Abstract
Background
A relationship between excessive daytime sleepiness (EDS) and poor treatment adherence has been suspected but not confirmed. We hypothesized that medication adherence would be poorer in adults with heart failure (HF) and EDS and that cognitive status would be the mechanism of effect.
Methods
A sample of 280 adults with chronic HF was enrolled into a prospective cohort comparison study. We identified a cohort with EDS and a control group without EDS and further divided both groups into those with and without mild cognitive decline. Data on medication adherence was obtained at baseline, 3- and 6-months using the Basel Assessment of Adherence Scale (BAASIS). Regression analysis was used to clarify the contribution of EDS and cognition to medication adherence and to assess relationships over six months after adjusting for age, enrollment site, gender, race, functional class, depression, and premorbid intellect.
Results
At baseline, 62% of subjects were nonadherent to their medication regime. Nonadherence was significantly more common in those with EDS, regardless of cognitive status (p=0.035). The odds of nonadherence increased by 11% for each unit increase in EDS (AOR=1.11, 95% CI=1.05–1.19, p=0.001). In longitudinal models there was a 10% increase in the odds of nonadherence for each unit increase in EDS (p=0.008). The only cognition measure significantly associated with medication adherence was attention (p=0.047).
Conclusion
Adults with HF and EDS are more likely to have problems adhering to their medication regimen than those without EDS, regardless of their cognitive status. Identifying and correcting factors that interfere with sleep may improve medication adherence.
Keywords: heart failure, sleep, excessive daytime sleepiness, cognition, vigilance, patient compliance, self-care
Introduction
Poor self-care—encompassing treatment adherence, symptom monitoring, and management of symptoms—remains the most common reason for unplanned hospitalization in adults with heart failure (HF).(1–3) Medication adherence, in particular, is integral to controlling volume overload and symptoms, improving functioning and quality of life, and preventing acute decompensation in adults with HF.(4–6) Yet, self-care remains poor in HF patients.(7, 8) Initiatives to improve HF self-care have had limited success,(9) suggesting that important operative factors have not been identified and considered.
A 2006 Institute of Medicine report called national attention to the devastating effects of daytime sleepiness, including potential problems with treatment adherence.(10) Excessive daytime sleepiness (EDS) is the term used to describe decreased attention and increased sleep propensity during wakeful states.(11, 12) Sleep propensity reflects the interaction of homeostatic mechanisms that regulate sleep intensity, and circadian rhythm, which regulates the timing of sleep.(13, 14) The relative strength of the sleep and wake drives reflects chronobiological and environmental factors such as physical activity, which stimulate arousal.(15) In adults with HF, contributors to EDS include older age, sleep disordered breathing, insomnia, depression, and polypharmacy, often with medications that cause somnolence as a primary side effect.(16–19)
Critical, yet unexplored, is the contribution of EDS to poor self-care in HF patients. We hypothesized that EDS may impair self-care through its effects on cognition.(20, 21) Inadequate sleep has been shown to impair information processing, memory, vigilance, judgment, motivation, and decision-making across a wide range of populations.(22–24) Most of these cognitive domains are also susceptible to decline in people with HF,(25, 26) 25% to 50% of whom have impaired cognition.(27) Therefore, the primary objective of this study was to determine if medication adherence differs in adults with HF and EDS compared to adults with HF but without EDS and to test cognition as the mechanism of the effect.
Methods
A prospective cohort comparison study was conducted to test the hypothesis that a cohort of adults with HF and EDS would, over time, experience more problems with medication adherence than a cohort with HF but without EDS. A consecutive sample of 280 adults with HF was enrolled from three outpatient settings in Philadelphia, Pennsylvania and Newark, Delaware. Data were collected between 2007 and 2010.
Inclusion criteria specified enrollment of adults with chronic Stage C HF confirmed based on echocardiographic and clinical evidence. Potential subjects were screened to discern their abilities to participate in the study (e.g., visual acuity sufficient to read study materials, hearing sufficient to engage in a dialogue, and English literacy sufficient to enable accurate cognition testing).(28) We included subjects with mild cognitive decline(29) as measured by the Telephone Interview of Cognitive Status (TICS).(30) The TICS is composed of 11 items, with a maximum score of 41; high scores indicate less impairment. A score in the range of 21–25 suggests mild cognitive decline. We sought to enroll individuals with mild cognitive decline so anyone with a TICS score ≥ 24 was included.
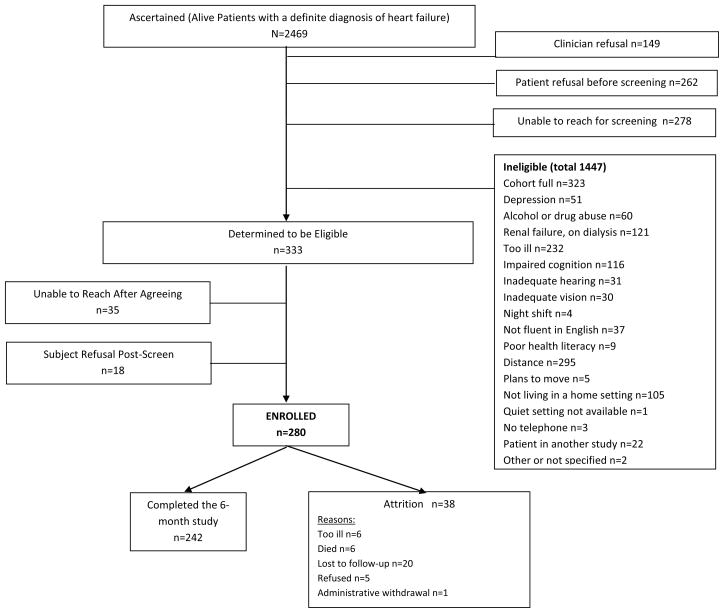
Otherwise eligible individuals were excluded if they lived in a long term care setting where self-care was not a reasonable expectation or worked nights or rotating shifts, or if they had renal failure requiring dialysis, an imminently terminal illness, plans to move out of the area, a history of serious drug or alcohol abuse within the past year, or major depression (Figure 1). Major depression was identified in two ways: patients described in the medical record as having major depressive illness were not contacted. In addition, everyone was screened with the 9-item Patient Health Questionnaire (PHQ-9).(31) We excluded anyone reporting 5 or more of the 9 symptoms more than half the days in the past 2 weeks; 1 of the symptoms had to be depressed mood or anhedonia. For those who passed screening, we continued to use a subset of data from the PHQ-9 (i.e., the PHQ-2) at each testing interval as an indicator of mild depressive symptoms, as described below. Most of the data were collected during home visits by trained research assistants. Clinical information was abstracted from the medical record by registered nurses. Sleep disordered breathing was not an exclusion criterion because our focus was on identifying the effect of EDS on self-care, regardless of the cause.
