCI.09-04-01213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Hutchings ME, Meyer SE. Binaural masking level differences in children with a history of otitis media. Audiology. 1991;30:91–101. doi: 10.3109/00206099109072874. [DOI] [PubMed] [Google Scholar]
- Moore DR, Hine JE, Jiang ZD, Matsuda H, Parsons CH, King AJ. Conductive hearing loss produces a reversible binaural hearing impairment. J Neurosci. 1999;19:8704–8711. doi: 10.1523/JNEUROSCI.19-19-08704.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Hartley DE, Hogan SC. Effects of otitis media with effusion (OME) on central auditory function. Int J Pediatr Otorhinolaryngol. 2003 Dec;67 Suppl 1:S63–S67. doi: 10.1016/j.ijporl.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Musicant AD, Butler RA. The psychophysical basis of monaural localization. Hear Res. 1984;14:185–190. doi: 10.1016/0378-5955(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Oldfield SR, Parker SP. Acuity of sound localisation: a topography of auditory space. III. Monaural hearing conditions. Perception. 1986;15(1):67–81. doi: 10.1068/p150067. [DOI] [PubMed] [Google Scholar]
- Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–333. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- Parsons CH, Lanyon RG, Schnupp JWH, King AJ. Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets. J. Neurophysiol. 1999;82:2294–2309. doi: 10.1152/jn.1999.82.5.2294. [DOI] [PubMed] [Google Scholar]
- Patterson JH, Gautier IML, Carrier M, Curd DL, Hargett CE. Attenuation produced by foam earplugs worn by chinchilla. 1991 USAARL Report No 91-16. [Google Scholar]
- Pillsbury HC, Grose JH, Hall JW. Otitis media with effusion in children. Binaural hearing before and after corrective surgery. Arch Otolaryngol Head Neck Surg. 1991 Jul;117(7):718–723. doi: 10.1001/archotol.1991.01870190030008. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65(5):718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hear Res. 2004;195:103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Reale RA, Brugge JF, Chan JCK. Maps of auditory cortex in cats reared after unilateral cochlear ablation in the neonatal period. Dev Brain Res. 1987;34:281–290. doi: 10.1016/0165-3806(87)90215-x. [DOI] [PubMed] [Google Scholar]
- Rife DD, Vanderkooy J. Transfer-function measurement with maximum-length sequences. J Aud Eng Soc. 1989;37(6):419–444. [Google Scholar]
- Roth GL, Kochhar RK, Hind JE. Interaural time differences: Implications regarding the neurophysiology of sound localization. J Acoust Soc Am. 1980;68(6):1643–1651. doi: 10.1121/1.385196. [DOI] [PubMed] [Google Scholar]
- Schnupp JWH, King AJ, Carlile S. Altered spectral localization cues disrupt the development of the auditory space map in the superior colliculus of the ferret. J. Neurophysiol. 1998;79:1053–1069. doi: 10.1152/jn.1998.79.2.1053. [DOI] [PubMed] [Google Scholar]
- Schoonhoven R. Responses from the cochlea. In: Burkhard RF, Don M, Eggermont JJ, editors. Auditory evoked potentials. Lippincott Williams & Wilkins; 2007. pp. 180–198. [Google Scholar]
- Shaw EA. Transformation of sound pressure level from the free field to the eardrum in the horizontal plane. J Acoust Soc Am. 1974 Dec;56(6):1848–1861. doi: 10.1121/1.1903522. [DOI] [PubMed] [Google Scholar]
- Shekelle P, et al. Evidence Report/Technology Assessment No. 55. Rockville, MD: Agency for Healthcare Research and Quality; 2003. May, Diagnosis, Natural History, and Late Effects of Otitis Media with Effusion. AHRQ Publication No. 03-E023. [Google Scholar]
- Silva PA, Chalmers D, Stewart I. Some Audiological, Psychological, Educational and Behavioral Characteristics Of Children With Bilateral Otitis Media With Effusion: A Longitudinal Study. J Learn Disabil. 1986;19(3):165–169. doi: 10.1177/002221948601900307. [DOI] [PubMed] [Google Scholar]
- Silverman MS, Clopton BM. Plasticity of binaural interaction. I. Effect of early auditory deprivation. J. Neurophysiol. 1977;40:1266–1274. doi: 10.1152/jn.1977.40.6.1266. [DOI] [PubMed] [Google Scholar]
