Abstract
Pulmonary arterial hypertension is characterized by elevated pulmonary artery pressure and vascular resistance. In women the incidence is 4 fold greater than that in men. Studies suggest sustained vasoconstriction is a factor in increased vascular resistance. Possible vasoconstrictor mediators include arachidonic acid-derived lipoxygenase metabolites. Our studies in rabbits showed enhanced endothelium-dependent contractions to arachidonic acid in pulmonary arteries from females compared to males. Because treatment with a non-specific lipoxygenase inhibitor reduced contractions in females but not males, the present study identified which lipoxygenase isoform contributes to sex-specific pulmonary artery vasoconstriction. 15- and 5- but not 12-lipoxygenase protein expression was greater in females. Basal and A23187-stimulated release of 15-, 5- and 12-hydroxyeicosatetraenoic acid from females and males was measured by liquid chromatography/mass spectrometry. Only 15-hydroxyeicosatetraenoic acid synthesis was greater in females compared to males under both basal and stimulated conditions. Vascular contractions to 15-hydroxyeicosatetraenoic acid were enhanced in females compared to males (maximal contraction; 44 ± 6% vs 25 ± 3%). The specific 15-lipoxygenase inhibitor PD146176 (12 μmol/L) decreased arachidonic acid-induced contractions in females (maximal contraction; 93 ± 4% vs 57 ± 10%). If male pulmonary arteries were incubated with estrogen (1 μmol/L, 18 hrs), protein expression of 15-lipoxygenase, and 15-hydroxyeicosatetraenoic acid production increased. Mechanisms to explain the increased incidence of pulmonary hypertension in women are not known. Results suggest the 15-lipoxygenase pathway is different between females and males and is regulated by estrogen. Understanding this novel sex-specific mechanism may provide insight into the increased incidence of pulmonary hypertension in females.
Keywords: arachidonic acid, lipoxygenase, vasoconstriction, pulmonary artery, estrogen
Introduction
Pulmonary arterial hypertension (PAH) encompasses a group of diseases characterized by high pulmonary artery pressure and pulmonary vascular resistance. One form of PAH presents in an idiopathic form, called IPAH. While relatively rare, IPAH is a medically significant disease that occurs more frequently in young women 1. The disease is usually catastrophic for those afflicted with a median survival time of 2.8 years post diagnosis. Mechanisms to explain sex-based differences in PAH have not been well studied.
The increased pulmonary vascular resistance in PAH has been attributed to two major factors: vasoconstriction and remodeling of the vascular wall. In severe progressive PAH, the fixed obstructive vascular remodeling rather than vasoconstriction is thought to be the major cause of elevated resistance ultimately resulting in right heart failure and death. However, recent studies in animal models of severe occlusive PAH showed that a sustained Rho-kinase-mediated vasoconstriction is the primary determinant of increased pulmonary vascular resistance 2. In patients with PAH that were refractory to other vasodilators, the administration of the Rho-kinase inhibitor, fasudil decreased pulmonary arterial pressure 3, 4. These recent studies are important because it suggests that the role of vasoconstriction in late stages of pulmonary hypertension may be underappreciated 5. Important to this concept is evidence that pulmonary vascular tone is modulated by factors released from the endothelium. Our previous studies used a rabbit model and demonstrated enhanced endothelium-dependent contractions to arachidonic acid and methacholine in pulmonary arteries from females compared to males 6. Several other studies suggested that a cyclooxygenase (COX) metabolite of arachidonic acid mediates endothelium-dependent contractions of blood vessels 7–12. However, in our previous study, pretreatment with the COX inhibitor, indomethacin only partially blocked contractions in the females compared to an almost complete attenuation in the males 6. The combined COX/lipoxygenase (LO) inhibitor, BW 755C completely blocked contractions in the females. Therefore, the current study was designed to more completely characterize the role of arachidonic acid metabolites in the sex-related differences in vascular responses in males versus females. Studies addressed the specific hypothesis that differences in arachidonic acid metabolism by LO(s) contributes to the increased endothelium-dependent pulmonary vasoconstriction in females compared to males. While not designed to specifically study PAH, the studies lay the fundamental groundwork to further investigate a novel vasoconstrictor signaling pathway that potentially contributes to the disease.
Methods
Animals
Animal protocol was approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin, and procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). Two month old female and male New Zealand White (NZW) rabbits were obtained from New Franken Rabbitry (New Franken, WI) or Kuiper Rabbit Ranch (Gary, IN). The animals were housed in the Medical College of Wisconsin Biomedical Research Facilities and maintained on a standard rabbit chow diet and given tap water ad libitum.
Vascular Reactivity
Pulmonary artery tissue was isolated as previously described 6. Briefly, female and male NZW rabbits were sacrificed (sodium pentobarbital, 120 mg/kg, iv), the heart and lungs removed as a unit, and placed immediately in a Kreb’s bicarbonate buffer of the following composition (mmol/L): NaCl 118, KCl 4, CaCl2 3.3, NaHCO3 24, KH2PO4 1.4, MgSO4 1.2 and glucose 11, pH 7.4. The main pulmonary artery was identified at its origin from the right ventricle, and both left and right pulmonary arteries were dissected to their most distal end. The pulmonary artery distal to the first branching of the left or right pulmonary artery was used, and this is referred to as the intrapulmonary artery. After dissection, the tissue was cleaned of adherent lung parenchyma and connective tissue using care not to disturb the endothelial layer. Rings of intrapulmonary artery were obtained (2–3 mm) and suspended in 5 ml organ baths containing Kreb’s bicarbonate buffer (warmed to 37° C) and continuously aerated with a 95% O2-5% CO2 mixture. Isometric tension was measured as previously described 6. Resting tension was adjusted to the length-tension maxima for each rabbit by increasing the length of the rings in a stepwise fashion and measuring the active tension generated by exposing the rings to 20 mmol/L KCl. The resting tension was 1.0 g and was not different between females and males. Vessels equilibrated at resting tension for 1 hr. Contractions were produced by raising the KCl concentration to 40 mmol/L. After vessels had reproducible, stable contractions to KCl, then cumulative concentration-response curves to arachidonic acid (10−8–10−5 mol/L), 15-HETE (10−9–10−5 mol/L), 5-HETE (10−9–10−5 mol/L) or 15-HETE in the presence of 5-HETE were determined. In some cases, the effect of a specific 15-LO inhibitor, PD146176 (12 μmol/L) was added prior to arachidonic acid. To standardize for any minor differences tissue size, contractile responses were expressed as a percentage of the maximal response to 40 mmol/L KCl. Maximal KCl contractions were not different between females and males (1.3 ± 0.1 gr vs 1.1 ± 0.1 gr; female vs male).