Figure 1.
Flow of Participants Through the Study
A total of 2469 adults with HF were considered for enrollment, but only 333 were eligible to participate. Major reasons for ineligibility were distance, illness severity, renal dialysis, dementia or prior stroke, or living in an institutional setting. Some of these issues reflect the university referral center where most patients were recruited. Further, because this was a cohort study, as cohorts were saturated, patients eligibility focused on criteria related to EDS and cognitive status, which eliminated 323 otherwise eligible individuals. A total of 280 individuals were enrolled and followed for six months. Attrition was only 13.6% (n=38) over the six months of follow-up with data on EDS, cognition, and medication adherence collected in person at baseline, 3- and 6-months..
Using data collected at baseline, we identified a cohort with EDS and a control group without EDS using the Epworth Sleepiness Scale. Both groups were further divided into those with and without mild cognitive decline, as described below.
Measurement
Daytime Sleepiness
Excessive daytime sleepiness was measured with the Epworth Sleepiness Scale (ESS), a validated research tool considered the gold-standard measure for the assessment of daytime sleepiness.(32) The ESS is an 8-item self-report scale on which respondents rated the likelihood of falling asleep in boring situations such as riding as a passenger in a car. Test-retest reliability (r = 0.82) and internal consistency (α = 0.88) have been established.(33) In this study, the alpha coefficient was .78. Responses on a 4-point Likert scale are summed, with higher scores indicating greater sleepiness. Others have shown that even HF patients with sleep disordered breathing report relatively low levels of EDS.(34) Therefore, we used a cut-point of more than 6 for the determination of EDS on the advice of the instrument author (Johns, personal communication, 2007).
Cognitive Decline
Sleep loss is known to affect simple and complex attention, processing speed, working memory, short-term memory, reasoning and crystallized cognitive ability, with simple attention most sensitive to sleep deprivation.(23) A neuropsychological test battery measuring these major cognitive domains was administered to all participants. The battery included the Psychomotor Vigilance Task (PVT)(35) (simple attention), the Trail Making Test B(36) (complex attention), the Digit Symbol Substitution Test(37) (processing speed), the Probed Recall Memory Task(38) (working memory), the Letter Number Sequencing test(37) (short-term memory), and crystallized cognitive ability or premorbid intellect (American National Adult Reading Test (ANART) (39) (Table 1). The number of tests on which subjects scored below their age-based norm was used as the measure of cognitive status.(37) Specifically, anyone scoring < 1.5 standard deviations on two or more of the paper-and-pencil cognition tests was judged to have a mild cognitive decline. The PVT is not influenced by practice, aptitude, or education, although it is influenced by age and gender.(40) Therefore, age and gender were used as covariates in the analysis, as discussed below. The ANART also was used as a covariate in analysis.(39)
Table 1.
Description of Each Measure in the Neuropsychological Battery
| Cognitive Domain | Neuropsychological Test | Brief Description of the Task |
|---|---|---|
| Simple attention | Psychomotor vigilance task (PVT) | Subjects press a button in response to a series of red digits “000” in an automated light emitting diode (LED) counter window of a small, portable device. The signals are presented at random intervals over a 10-minute period. The device flashes until the response button is pushed; the speed of response is measured. The subject makes an effort to keep response times as short as possible, but not to press before the stimulus appears. Lapses, defined as the number of times the subject failed to respond to the signal or failed to respond in a timely manner, is used to indicate problems with attention and psychomotor speed. Transformed lapses categorized at a cut-point of 4.69 (≥ 4.69 lapses [impaired] vs. < 4.69 lapses [not impaired]) were used in cohort assignment. |
| Complex attention | Trail Making Test B | Subjects connect randomly numbered and ordered circles in serial order by alternating as fast as possible between numbers and letters. The test emphasizes flexibility in organizing stimulus material, keeping two sequences in mind simultaneously, and accomplishing the task quickly. Time to completion is used in analysis. Age-specific norms were used to classify subjects as performing appropriately for their age [not impaired] or not [impaired].(36) |
| Processing speed | Digit Symbol Substitution task | Subjects were provided a series of numbers with blank boxes immediately below them. A key at the top shows each unique number paired with a different nonsense symbol. Subjects transcribe the appropriate symbol for each number in the lower part of the page. Speed is enhanced if the symbol that goes with each letter is recalled. The number correctly completed in 120 seconds is measured. Age-specific norms were used to classified subjects as performing appropriately for their age [not impaired] or not [impaired].(37) |
| Working memory | Probed Memory Recall | Subjects were presented with a list of 4 word pairs, which they studied for 30 seconds. Testing on other topics continued for 10 minutes. Then a list of 1 word in each pair (in a different order) was presented. The task was to recall all 4 of the paired words within 1 minute. The number of words recalled was categorized at a cut-point of 1 (≤ 1 word recalled [impaired] vs. >1 word [not impaired]) was used in cohort formation. |
| Short-term memory | Letter Number Sequencing subtest | Subjects reorder sequentially a series of numbers and letters initially presented orally in a specified random order. The subject must first remember the numbers and letters and then reorganize them into ascending or alphabetical order. Slowed processing speed, inability to remember earlier responses, and reduced capacity to ignore irrelevant information all contribute to decrements in performance. An age-adjusted scaled score derived from the number of correctly recalled sequences was used in the analysis.(37) |
| Crystallized cognitive ability | American National Adult Reading Test (ANART) | Subjects read a list of 50 phonetically irregular words aloud. The number of words pronounced correctly is used as the score.(39) The ANART was used as a covariate in analyses. |
Medication Adherence
Medication adherence was assessed with the Basel Assessment of Adherence Scale (BAASIS), a structured interview assessing general medication adherence over the past month.(41) This self-report measure was chosen over others available because it measures both taking and timing dimensions of the medication regimen as well as the occurrence of drug holidays; most other self-report surveys focus on medication taking behavior only. The four dichotomous items are easy to score and interpret. A positive answer on any of the questions classifies a patient as nonadherent with the medication regimen. This broad definition of nonadherence compensates for the underreporting of nonadherence common in self-reports.(42) Validity has been established in HIV infected persons (43) and renal transplantation (Schäfer et al., work in progress) patients.