- Slattery WH, III, Middlebrooks JC. Monaural sound localization: Acute versus chronic unilateral impairment. Hear Res. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Sterbing SJ, Hartung K, Hoffmann KP. Spatial tuning to virtual sounds in the inferior colliculus of the guinea pig. J Neurophysiol. 2003;90(4):2648–2659. doi: 10.1152/jn.00348.2003. [DOI] [PubMed] [Google Scholar]
- Teele DW, Klein JO, Chase C, et al. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J Infect Dis. 1990 Sep;162(3):685–694. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Koka K. Postnatal development of sound pressure transformations by the head and pinnae of the cat: Binaural characteristics. J Acoust Soc Am. 2009;126(6):3125–3136. doi: 10.1121/1.3257234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ. The development of sound localization mechanisms. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; 2010. pp. 262–282. [Google Scholar]
- Torres AI, Backous DD. Clinical assessment and surgical management of conductive hearing loss. In: Flint P, Haughey B, Lund V, Niparko JK, Richardson MA, Robbbins KT, Thomas JR, editors. Cummings Otolaryngology: Head and Neck Surgery Review. Baltimore: Mosby; 2010. Ch 143. [Google Scholar]
- Tucci DL, Cant NB, Durham D. Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hear Res. 2001;155:124–132. doi: 10.1016/s0378-5955(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Sound localization under perturbed binaural hearing. J Neurophysiol. 2007;97:715–726. doi: 10.1152/jn.00260.2006. [DOI] [PubMed] [Google Scholar]
- Volman SF, Konishi M. Spatial Selectivity and Binaural Responses in the Inferior Colliculus of the Great Horned Owl. J Neuroscience. 1989;9(9):3083–3098. doi: 10.1523/JNEUROSCI.09-09-03083.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrettakos PA, Dear SP, Saunders JC. Middle ear structure in the chinchilla: A quantitative study. Am J Otolaryng. 1988;9:58–67. doi: 10.1016/s0196-0709(88)80009-7. [DOI] [PubMed] [Google Scholar]
- Walger M, Laska M, Schneider I, Diekmann H, von Wedel H. Maturation of auditory evoked potentials in young guinea pigs with binaural conductive hearing loss. Eur Arch Otorhinolaryngol. 1993;250(6):362–365. doi: 10.1007/BF00188388. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M. Neonatal sound deprivation affects brain stem auditory nuclei. Arch Otolaryngol. 1977 Jul;103(7):392–396. doi: 10.1001/archotol.1977.00780240050006. [DOI] [PubMed] [Google Scholar]
- Webster DB. Conductive Hearing loss affects the growth of the cochlear nuclei over an extended period of time. Hear Res. 1988;32:185–192. doi: 10.1016/0378-5955(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Welsh LW, Welsh JJ, Healy MP. Effect of sound deprivation on central hearing. Laryngoscope. 1983;93:1569–1575. doi: 10.1288/00005537-198312000-00010. [DOI] [PubMed] [Google Scholar]
- Weiderhold ML, Zajtchuk JT, Vap JG, Paggi RE. Hearing loss in relation to physical properties of middle ear e!usions. Ann. Otol. Rhinol. Laryngol. 1980;89:185–189. doi: 10.1177/00034894800890s343. [DOI] [PubMed] [Google Scholar]
- Wever EG, Lawrence M. Physiological Acoustics. Princeton Univ. Press; 1954. p. 137. [Google Scholar]
- Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. J Acoust Soc Am. 1992 Mar;91(3):1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- Winskel H. The effects of an early history of otitis media on children’s language and literacy skill development. Br J Educ Psychol. 2006;76:727–744. doi: 10.1348/000709905X68312. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental Psychology. New York: H. Holt and Company; 1938. [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27(35):9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998 Nov;102(5):1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]