14C-Arachidonic Acid Metabolism
In order to identify the arachidonic acid metabolites produced by female and male pulmonary arteries, segments of intrapulmonary artery (70 mg of wet weight) were incubated in HEPES buffer (in mmol/L: HEPES 10, NaCl 150, KCl 5, CaCl2 2, MgCl2 1, and glucose 6, pH 7.4) containing 14C-arachidonic acid (0.05 μCi, 10−7 mol/L) and the calcium ionophore A23187 (20 μmol/L) for 15 min at 37°C. After incubation, HEPES buffer was removed, acidified to pH 2.0 with glacial acetic acid, and extracted over BondElut octadecylsilica extraction columns as previously described 13. Arachidonic acid metabolites were eluted with 6 ml ethyl acetate, evaporated to dryness under nitrogen, and stored at −40° C until analysis by reverse phase high pressure liquid chromatography (RP-HPLC) (Beckman Instruments, Fullerton, CA). Recovery of arachidonic acid metabolites by this extraction procedure is greater than 95%. Separation of metabolites of arachidonic acid was accomplished with RP-HPLC and solvent system I, where solvent A was water and solvent B was acetonitrile containing 0.1% glacial acetic acid. The program consisted of a 40 minute linear gradient from 50 % B in A to 100% B. RP separations used a Nucleosil C18 column (5μM, 4.6 × 250 mm, Phenomenox Inc., Torrance, CA), and the flow rate was 1 ml/min. Radioactivity of column eluate was collected in 0.2 ml aliquots and measured by liquid scintillation spectrometry. In order to identify HETE metabolites produced by males and females, fractions 95–120 collected from RP-HPLC using solvent system I were pooled, acidified, extracted with cyclohexane/ethyl acetate (50/50) and rechromatographed on normal phase HPLC using solvent system II. Solvent A was hexane containing 0.1% glacial acetic acid and solvent B was hexane containing 0.1% glacial acetic acid and 2% isopropanol. The program consisted of a 40 min linear gradient from 25% solvent B in A to 75% B in A. The flow was 3 ml/min. Radioactivity of column eluate was collected in 0.6 ml aliquots and measured by liquid scintillation spectrometry.
Liquid Chromatography/Electrospray Ionization/Mass Spectrometry
The production of 15-, 12- and 5-HETE were quantified using LC–electrospray ionization (ESI)–Mass Spectometry (MS) (Agilent 1100 LC/MSD, SL model). For these studies, pulmonary artery (10–30 mg) from females and males were incubated for 15 min at 37° C in HEPES buffer containing vehicle or arachidonic acid (10−6 mol/L) plus A23187 (20 μmol/L). After incubation, HEPES buffer was removed, internal standard ([2H4]15-HETE) was added, the buffer acidified to pH 3.0 with glacial acetic acid, and extracted over BondElut octadecylsilica extraction columns as described above. The HETEs were eluted with 6 ml ethyl acetate, then were evaporated to dryness under N2 and stored at −80°C until analysis. Samples were analyzed by LC-ESI-MS using a modification of a method previously described by Nithipatikom et al. 14. Briefly, the samples column (Kromasil, 250×2 mm) using were separated on a RP C18 water/acetonitrile with 0.01% acetic acid as a mobile phase at a flow rate of 0.300 mL/min. The mobile phase started at 35% acetonitrile for 1 minute, increased linearly to 68% acetonitrile in 18 minutes, increased to 100% acetonitrile in 15 minutes, and held for 10 minutes. Drying gas flow was 12 L/min, drying gas temperature was 350°C, nebulizer pressure was 35 psi gauge, vaporizer temperature was 325°C, capillary voltage was 3000 V, and fragmenter voltage was 90 V. Detection was made in the negative mode. For quantitative measurements, m/z=319 and 327 ions were used for 15-HETE and [2H8]15-HETE, respectively. Standard curves were typically constructed over a range of 5 to 500 pg per injection. The concentrations of 15-, 12- and 5-HETE in samples were calculated by comparing their ratios of peak areas with the standard curves. The results were normalized to wet tissue weight and expressed as pg/mg.