Sociodemographic Characteristics
Sociodemographic characteristics were self-reported. Education was categorized as less than high school, high school, or at least some college. Subjects were asked to rate their financial income as: 1) comfortable, have more than enough to make ends meet; 2) have enough to make ends meet; or 3) do not have enough to make ends meet.
Most clinical information (e.g., comorbid illnesses, HF type and duration, left ventricular ejection fraction) was gathered from the medical record by a registered nurse. Comorbidity was scored using the Charlson Index;(44) higher scores indicate more comorbid illnesses.(44) In addition to the score derived from the Charlson Index, we added the number of major comorbid conditions identified in the medical record. Information on New York Heart Association (NYHA) functional class was gathered by trained research assistants using a structured interview.(45) A single board-certified cardiologist scored functional class in every subject. Length of time with HF was calculated after a historical search of each record to identify the first mention of HF.
A physician-diagnosis of sleep disordered breathing (SDB) based on polysomnography was obtained from the medical record at the time of enrollment. If no documentation of recent sleep testing was found in the medical record, sleep was assessed in the home using an unattended sleep study device, the Embletta (Medcare, Buffalo, NY). Individuals with an apnea hypopnea index ≥ 5 were classified as having SDB.(46)
Depressive symptoms were measured over the course of the study using the PHQ-2.(31) The PHQ-2 was used rather than the PHQ-9 to avoid symptoms related to EDS. Symptoms of insomnia were ascertained using the Pittsburgh Sleep Quality Index.(47) Scores on subscales for quality, latency, duration, and efficiency were added; higher scores indicate more insomnia. Perceived or self-reported health status was measured with a single item from the Medical Outcomes Study. Although commonly unrelated to objective ratings of health by a physician, others have documented that HF patients who perceived their health to be poor were more likely to be hospitalized over a 12 week period.(48) Still others have found that perceived health was an independent predictor of cardiovascular mortality and quality of life in older adults with HF.(49, 50)
Analysis
Demographic differences between the sleep and cognition cohorts were tested with Chi-square or ANOVA. The primary analysis used the sleep and cognition cohorts at baseline. Differences in medication adherence between the sleep and cognition cohorts were assessed at baseline and at each point longitudinally (3- and 6-month follow-up points). For baseline associations, chi square analysis followed by logistic regression analysis was used to clarify the contribution of EDS and mild cognitive decline to medication adherence. Longitudinally, logistic mixed effects regression models were used to assess the relationships over time.
Secondary analyses examined the Epworth Sleepiness Scale score along with each of the independent cognition test scores. Regression models were adjusted for age, enrollment site, gender, race, NYHA functional class, and premorbid intellect measured with the ANART. In addition, depression was adjusted in each analysis because depression is an important contributor to EDS (51). All analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Demographic characteristics at baseline by EDS and cognition cohorts are shown in Table 2. The average subject tended to be a white (63%) male (64%) aged 62±12 years. Approximately half (54%) of the sample had SDB. The cohorts without mild cognitive decline tended to be younger (p<.001), have more white subjects (p=0.008), have a higher level of education (p=0.009), not be taking a diuretic (p=0.042), and to have higher ANART scores (p=0.001). A summary of scores on the various cognition tests are given in Table 3.
Table 2.
Baseline characteristics of the sample by sleep and cognition cohorts. Mean ± standard deviation or n (%) is reported.