Polyacrylamide Gel Electrophoresis and Western Blotting
Pulmonary artery was obtained from rabbits as described above. Lysates were prepared by homogenizing samples in a buffer containing 20 mmol/L HEPES, 255 mmol/L sucrose, 1 mmol/L EDTA and 100 μmol/L phenylmethylsulfonylfluoride, pH 7.4. Protein lysates (30 μg) were analyzed by SDS-PAGE by the method of Laemmli 15 using a 4% acrylamide stacking gel and 10% acrylamide resolving gel. Proteins were electrophoretically transferred to nitrocellulose and membrane was blocked for 2 hr at RT with 2% nonfat dry milk in Tris-buffered saline (20 mmol/L TRIZMA hydrochloride; 500 mmol/L NaCl, pH 7.5) with Tween-20 (TTBS) before incubation with a polyclonal 15-LO antibody, a 5-LO antibody or a 12-LO antibody. Primary antibodies were used at a dilution of 1:1000 (15- and 12-LO) or 1:250 (5-LO) overnight at 4°C. Following washing, blots were incubated for 1 hr with secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG antibody for 12 and 5-LO or horseradish peroxidase-conjugated donkey anti-sheep IgG antibody for 15-LO) at a dilution of 1:5000. After again washing with TTBS, blots were incubated for 2 min with Thermo Scientific Pierce ECL Western blotting substrate. Membranes were subsequently exposed to Kodak Biomax MR imaging film and developed. Prestained protein markers (Sigma) were used for molecular mass determination. The anti-15-, 12- and 5-LO antibodies give immunoreactive bands corresponding to 75 16, 75 17 and 78 18 kDa proteins, respectively. Membranes were reprobed with mouse anti-β-actin as a loading control. Immunoreactive bands (37 kDa) were identified, and a densitometric analysis (ImageJ) was performed by comparing band intensity of respective LO to loading control.
Role of Estrogen on 15-LO Expression and 14C-Arachidonic Acid Metabolism
Male pulmonary arteries were isolated as described above. Vessels were incubated in HEPESβ buffer containing vehicle or 17β-estradiol (1 μmol/L) for 18 hr at 37°C. Lysates were prepared for analysis of 15-LO protein by Western blotting or intact vessel segments were incubated with 14C-arachidonic acid for identification of 15-HETE by RP-HPLC.
Materials
[14-C]-arachidonic acid was obtained from New England Nuclear (Boston, MA); Arachidonic acid, A23187 and 17β-estradiol were from Sigma Chemical Co (St. Louis, MO). 15-, 12- and 5- LO antibodies, 15-HETE, 5-HETE and PD146176 were from Cayman Chemical (Ann Arbor, MI). Arachidonic acid, A23187, PD146176 and HETEs were prepared in ethanol previously sparged with nitrogen. Stock solution and dilutions were made fresh for each experiment, kept on ice and under a nitrogen atmosphere.
Statistics
Data are expressed and the mean + SEM. Statistical analysis of the vascular reactivity data was performed by a two-way ANOVA followed by Bonferroni’s posttest. A Student’s t-test was used to analyze the band intensities from immunoblots and the LC-MS data. Values were considered significant at P < 0.05.
Results
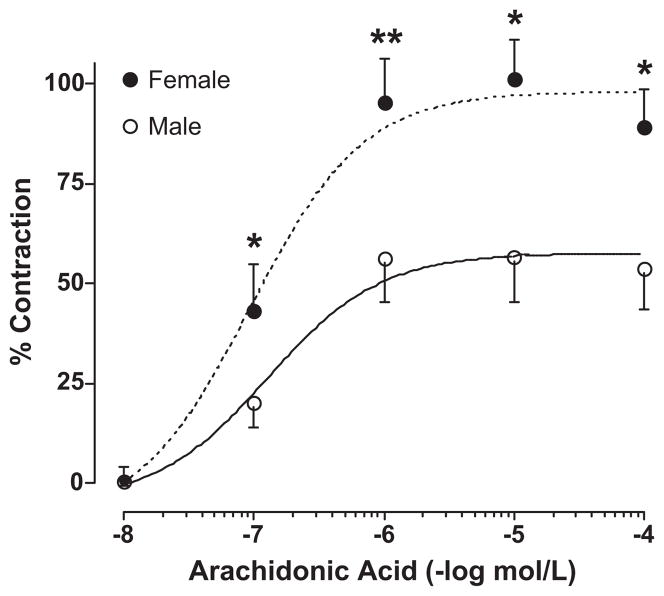
Arachidonic acid caused endothelium-dependent, concentration-related contractions of rabbit pulmonary artery from both female and male rabbits (Figure 1) confirming our earlier observations 6. The maximum contractions were greater in the female rabbits (maximal response, 101 ± 10 % vs 57 ± 11 %, female vs male, figure 1). The EC50 for arachidonic acid-induced contractions was 0.26 ± 0.09 μM and 0.36 ± 0.24 μM for female and male, respectively. The contractions were inhibited by removal of the endothelium to a similar extent in both females and males (data not shown, 6).
Figure 1.
Contractile responses to arachidonic acid in female and male rabbit pulmonary arteries. Results are expressed as percent KCl (40 mM) contractions, and data points are the mean ± SEM for n = 8–12. * p < 0.05; ** p< 0.01, female vs male.
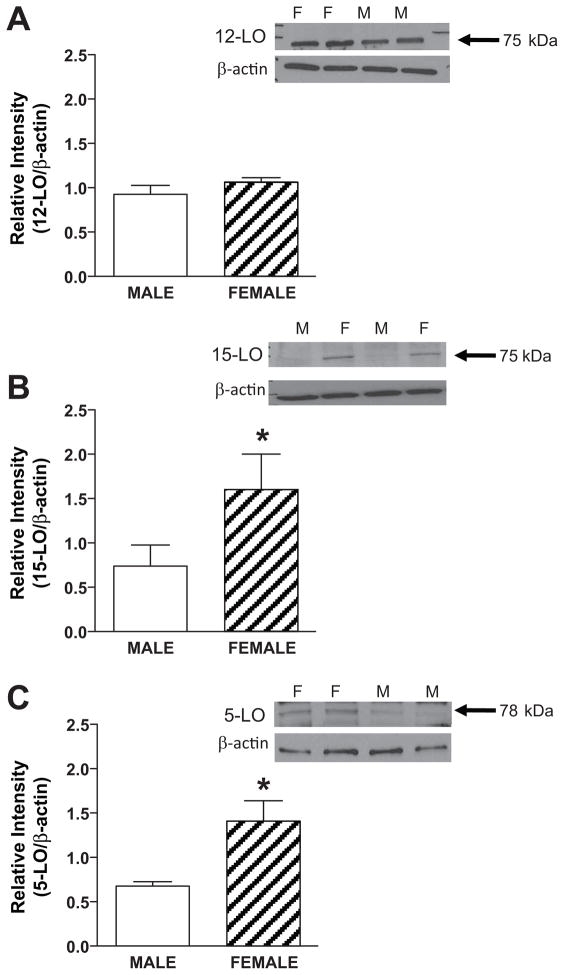
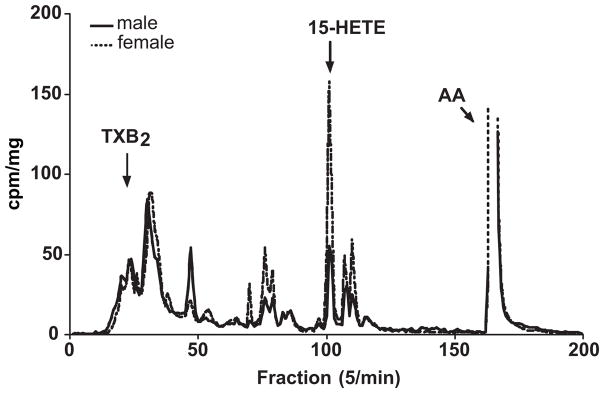
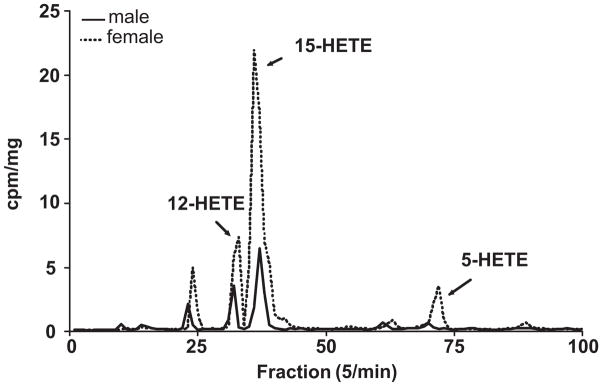
Studies next determined if the protein expression of LOs were different in pulmonary lysates obtained from females and males. Immunoblots revealed similar 12-LO expression (Figure 2A) while there was a greater expression of both 15-LO (Figure 2B) and 5-LO (figure 2C) in females compared to males. Segments of pulmonary artery from female and male rabbits were incubated with 14C-arachidonic acid and the metabolites were resolved by reverse phase HPLC using solvent system I. A representative chromatogram is shown in figure 3. Female and male rabbit pulmonary arteries synthesized radioactive products that comigrated with the prostaglandins (fractions 10–50), dihydroxyeicosatrienoic acids (DHETs) and dihydroxyeicosatetranoic acids (DiHETEs) (fractions 70–85), and the HETEs (fractions 90–120). In the females, there was increased production of products migrating with the HETEs and DHETs/diHETEs. Fractions corresponding to HETES were collected, extracted with cyclohexane/ethyl acetate and rechomatographed on normal phase HPLC using solvent system II. A representative chromatogram is shown in figure 4. The major HETEs produced by the rabbit pulmonary artery comigrated with 12-, 15- and 5-HETE. The production of all three HETEs appeared greater in females compared to males. However, a better method for quantifying the HETEs is to use LC/ESI/MC and results from this method are summarized in Table 1. The basal production of 15-HETE was greater in females compared to males. No differences in the basal production of 12- and 5-HETE was measured in females and males. A23187 increased the production of all the measured HETEs in both females and males. The increased production of 15-HETE by A23187 was greater in females compared to males. While there was a trend for 5-HETE to be greater in female vessels incubated with A23187 compared to males, the increase was not significant. A23187-stimulated 12-HETE production was not different between female and males.
Figure 2.
LO protein expression in female and male rabbit pulmonary artery. Lysates were prepared and separated on 10% SDS gel and analyzed with antibody against 12-LO (A), 15-LO (B) and 5-LO (C). Representative Western immunoblots for each LO and β-actin in lysates (30 μg protein) are shown as an inset for each bar graph. The bar graph shows the LO band density normalized to the β-actin band density. Values represent mean ± SEM for n = 6.
Figure 3.
Representative chromatogram showing the metabolism of 14C-arachidonic acid by female and male pulmonary arteries. Segments of pulmonary arteries were incubated with 14C-arachidonic acid for 15 min, and the metabolites separated using reverse phase HPLC using solvent system I (panel A). Migration times of known standard eicosanoids are shown above the chromatogram. This experiment was repeated three times with similar results. Results are presented as cpm/mg of tissue in order to control for vessel weight in the incubations.
Figure 4.
Representative chromatograms showing the metabolism of 14C-HETEs by female and male pulmonary arteries. Pulmonary arteries were incubated with 14C-arachidonic acid, and metabolites separated using reverse phase HPLC with solvent system I (figure 3). Metabolites corresponding to fractions 95–120 (19 – 24 min) were collected, extracted using cyclohexane/ethyl acetate, and rechromatographed using normal phase HPLC. Migration times of known standard eicosanoids are shown above the chromatogram. This experiment was repeated three times with similar results. Results are presented as cpm/mg of tissue in order to control for vessel weight in the incubations.