| Total Sample (N=280) | +EDS +MCD (n=82) | +EDS no MCD (m=76) | no EDS +MCD (n=59) | no EDS no MCD (n=63) | p-value1 | |
|---|---|---|---|---|---|---|
| Age (years) | 61.9±12.5 | 62.2±12.9 | 59.6±11.8 | 67.3±11.9 | 59.6±11.9 | 0.001 |
| Male | 180 (64.3) | 58 (70.7) | 41 (54.0) | 40 (67.8) | 41 (65.1) | 0.148 |
| Race/Ethnicity | 0.021 | |||||
| White | 175 (62.5) | 43 (52.4) | 54 (71.1) | 31 (52.5) | 47 (74.6) | |
| Black | 96 (34.3) | 36 (43.9) | 20 (26.3) | 24 (40.7) | 16 (25.4) | |
| Other2 | 9 (3.2) | 3 (3.7) | 2 (2.6) | 4 (6.8) | 0 (0.0) | |
| Education | 0.008 | |||||
| Less than high school | 27 (9.6) | 10 (12.2) | 4 (5.3) | 11 (18.6) | 2 (3.2) | |
| High school | 102 (36.4) | 31 (37.8) | 26 (34.2) | 26 (44.1) | 19 (30.2) | |
| At least some college | 151 (53.9) | 41 (50.0) | 46 (60.5) | 22 (37.3) | 42 (66.7) | |
| Smoking | 0.212 | |||||
| Current | 30 (10.7) | 12 (14.6) | 6 (7.9) | 9 (15.3) | 3 (4.8) | |
| Former | 155 (55.4) | 44 (53.7) | 38 (50.0) | 34 (57.6) | 39 (61.9) | |
| Never | 95 (33.9) | 26 (31.7) | 32 (42.1) | 16 (27.2) | 21 (33.3) | |
| Body mass index (BMI) | 31.0± 7.9 | 30.5±6.9 | 31.6±9.1 | 30.4±7.6 | 31.3±8.1 | 0.751 |
| Perceived health | <.001 | |||||
| Excellent/Very Good | 34 (12.1) | 28 (34.2) | 48 (63.2) | 25 (42.4) | 27 (42.9) | |
| Good | 94 (33.6) | 33 (40.2) | 23 (30.3) | 28 (47.5) | 31 (49.2) | |
| Fair/Poor | 152 (54.3) | 21 (25.6) | 5 (6.6) | 6 (10.2) | 5 (7.9) | |
| Months with heart failure | 73.2± 71.0 | 65.8± 57.3 | 65.4± 60.6 | 68.5± 65.7 | 98.6± 96.7 | 0.172 |
| Charlson Comorbidity Index score | 2.8± 1.7 | 3.2±1.9 | 2.4±1.5 | 3.0±1.7 | 2.4±1.4 | 0.006 |
| Number of comorbid illnesses | 3.2±2.1 | 3.5±2.1 | 2.5±2.1 | 3.9±2.2 | 2.8±1.7 | 0.000 |
| Atrial fibrillation | 93(33.3) | 25 (31.7) | 18 (24.0) | 25 (42.4) | 24 (38.1) | 0.119 |
| Heart failure type3 | 0.959 | |||||
| • systolic/mixed | 226(81.0) | 67 (81.7) | 60 (79.0) | 48 (81.4) | 51 (82.3) | |
| • diastolic | 53(19.0) | 15 (29.5) | 16 (21.1) | 11 (18.6) | 11 (17.7) | |
| Heart failure etiology3 | 0.02 | |||||
| • ischemic | 102 (36.6) | 30 (36.6) | 24 (32.0) | 31 (52.5) | 17 (27.0) | |
| • nonischemic | 177(63.4) | 52 (63.4) | 51 (68.0) | 28 (47.5) | 46 (73.0) | |
| NYHA functional class | 0.039 | |||||
| • Class I & II | 66 (23.6) | 12 (14.6) | 18 (23.7) | 19 (32.2) | 17 (27.0) | |
| • Class III | 164 (58.6) | 47 (57.3) | 45 (59.2) | 32 (54.2) | 40 (63.5) | |
| • Class IV | 50 (17.9) | 23 (28.1) | 13 (17.1) | 8 (13.6) | 6 (9.5) | |
| Left ventricular ejection fraction | 35.4 ± 16.9 | 36.6 ± 18.0 | 34.5 ± 15.2 | 32.5 ± 17.7 | 37.6 ± 16.9 | 0.34 |
| Sleep Disordered Breathing (SDB) | 153 (55) | 42 (51.2) | 46 (60.5) | 32 (54.2) | 33 (52.4) | 0.66 |
| Total number of prescription medications taken | 9.8 ± 3.9 | 10.4 ± 3.9 | 9.4 ± 4.1 | 10.1 ± 4.6 | 9.3 ± 3.8 | 0.24 |
| Angiotensin-Converting Enzyme (ACE) inhibitor | 162 (57.9) | 49 (59.8) | 50 (65.8) | 31 (52.5) | 32 (50.8) | 0.256 |
| Angiotensin II Receptor Blocker (ARB) | 83 (29.6) | 24 (29,3) | 17 (22.4) | 20 (33.9) | 22 (34.9) | 0.349 |
| Diuretic (not including spironolactone/eplerenone) | 226 (80.7) | 68 (82.9) | 55 (72.4) | 54 (91.5) | 49 (77.8) | 0.038 |
| Beta-Blocker | 259 (92.5) | 76 (92.7) | 70 (32.1) | 55 (93.2) | 58 (92.1) | 0.994 |
| Depression measured with the Patient Health Questionnaire (PHQ-2) | 0.82 ± 1.0 | 1.11±1.3 | 0.80±1.0 | 0.71±0.9 | 0.57±0.8 | 0.048 |
| Insomnia symptoms | 4.5 ± 3.1 | 5.3 ± 3.3 | 4.2 ± 2.6 | 4.6 ± 3.3 | 3.6 ± 2.8 | 0.009 |
| ANART Score | 29.9 ± 11.8 | 26.2± 12.5 | 32.9± 11.7 | 28.5± 11.9 | 32.0± 9.4 | 0.001 |
Comparison of groups via ANOVA, Kruskal-Wallis or Chi-square tests.
Other category combined with Black for analysis.
n=279
ANART = American National Adult Reading Test; EDS = excessive daytime sleepiness, MCD = mild cognitive decline, NYHA = New York Heart Association;
Table 3.