Table 1.
15-HETE, 12-HETE and 5-HETE production in pulmonary artery from male and female rabbits
| 15-HETE (pg/mg) | 12-HETE (pg/mg) | 5-HETE (pg/mg) | ||||
|---|---|---|---|---|---|---|
| SEX | Basal | +A23187 | Basal | +A23187 | Basal | +A23187 |
| Male | 707 ± 220 | 1506 ± 522 * | 226 ± 66 | 841 ± 254 * | 30 ± 8 | 190 ± 46 * |
| Female | 1713 ± 538 † | 4097 ± 921 *† | 240 ± 29 | 621 ± 102 * | 30 ± 7 | 251 ± 28 * |
Segments of pulmonary arteries from male and female rabbits were incubated with and without A23187 (20 μMol/L) for 15 minutes at 37°C. The production of 15-, 12- and 5-HETE were measured in the incubation media using LC/ESI/MS. Values are mean ± SEM for n = 6.
p < 0.05, Basal vs A23187;
p < 0.05 Female vs Male
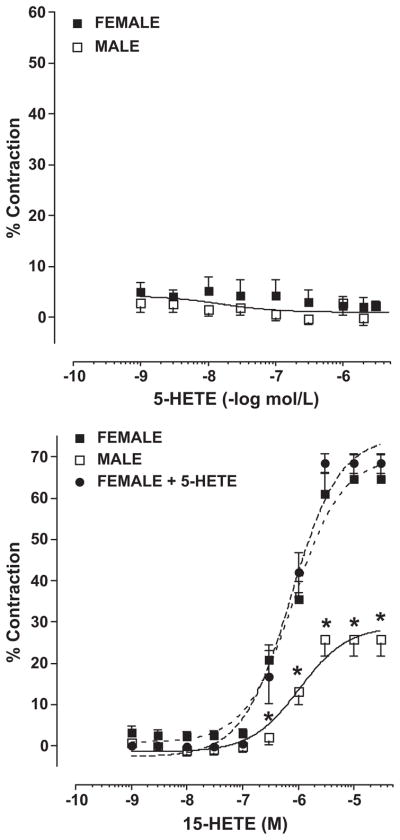
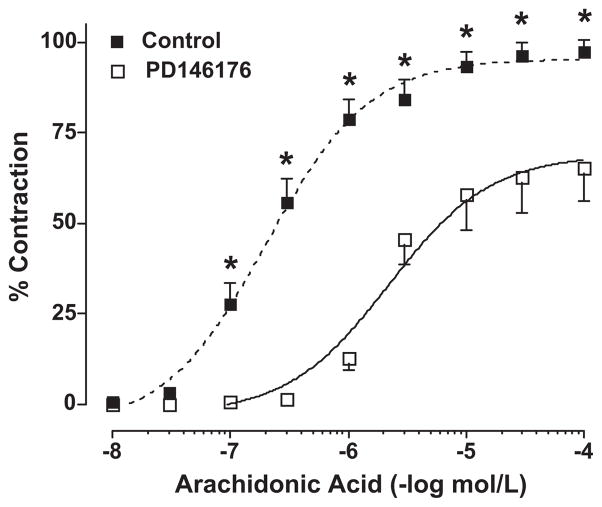
The ability of 5- and 15-HETE to contract pulmonary arteries from female and male rabbits is shown in Figure 5. There was no effect of 5-HETE to elicit contraction in pulmonary arteries (top panel). In contrast, 15-HETE produced a concentration-dependent vasoconstriction that was significantly greater in females compare to males (bottom panel). Additional studies tested the hypothesis that 5-HETE may potentiate contractions to 15-HETE. When 15-HETE contractions were repeated in the presence of 5-HETE, no enhancement of contraction was seen when compared to 15-HETE alone (bottom panel). To further establish 15-HETE as the mediator contributing to enhanced arachidonic acid-induced contractions in the females compared to males, the effect of the specific 15-LO inhibitor, PD 146176 (12 μmol/L) was tested on vascular reactivity and arachidonic acid metabolism in pulmonary arteries from female rabbits. As shown in figure 6, PD146176 attenuated arachidonic acid-induced contractions (maximal contraction; 93 ± 4% vs 57 ± 10%; control vs PD146176). The maximal contraction in the presence of the 15-LO inhibitor was similar to that observed in untreated male pulmonary arteries (figure 1). HPLC analysis showed that this concentration of PD146176 also decreased 15-HETE production (data not shown).
Figure 5.
Contractile responses to 5-HETE and 15-HETE in female and male rabbit pulmonary arteries. Results are expressed as percent KCl (40 mM) contractions, and data points are the mean ± SEM for n = 8–12. * p < 0.05; female vs male
Figure 6.
Effect of 15-LO inhibitor on contractile responses to arachidonic acid in female rabbit pulmonary arteries. Results are expressed as percent KCl (40 mM) contractions, and data points are the mean ± SEM for n = 8. * p< 0.01, PD146176 vs vehicle.