Baseline ESS and cognitive test score, overall and by EDS and mild cognitive decline groups. Mean ± standard deviation or n (%) is reported.
| Total Sample (n=280) | +EDS +MCD (n=82) | +EDS no MCD (n=76) | no EDS + MCD (n=59) | no EDS no MCD (n=63) | |
|---|---|---|---|---|---|
| Epworth Total Score (< 11 normal) | 7.0±4.6 | 10.8±3.9 | 9.2±3.1 | 3.0±1.5 | 2.9±1.8 |
| Pvt Cnt Trans1 (<4.69 normal) | 5.0±3.5 | 7.0±4.1 | 3.1±2.0 | 6.3±2.7 | 3.4±2.6 |
| Digit Symbol Substitution Test2 (Age-specific norms) | 53.4±17.5 | 47.1±15.4 | 62.6±15.3 | 44.4±17.9 | 59.1±14.8 |
| Probed Memory Recall task2 (>1 of 4 words normal) | 2.0±1.2 | 1.5±1.2 | 2.7±0.9 | 1.2±1.1 | 2.6±0.9 |
| Letter Number Sequencing Subtest3 (Age-specific norms) | 1.0±0.2 | 1.07±0.4 | 1.0±<.01 | 1.0±<.01 | 1.03±0.3 |
| Trial Making Test B2 (Age-specific norms) | 111.2±59.1 | 126.7±65.5 | 89.9±34.5 | 132.5±72.0 | 96.8±48.0 |
n=274,
n=278,
n=279
EDS = excessive daytime sleepiness; MCD = mild cognitive decline
At baseline, 62% (n=171) of subjects were found to be nonadherent to their HF medication regime (Table 4). Specific issues reported were taking medicines 2 or more hours late (timing adherence) (46.8%), forgetting to take medicines (dosing adherence) (35.5%), reducing the amount taken (taking adherence) (7.2%), and drug holidays or skipping medicine doses altogether (6.8%). Medication nonadherence was significantly more common in those with EDS, regardless of cognitive status (p=0.033) even after adjustment for important covariates (Figure 2). Compared to subjects without EDS or cognitive decline, subjects with EDS and mild cognitive decline were 2.5 times more likely to be nonadherent (AOR=2.52, 95% CI=1.18–5.38, p=0.017), while the group with EDS but without cognitive decline was twice as likely to be nonadherent (AOR=2.36, 95% CI=1.12–4.99, p=0.025). Secondary models using the Epworth Sleepiness Scale score and the individual cognition test scores showed that the odds of nonadherence increased by 11% for each unit increase in EDS (AOR=1.11, 95% CI=1.04–1.19, p=0.001) while cognitive status was not significantly associated with medication adherence (p=0.752).
Table 4.
Baseline medication adherence by sleep and cognition cohorts.
| +EDS, +MCD | +EDS, no MCD | no EDS, +MCD | no EDS, no MCD | |
|---|---|---|---|---|
| Nonadherent to medications (61.4%) | 56 (68.3%) | 56 (73.7%) | 29 (49.1%) | 31 (49.2%) |
| Adherent to medications (38.6%) | 26 (31.7%) | 20 (26.3%) | 30 (50.9%) | 32 (50.8%) |
EDS = excessive daytime sleepiness
MCD = mild cognitive decline
Figure 2. Baseline medication adherence by EDS and mild cognitive decline cohorts.
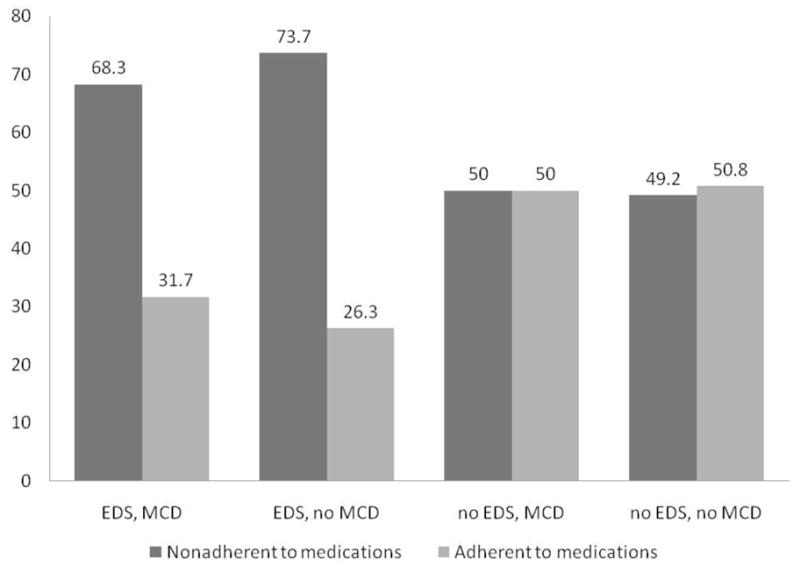
Illustration of the finding that reported medication nonadherence was significantly higher in the two cohorts with excessive daytime sleepiness, regardless of whether or not they had a mild cognitive decline.
EDS = excessive daytime sleepiness
MCD = mild cognitive decline
Mixed effect logistic regression models were then used to assess the changes in medication adherence over the entire course of the study (baseline to 6-months in three month intervals). In adjusted models (Table 5), the EDS groups were almost twice as likely to be nonadherent compared to the cohort without either EDS or a mild cognitive decline; subjects with EDS and mild cognitive decline were 1.6 times more likely to be nonadherent (AOR=1.61, 95% CI=1.03–2.50, p=0.037), while the group with EDS but without cognitive decline was twice as likely to be nonadherent (AOR=1.72, 95% CI=1.11–2.70, p=0.014).
Table 5.
Final mixed effects logistic regression model for longitudinal medication adherence. Odds of being adherent are shown.
| Variable | AOR1 | 95% CI | p-value |
|---|---|---|---|
| Sleep and cognition cohorts | |||
| no EDS, no MCD | (ref) | ||
| no EDS, + MCD | 1.01 | 0.63–1.61 | 0.975 |
| +EDS, no MCD | 0.58 | 0.37–0.90 | 0.014 |
| +EDS, + MCD | 0.62 | 0.40–0.97 | 0.037 |
| Time | 0.99 | 0.93–1.05 | 0.824 |
| NYHA functional class | |||
| NYHA class I or II | (ref) | ||
| NYHA class III | 1.25 | 0.86–1.82 | 0.234 |
| NYHA class IV | 1.27 | 0.76–2.12 | 0.364 |
| PHQ2 Depression Score | 0.91 | 0.79–1.04 | 0.160 |
Model also adjusted for site, age, gender, and race. (ref=reference group for AOR).
EDS = excessive daytime sleepiness
MCD = mild cognitive decline
Secondary models (Table 6) using the Epworth Sleepiness Scale score found a 9% increase in the odds of nonadherence for each unit increase in EDS (p=0.001). Of the individual cognition measures, only the PVT was significantly associated with medication adherence, with a 9% increase in the odds of nonadherence for each unit increase in the number of transformed lapses detected over a 10-minute testing interval (p=0.024).
Table 6.
Mixed effects logistic regression models for longitudinal medication adherence based on individual cognitive tests. Odds of being adherent are shown.
| Variable | AOR1 | 95% CI | p-value |
|---|---|---|---|
| Model 1: | |||
| Epworth Total Score | 0.93 | 0.89–0.97 | 0.001 |
| PVT Transformed Lapses | 0.94 | 0.89–0.99 | 0.024 |
| Model 2: | |||
| Epworth Total Score | 0.92 | 0.88–0.95 | <.001 |
| Digit Symbol Substitution Test | 0.99 | 0.98–1.01 | 0.337 |
| Model 3: | |||
| Epworth Total Score | 0.92 | 0.89–0.96 | <.001 |
| Probed Memory Recall task | 1.01 | 0.88–1.17 | 0.837 |
| Model 4: | |||
| Epworth Total Score | 0.92 | 0.89–0.96 | <.001 |
| Letter Number Sequencing Subtest | 0.75 | 0.39–1.44 | 0.381 |
| Model 5: | |||
| Epworth Total Score | 0.92 | 0.88–0.96 | <.001 |
| Trial Making Test B | 1.00 | 1.00-1.00 | 0.757 |
Models also adjusted for site, age, gender, race, ANART score, NYHA class, PHQ2 depression score, diuretic use, and visit time.
Discussion
The major finding of this study was that self-reported medication nonadherence is significantly higher in adults with HF who have even relatively mild rates of EDS (using a cut-point of ≥ 6 rather than the usual cut-point of ≥ 11 on the Epworth Sleepiness Scale). The other major finding was that the relationship between EDS and medication nonadherence may be explained by selective attention. That is, adults with HF and EDS appear to be missing medication doses or taking their medicines late because of poor attention and a lack of vigilance.
The link between EDS and medication nonadherence is compelling. Most HF investigators cite medication nonadherence rates between 40% and 60%,(52–59) which is consistent with the rate of nonadherence identified in this study. Medication nonadherence has been shown to be associated with hospitalization and death in adults with HF. For example, Murray and colleagues found that hospitalization was higher in HF patients low in medication taking adherence compared to patients who were adherent with taking their medications.(60) Wu et al documented that medication adherence predicted event-free survival before and after controlling for age, gender, ejection fraction, NYHA class, angiotensin-converting enzyme inhibitor use, and beta-blocker use.(61) The findings of these authors demonstrate the importance of medication adherence, while the results of the current study provide a focus for interventions that may improve it.
In a recent review of the factors associated with medication nonadherence, forgetfulness was one of the most common barriers to adherence identified.(62) This result is supported and explained by our finding that attention may be the mechanism by which EDS influences medication nonadherence. Lim and Dinges argue that the ability to sustain attention is fundamental to all other aspects of cognition and that vigilance is the process most affected by sleep deprivation.(63) If alertness and sustained attention are necessary for successful information processing, memory, judgment, and decision-making, HF patients with EDS may not be able to sustain the attention needed to remember to take their medicines with any regularity.
Only one prior study of sleepiness and medication adherence was located. In a study of 173 HIV infected women, Phillips et al.(64) found a significant difference in adherence to highly active antiretroviral therapy between good sleepers and poor sleepers. Elements of cognition such as vigilance were not tested as mediators in this study. They did find, however, that depression mediated the relationship between sleep and medication adherence, which was not surprising in this population. The women were single, low income, African-American women, 39 years of age, on average, living in a rural community. In our HF population, both depression and impaired cognition are common issues and depression was adjusted in our analyses. But, future research should explore the contribution of depression to the relationship between EDS and adherence.
Strengths of the current study include a large sample enrolled from three diverse sites and the relatively large sample of women and minorities. The primary limitation of this study is the self-report method of assessing medication adherence, although self-report is generally considered a central component of adherence assessment. It is possible that subjects with a mild cognitive decline may not remember if they took their medications. However, inaccuracies in the self-report approach most likely would under report the occurrence of skipped doses and late administrations. Thus, true medication nonadherence is most likely even higher than the self-reported rate presented here. In addition, demographic differences in age, education, race, and premorbid intellect between the cohorts is a limitation that is difficult to account for statistically and complicates our interpretation of the cognitive tests. Selection bias is possible also, as many patients were ineligible for inclusion in the study. Further, it is plausible that subjects with cognitive decline may have been adherent to the medication regimen because a caregiver assured adherence. This possibility was not accounted for in the analysis.
Further research is needed to describe the factors other than sleep quality influencing simple attention or vigilance in this population; it may be that these factors could be amenable to intervention. It would also be useful to test an association between EDS and other forms of self-care prescribed for adults with HF. It may be that EDS is also associated with failure to comply with daily weighing, a sodium restricted diet, and the response to symptoms when they occur. Research is also needed to describe how EDS changes over time in adults with HF. It has already been established that persons with HF do not experience as much EDS as other adults in the same age group.(34) However, further research is needed to explain this observation.
In summary, we found that even a relatively low level of EDS is associated with significantly more self-reported medication nonadherence in adults with HF. An inability to maintain vigilance and pay attention may be the mechanism responsible for the link between EDS and medication nonadherence in adults with HF.