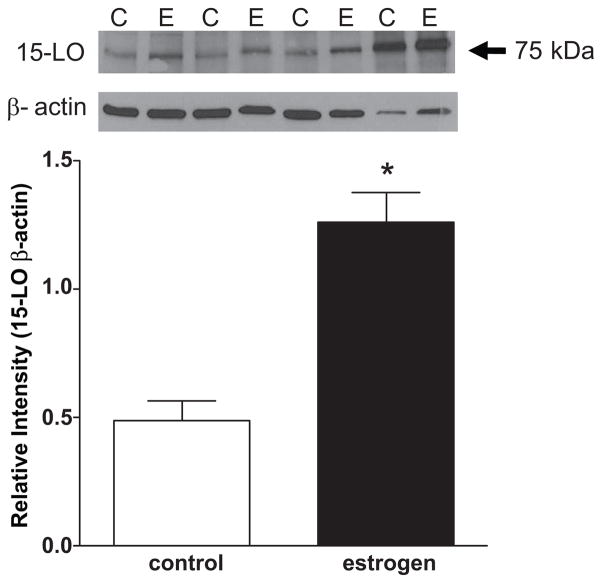
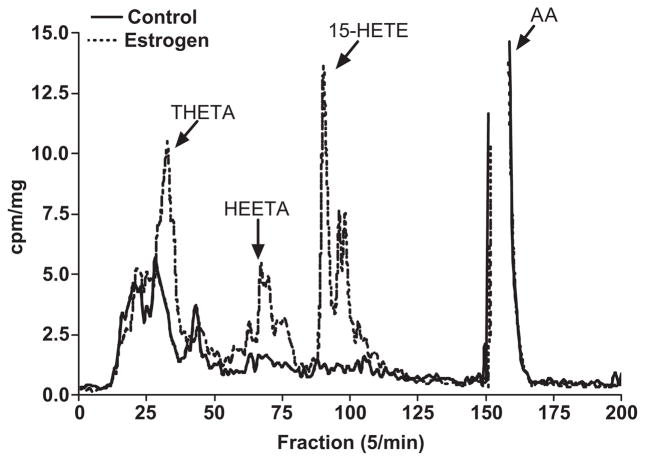
In a final series of experiments, pulmonary arteries obtained from male rabbits were treated for 18 hours with 17β-estradiol (1 μmol/L) and then incubated with 14C-arachidonic acid as described above. Results clearly showed an increase in LO products in the 17β-estradiol-treated vessels (dotted line) compared to untreated control. Interestingly, there were also other peaks that increased after estrogen treatment and some of these same peaks (DHETs/DiHETEs) were greater in females compared to males (figure 3). Furthermore, the protein expression of 15-LO increased after 17β-estradiol treatment (figure 8).
Figure 8.
15-LO expression in male pulmonary arteries incubated with 17β-estradiol (1 μmol/L, 18 hrs, 37 °C). Representative Western immunoblots for 15-LO and β-actin in lysates (30 μg protein) is shown as an inset for the bar graph. The bar graph shows the 15-LO band density normalized to the β-actin band density. Values represent mean ± SEM for n = 6. * p < 0.05
Discussion
Our previous studies suggested that enhanced pulmonary artery contractions to arachidonic acid in females compared to males was mediated by a LO metabolite 6. The purpose of the current study was to systematically characterize the LO pathways in female and male pulmonary arteries. First, it was shown that the protein expression of both 15- and 5-LO was greater in females compared to males. While pulmonary arteries from both male and females produced the corresponding LO metabolites, 15- and 5-HETE, only the synthesis of 15-HETE was enhanced in females. The increase in 15-HETE was correlated with an increased 15-HETE-mediated pulmonary artery vasoconstriction in females compared to males. These findings are significant because it is the first report that the 15-LO pathway is regulated in a sex-specific way. These results may impact what is known about the sex-differences in the incidence of PAH.
The enhanced vasoconstriction in females compared to males could be due to increased production of the vasoconstrictor compound, 15-HETE. Alternative explanations are also possible. The arachidonic acid metabolism studies showed that there are other radioactive peaks migrating with DHETs/diHETEs that are greater in females compared to males. These products were not tested for vascular responses but could contribute to enhanced response in females compared to males. Our previous work identified thromboxane as an endothelium-dependent contracting factor in male pulmonary arteries 10. Our previous work showed that thromboxane also contributes to a portion of the vasoconstrictor response in females as the COX inhibitor indomethacin partially blocked contractions to both arachidonic acid and methacholine in females 6. Male rabbits not only produced less 15-HETE than females but also had decreased maximal vasoconstrictor responses suggesting that another possible explanation for differences in the responses in males and females may relate to a decreased sensitivity of male vessels to 15-HETE.
In women, the incidence of certain forms of PAH has been most recently reported to be four fold greater than that observed in men 1. Results from the REVEAL registry which enrolled over 2500 patients with PAH from across the United States found that approximately 80% were females. Mechanisms to explain the increased incidence are scarce but there are suggestions that hormonal changes may contribute to the pathogenesis of the disease. This is based on studies which showed that in a small percentage of women taking oral contraceptives, pulmonary hypertension developed 19 20. There is also evidence that PAH was more prevalent in postmenopausal women receiving hormone replacement therapy 21. Others studies have refuted the association of oral contraceptives and PAH 22, 23. Despite these controversies, it is well known that the sex hormone, estrogen, modulates vascular reactivity in a variety of different vascular beds (for review, see 24). In the isolated perfused rat lung circulation, Farhat and Ramwell 25 showed that the TXA2 mimetic, U46619 produced a greater pressor response in females compared to males. Infusion of 17β-estradiol into the rat lung intensified the pressor response to U46619. Chan and coworkers also reported a sex difference in isolated rat pulmonary arteries to raloxifene, a selective estrogen receptor modulator 26. Raloxifene produced greater relaxations in males compared to females. However, other studies in animal models of pulmonary hypertension have shown beneficial effects of estrogen in that females were less likely to develop PAH and/or removal of estrogen by ovariectomy prevented the development of pulmonary hypertension 27–29. Thus there remains an obvious inconsistency regarding the role of estrogen in pulmonary hypertension 30. It should be emphasized the rabbits for the present study were young, unmated males and females. Mean concentrations of 17β-estradiol and progesterone in female rabbits are 29.9 ± 11.4 pg/ml and 17.0 ± 2.05 ng/ml, respectively. These sex hormones are not detected in males. However, when male pulmonary arteries were incubated in vitro with 17β-estradiol, the expression of 15-LO and 15-HETE production increased compared to untreated control vessels. These data are important because it suggests that one mechanism to explain the increased vascular responses in females compared to males involves a regulation of the 15-LO by estrogen. Estrogen also increased other products in the arachidonic acid pathway and additional studies are needed to address whether these products contribute to pulmonary vascular function.