Acknowledgments
This work was funded by a grant from the National Heart, Lung & Blood Institute (RO1 HL084394-01A1) and by the Philadelphia Veterans Affairs Medical Center, VISN 4 Mental Illness Research, Education, and Clinical Center (MIREC).
The authors gratefully acknowledge Thomas A. Gillespie, MD, FACC for scoring the New York Heart Association (NYHA) interviews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barbara Riegel, University of Pennsylvania School of Nursing.
Stephen T. Moelter, University of the Sciences in Philadelphia.
Sarah J. Ratcliffe, University of Pennsylvania School of Medicine.
Susan J. Pressler, University of Michigan School of Nursing.
Sabina De Geest, Institute of Nursing Science, University of Basel, Switzerland & Center of Health Services and Nursing Research, Katholieke Universiteit Leuven (Belgium).
Sheryl Potashnik, University of Pennsylvania School of Nursing.
Desiree Fleck, Hospital of the University of Pennsylvania.
Daohang Sha, School of Medicine, University of Pennsylvania.
Steven L. Sayers, University of Pennsylvania School of Medicine and Philadelphia Veterans Affairs Medical Center.
William Weintraub, Director of Christiana Care Center for Outcomes Research, Christiana Care Health System, Newark, DE.
Terri E. Weaver, College of Nursing, University of Illinois at Chicago.
Lee R. Goldberg, University of Pennsylvania School of Medicine.
References
- 1.Welsh JD, Heiser RM, Schooler MP, Brockopp DY, Parshall MB, Cassidy KB, et al. Characteristics and treatment of patients with heart failure in the emergency department. Journal of Emergency Nursing. 2002;28(2):126–31. doi: 10.1067/men.2002.123074. [DOI] [PubMed] [Google Scholar]
- 2.Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. American Journal of Medicine. 2003;114:625–30. doi: 10.1016/s0002-9343(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz CR, Rein SB, Leventhal H. A story of maladies, misconceptions and mishaps: effective management of heart failure. Social Science and Medicine. 2004 Feb;58(3):631–43. doi: 10.1016/s0277-9536(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett SJ, Cordes DK, Westmoreland G, Castro R, Donnelly E. Self-care strategies for symptom management in patients with chronic heart failure. Nurs Res. 2000 May–Jun;49(3):139–45. doi: 10.1097/00006199-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoes AW, Leufkens HG. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003 Oct;9(5):404–11. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 6.Hope CJ, Wu J, Tu W, Young J, Murray MD. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm. 2004 Oct 1;61(19):2043–9. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]
- 7.Gary R. Self-care practices in women with diastolic heart failure. Heart Lung. 2006 Jan–Feb;35(1):9–19. doi: 10.1016/j.hrtlng.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Riegel B, Driscoll A, Suwanno J, Moser DK, Lennie TA, Chung ML, et al. Heart failure self-care in developed and developing countries. J Card Fail. 2009 Aug;15(6):508–16. doi: 10.1016/j.cardfail.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler DM, Everett W. Thinking outside the pillbox--medication adherence as a priority for health care reform. N Engl J Med. 2010 Apr 29;362(17):1553–5. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Institute of Medicine; 2006. [PubMed] [Google Scholar]
- 11.Young TB. Epidemiology of daytime sleepiness: definitions, symptomatology, and prevalence. Journal of Clinical Psychiatry. 2004;65( Suppl 16):12–6. [PubMed] [Google Scholar]
- 12.Ohayon MM. From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev. 2008 Apr;12(2):129–41. doi: 10.1016/j.smrv.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004 Mar;75(3 Suppl):A37–43. [PubMed] [Google Scholar]
- 14.Achermann P, Borbely AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol Cybern. 1994;71(2):115–21. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- 15.Cluydts R, De Valck E, Verstraeten E, Theys P. Daytime sleepiness and its evaluation. Sleep Med Rev. 2002 Apr;6(2):83–96. doi: 10.1053/smrv.2002.0191. [DOI] [PubMed] [Google Scholar]
- 16.Redeker NS. Sleep disturbance in people with heart failure: implications for self-care. J Cardiovasc Nurs. 2008 May–Jun;23(3):231–8. doi: 10.1097/01.JCN.0000305094.20012.76. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008 Feb 15;4(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Rich MW. Management of heart failure in the elderly. Heart Failure Reviews. 2002;7(1):89–97. doi: 10.1023/a:1013706023974. [DOI] [PubMed] [Google Scholar]
- 19.Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003 Jan–Feb;65(1):119–28. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 20.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002 Mar;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 21.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. May;12(5):508–15. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 22.Bruck D, Pisani DL. The effects of sleep inertia on decision-making performance. J Sleep Res. 1999 Jun;8(2):95–103. doi: 10.1046/j.1365-2869.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 23.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010 May;136(3):375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinges DF. An overview of sleepiness and accidents. Journal of Sleep Research. 1995;4(S2):4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 25.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010 Mar–Apr;59(2):127–39. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoth KF, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol. 2008 Jun;21(2):65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- 27.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002–July 2007) J Cardiovasc Nurs. 2008 May–Jun;23(3):239–49. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 28.Chew LD, Bradley KA, Flum DR, Cornia PB, Koepsell TD. The impact of low health literacy on surgical practice. Am J Surg. 2004 Sep;188(3):250–3. doi: 10.1016/j.amjsurg.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Diagnostic criteria for research. Geneva: 1993. The ICD-10 classification of mental and behavioral disorders. [Google Scholar]
- 30.Brandt J, Folstein MF. Telephone Interview for Cognitive Status. Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 34.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea.[see comment] Archives of internal medicine. 2006 Sep 18;166(16):1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 35.Dinges D, Kribbs N, Bates B, Carlin M. A very brief probed recall memory task: sensitivity to sleep loss. Sleep Research. 1993;22:330. [Google Scholar]
- 36.Reitan RM. Trail Making Test. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 37.Corporation TP. WAIS-III Technical Manual. Harcourt Assessment Co; 2002. [Google Scholar]
- 38.Lezak M, Howieson D, Lorig D. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 39.Gladsjo JA, Heaton RK, Palmer BW, Taylor MJ, Jeste DV. Use of oral reading to estimate premorbid intellectual and neuropsychological functioning. J Int Neuropsychol Soc. 1999 Mar;5(3):247–54. doi: 10.1017/s1355617799533079. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007 Oct 1;30(10):1309–16. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobbels F, Berben L, De Geest S, Drent G, Lennerling A, Whittaker C, et al. The Psychometric Properties and Practicability of Self-Report Instruments to Identify Medication Nonadherence in Adult Transplant Patients: A Systematic Review. Transplantation. 2010 Jul 27;90(2):205–19. doi: 10.1097/TP.0b013e3181e346cd. [DOI] [PubMed] [Google Scholar]
- 42.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical Therapeutics. 1999;21(6):1074–90. doi: 10.1016/S0149-2918(99)80026-5. discussion 3. [DOI] [PubMed] [Google Scholar]
- 43.Deschamps AE, De Geest S, Vandamme AM, Bobbaers H, Peetermans WE, Van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS Patient Care STDS. 2008 Sep;22(9):735–43. doi: 10.1089/apc.2007.0229. [DOI] [PubMed] [Google Scholar]
- 44.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 45.Kubo S, Schulman S, Starling R, Jessup M, Wentworth D, Burkhoff D. Development and validation of a patient questionnaire to determine New York Heart Association Classification. Journal of Cardiac Failure. 2004;10(3):228–35. doi: 10.1016/j.cardfail.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009 Feb 1;32(2):150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buysse D, Reynolds CF, 3d, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 48.Havranek EP, Lapuerta P, Simon TA, L’Italien G, Block AJ, Rouleau JL. A health perception score predicts cardiac events in patients with heart failure: results from the IMPRESS trial. Journal of Cardiac Failure [Multicenter Study] 2001 Jun;7(2):153–7. doi: 10.1054/jcaf.2001.24121. [DOI] [PubMed] [Google Scholar]
- 49.Johansson P, Brostrom A, Dahlstrom U, Alehagen U. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. Eur J Heart Fail. 2008 Oct;10(10):1040–7. doi: 10.1016/j.ejheart.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Johansson P, Brostrom A, Dahlstrom U, Alehagen U. Global perceived health and health-related quality of life in elderly primary care patients with symptoms of heart failure. Eur J Cardiovasc Nurs. 2008 Dec;7(4):269–76. doi: 10.1016/j.ejcnurse.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Redeker NS. Somatic symptoms explain differences in psychological distress in heart failure patients vs a comparison group. Prog Cardiovasc Nurs. 2006 Fall;21(4):182–9. doi: 10.1111/j.0889-7204.2006.05643.x. [DOI] [PubMed] [Google Scholar]
- 52.Cline CM, Bjorck-Linne AK, Israelsson BY, Willenheimer RB, Erhardt LR. Non-compliance and knowledge of prescribed medication in elderly patients with heart failure. European Journal of Heart Failure. 1999;1(2):145–9. doi: 10.1016/s1388-9842(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez B, Lupon J, Parajon T, Urrutia A, Altimir S, Coll R, et al. Nurse evaluation of patients in a new multidisciplinary Heart Failure Unit in Spain. European Journal of Cardiovascular Nursing. 2004;3(1):61–9. doi: 10.1016/j.ejcnurse.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Rodgers PT, Ruffin DM. Medication nonadherence: Part II--A pilot study in patients with congestive heart failure. Manag Care Interface. 1998 Sep;11(9):67–9. 75. [PubMed] [Google Scholar]
- 55.Bhagat K, Mazayi-Mupanemunda M. Compliance with medication in patients with heart failure in Zimbabwe. East Afr Med J. 2001 Jan;78(1):45–8. doi: 10.4314/eamj.v78i1.9112. [DOI] [PubMed] [Google Scholar]
- 56.Bohachick P, Burke LE, Sereika S, Murali S, Dunbar-Jacob J. Adherence to angiotensin-converting enzyme inhibitor therapy for heart failure. Prog Cardiovasc Nurs. 2002 Fall;17(4):160–6. doi: 10.1111/j.0889-7204.2002.01643.x. [DOI] [PubMed] [Google Scholar]
- 57.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Avorn J. Noncompliance with congestive heart failure therapy in the elderly. Archives of Internal Medicine. 1994;154:433–7. [PubMed] [Google Scholar]
- 58.Artinian NT, Harden JK, Kronenberg MW, Vander Wal JS, Daher E, Stephens Q, et al. Pilot study of a Web-based compliance monitoring device for patients with congestive heart failure. Heart Lung. 2003 Jul–Aug;32(4):226–33. doi: 10.1016/s0147-9563(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 59.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure.[erratum appears in Heart Lung 2001 Nov–Dec;30(6):476–8] Heart & Lung: Journal of Acute & Critical Care. 2001;30(4):294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 60.Murray MD, Tu W, Wu J, Morrow D, Smith F, Brater DC. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clin Pharmacol Ther. 2009 Jun;85(6):651–8. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu JR, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail. 2008 Apr;14(3):203–10. doi: 10.1016/j.cardfail.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am. 2008 Mar;43(1):133–53. vii–viii. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 64.Phillips KD, Moneyham L, Murdaugh C, Boyd MR, Tavakoli A, Jackson K, et al. Sleep disturbance and depression as barriers to adherence. Clin Nurs Res. 2005 Aug;14(3):273–93. doi: 10.1177/1054773805275122. [DOI] [PubMed] [Google Scholar]