15-LO is implicated in the pathogenesis of several diseases, including atherosclerosis, asthma and cancer (for review 31). There is less known about the role of 15-LO in the pulmonary circulation. However, 15-LO is expressed in many cell types found in the human lung, including airway epithelium, eosinophils, reticulocytes, macrophages and pulmonary artery 32–36. Zhu and coworkers showed that neonatal rabbit pulmonary arteries exposed to chronic hypoxia produced 15-HETE 37. Other studies in humans indicated that an increase in oxidative stress in severe PAH was associated with an increased production of 5-, 15- and 12-HETE in lung tissue extracts 38. Genetic studies have identified a mutation in the bone morphogenetic protein receptor 2 (BMPR-2) in most cases of familial PAH 39, 40. Since only 10–20% of individuals with this mutation develop PAH, it is likely that other genes and/or environmental factors are necessary to trigger the disease 41. A recent study using heterozygous BMPR-2-mutant mice reported that increased susceptibility to PAH occurred only in mice treated with recombinant adenovirus overexpressing 5-LO 42. Interestingly, the female rabbits in the current study also had increased protein expression of 5-LO and a trend for greater concentrations of 5-HETE. While 5-HETE did not contract pulmonary arteries of either males or females, arachidonic acid is metabolized by 5-LO to the leukotrienes which are potent inflammatory compounds involved in allergy and asthma. The cysteinyl-leukotrienes constrict bronchial and pulmonary smooth muscle 43. There is no evidence that pulmonary artery endothelial cells produce leukotrienes 44. The present work showed that the combination of 5- and 15-HETE did not produce greater vasoconstriction than that of 15-HETE alone. While speculative at this point, it is still possible that products of 5- and 15-LO may contribute to other aspects related to the development of PAH, like vascular remodeling. This idea is supported by studies showing that both 15-HETE and 5-HETE increase cell proliferation 45, 46.
Perspective
In summary, PAH is a relatively rare but medically significant disease that occurs more frequently in young women and is usually catastrophic for those afflicted. Mechanisms to explain the predominance in women are not clearly understood. Using a rabbit model, our data indicate that the mechanism of endothelium-dependent pulmonary vasoconstriction is different in females compared to males. The mechanisms contributing to sex-specific differences in vascular function are diverse and complex. While mammalian LOs have been implicated in the pathogenesis of a number of diseases, including asthma, diabetes, atherosclerosis and cancer, there are no known studies which have carefully characterized the LO pathways in a sex-specific manner. Our studies provide the first evidence of a sex-specific difference in 15-LO in pulmonary arteries. Future studies designed to understand the mechanisms regulating this pathway may provide insight into the increased incidence of PAH in females.
Figure 7.
Representative chromatogram showing the effect of 17β-estradiol (1 μmol/L, 18 hrs, 37 °C) on the metabolism of 14C-arachidonic acid by male pulmonary arteries. Migration times of known standard eicosanoids are shown. This experiment was repeated three times with similar results. Results are presented as cpm/mg of tissue in order to control for vessel weight.
Acknowledgments
The technical assistance of Thivashnee Pillay and Marilyn Isabel is greatly appreciated.
Sources of Funding
This study was supported by grants from the National Institute of Health (HL093181) and American Heart Association (0151421Z).
Footnotes
Disclosures
None
References
- 1.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 2.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 3.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 4.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res. 2005;97:95–98. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- 6.Pfister SL, Campbell WB. Role of endothelium-derived metabolites of arachidonic acid in enhanced pulmonary artery contractions in female rabbits. Hypertension. 1996;27:43–48. doi: 10.1161/01.hyp.27.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Vanhoutte PM. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S328–331. doi: 10.1097/00005344-199322008-00086. [DOI] [PubMed] [Google Scholar]
- 8.Dai FX, Skopec J, Diederich A, Diederich D. Prostaglandin H2 and thromboxane A2 are contractile factors in intrarenal arteries of spontaneously hypertensive rats. Hypertension. 1992;19:795–798. doi: 10.1161/01.hyp.19.6.795. [DOI] [PubMed] [Google Scholar]
- 9.Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Feletou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. Am J Physiol Heart Circ Physiol. 2006;291:H2255–2264. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- 10.Buzzard CJ, Pfister SL, Campbell WB. Endothelium dependent contractions in rabbit pulmonary artery are mediated by thromboxane A2. CircRes. 1993;72:1023–1034. doi: 10.1161/01.res.72.5.1023. [DOI] [PubMed] [Google Scholar]
- 11.Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- 12.Miller VM, Vanhoutte PM. Endothelium-dependent contractions to arachidonic acid are mediated by products of cyclooxygenase. AmJPhysiol. 1985;248:H432–H437. doi: 10.1152/ajpheart.1985.248.4.H432. [DOI] [PubMed] [Google Scholar]
- 13.Pfister SL, Schmitz JM, Willerson JT, Campbell WB. Characterization of arachidonic acid metabolism in Watanabe Heritable Hyperlipidemic (WHHL) and New Zealand White (NZW) rabbit aortas. Prostaglandins. 1988;36:515–531. doi: 10.1016/0090-6980(88)90047-0. [DOI] [PubMed] [Google Scholar]
- 14.Nithipatikom K, Grall A, Holmes B, Harder D, Falck J, Campbell WB. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem. 2001;298:327–336. doi: 10.1006/abio.2001.5395. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Sigal E, Grunberger D, Craik CS, Caughey GH, Nadel JA. Arachidonate 15-lipoxygenase (w-6 lipoxygenase) from human leukocytes. J Biol Chem. 1988;263:5328–5332. [PubMed] [Google Scholar]
- 17.Chen XS, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J Biol Chem. 1994;269:13979–13987. [PubMed] [Google Scholar]
- 18.Komatsu N, Natsui K, Ueda N, Watanabe K, Yamamoto S. Immunohistochemical study on arachidonate 5-lipoxygenase in porcine leukocytes and other tissues. J Histochem Cytochem. 1991;39:655–662. doi: 10.1177/39.5.2016515. [DOI] [PubMed] [Google Scholar]
- 19.Kleiger RE, Boxer M, Ingham RE, Harrison DC. Pulmonary hypertension in patients using oral contraceptives. Chest. 1976;69:143–147. doi: 10.1378/chest.69.2.143. [DOI] [PubMed] [Google Scholar]
- 20.Irey NS, Norris HJ. Intimal vascular lesions associated with female reproductive steroids. Arch Pathol. 1973;96:227–234. [PubMed] [Google Scholar]
- 21.Sweeney L, Voelkel NF. Estrogen exposure, obesity and thyroid disease in women with severe pulmonary hypertension. Eur J Med Res. 2009;14:433–442. doi: 10.1186/2047-783X-14-10-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 23.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 24.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhat MY, Ramwell PW. Estradiol potentiates the vasopressor response of the isolated perfused rat lung to the thromboxane mimic U-46619. J Pharmacol Exp Ther. 1992;261:686–691. [PubMed] [Google Scholar]
- 26.Chan YC, Leung FP, Yao X, Lau CW, Vanhoutte PM, Huang Y. Raloxifene relaxes rat pulmonary arteries and veins: roles of gender, endothelium, and antagonism of Ca2+ influx. J Pharmacol Exp Ther. 2005;312:1266–1271. doi: 10.1124/jpet.104.077990. [DOI] [PubMed] [Google Scholar]
- 27.Lahm T, Crisostomo P, Markel T, Wang M, Wang Y, Weil B, Meldrum D. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock. 2008;30:660–667. doi: 10.1097/SHK.0b013e31816f239f. [DOI] [PubMed] [Google Scholar]
- 28.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol. 2001;280:L88–97. doi: 10.1152/ajplung.2001.280.1.L88. [DOI] [PubMed] [Google Scholar]
- 29.Tofovic SP, Salah EM, Mady HH, Jackson EK, Melhem MF. Estradiol metabolites attenuate monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2005;46:430–437. doi: 10.1097/01.fjc.0000175878.32920.17. [DOI] [PubMed] [Google Scholar]
- 30.Sakao S, Tanabe N, Tatsumi K. The estrogen paradox in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L435–438. doi: 10.1152/ajplung.00057.2010. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-LOs. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, Wenzel SE. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32:1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 33.Gulliksson M, Brunnstrom A, Johannesson M, Backman L, Nilsson G, Harvima I, Dahlen B, Kumlin M, Claesson HE. Expression of 15-lipoxygenase type-1 in human mast cells. Biochim Biophys Acta. 2007;1771:1156–1165. doi: 10.1016/j.bbalip.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Kumlin M, Ohlson E, Bjorck T, Hamberg M, Granstrom E, Dahlen B, Zetterstrom O, Dahlen SE. 15(S)-hydroxyeicosatetraenoic acid (15-HETE) is the major arachidonic acid metabolite in human bronchi. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:441–444. [PubMed] [Google Scholar]
- 35.Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon VR, Crouch EC, Takahashi Y, Ueda N, Yamamoto S, Holtzman MJ. Related expression of arachidonate 12- and 15-lipoxygenases in animal and human lung tissue. Am J Physiol. 1991;261:L399–405. doi: 10.1152/ajplung.1991.261.6.L399. [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res. 2003;92:992–1000. doi: 10.1161/01.RES.0000070881.65194.8F. [DOI] [PubMed] [Google Scholar]
- 38.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 39.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 41.Newman JH, Fanburg BL, Archer SL, Badesch DB, Barst RJ, Garcia JG, Kao PN, Knowles JA, Loyd JE, McGoon MD, Morse JH, Nichols WC, Rabinovitch M, Rodman DM, Stevens T, Tuder RM, Voelkel NF, Gail DB. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation. 2004;109:2947–2952. doi: 10.1161/01.CIR.0000132476.87231.6F. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Jones JE, Beppu H, Keaney JFJ, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rådmark O, Samuelsson B. Regulation of the activity of 5-Llipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 44.Zhang YY, Walker JL, Huang A, Keaney JF, Clish CB, Serhan CN, Loscalzo J. Expression of 5-lipoxygenase in pulmonary artery endothelial cells. Biochem J. 2002;361:267–276. doi: 10.1042/0264-6021:3610267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Setty B, Graeber JE, Stuart MJ. The mitogenic effect of 15- and 12-hydroxyeicosatetraenoic acid on endothelial cells may be mediated via diacylglycerol kinase inhibition. J Biol Chem. 1987;262:17613–17622. [PubMed] [Google Scholar]
- 46.Walker JL, Loscalzo J, Zhang YY. 5-lipoxygenase and human pulmonary artery endothelial cell proliferation. Am J Physiol Heart Circ Physiol. 2002;282:H585–593. doi: 10.1152/ajpheart.00003.2001. [DOI] [PubMed] [Google Scholar]