Abstract
H2S, the newly discovered gasotransmitter, plays important roles in biological systems. However, the research on H2S has been hindered by lacking controllable H2S donors which could mimic the slow and continuous H2S generation process in vivo. Herein we report a series of cysteine-activated H2S donors. Structural modifications on these molecules can regulate the rates of H2S generation. These compounds can be useful tools in H2S research.
Hydrogen sulfide (H2S) is a noxious gas with the characteristic smell of rotten eggs. Recent studies recognized H2S as the third gaseous transmitter beside nitric oxide (NO) and carbon monoxide (CO) that influence various physiological processes.1 H2S has been shown to relax vascular smooth muscles, mediate neurotransmission, elicit hibernation, inhibit insulin signalling, regulate inflammation and blood vessel caliber.1 Endogenous formation of H2S is achieved by enzymes such as cystathionine-β-synthase (CBS) in the brain and cystathionine γ-lyase (CSE) in liver, vascular and non-vascular smooth muscle. Although its exact chemical and biochemical modes of action are still not fully understood, levels of H2S in the brain and vasculature have unambiguously been associated with human health and disease.1
To study the physiological and pathophysiological properties of H2S, the direct use of H2S gas or NaHS in aqueous solutions are typical. However, the therapeutic potential of H2S gas seems to be limited due to difficulties in obtaining precisely controlled concentrations and possible toxic impact of H2S excess. NaHS, although widely used as a research tool, is a short-lasting donor which does not mimic the slow and continuous process of H2S generation in vivo. In addition, NaHS in aqueous solution can be rapidly oxidized by O2. Modifications that are made between the time that a solution is prepared and the time that the biological effect is measured can dramatically affect results. Due to these limitations, H2S-releasing agents (i.e. H2S donors) are considered useful tools in the study of H2S.1,2 However, currently available H2S donors are very limited.1,2 Besides NaHS, only three types of H2S donors have been reported (Scheme 1): 1) garlic-derived polysulfide compounds, such as diallyl trisulfide (DATS). H2S release from DATS was suggested to mediate the vasoactivity of garlic.3 2) GYY4137, a Lawesson’s reagent derivative, is a synthetic H2S donor.4 This molecule decomposes spontaneously in aqueous buffers to release H2S. 3) A dithiolthione moiety as a H2S donor has been used to prepare H2S- nonsteroidal anti-inflammatory drug hybrids like S-diclofenac.5 In addition, biological thiols such as cysteine and glutathione can be H2S donors upon enzymatic or thermal treatment.6 A limitation of these known donors is that H2S release is too fast to mimic biological H2S generation. Given the structural characters of these compounds, little can be done to modify their structures to control the release of H2S. Therefore, developing new H2S donors with controllable H2S generation capability is critical for this field. Ideal H2S donors, from therapeutic point of view and for the applications in H2S-related biological research, should release H2S slowly and in moderate amounts.2 The donors should also be stable compounds that can be easily handled by researchers.
Scheme 1.
Current H2S donors.
In our recent studies of S-nitrosothiols,7 we noticed that S-N bonds are unstable and easy to break under certain conditions. Such a property triggered our idea to develop controllable H2S donors based on S-N bonds. We envisioned that N-mercapto compounds like 1 could be potential H2S donors (Scheme 2). As N-SH derivatives are unstable species, we expected a protecting group on SH should enhance the stability. In addition, the protecting group could allow us to design different activation strategies to generate 1, therefore achieving controllable H2S release.
Scheme 2.
N-Mercapto compounds as H2S donors.
In our first generation design of N-mercapto based H2S donors, we decided to use acyl groups as the protecting group. As shown in Scheme 3, we expected compounds like 2 should react with cellular cysteine via a native chemical ligation (NCL) to produce N-SH 1 and then the cleavage of S-N bond of 1 should produce H2S.
Scheme 3.
Proposed cysteine-activated H2S donors.
To test this idea, a series of N-(benzoylthio)benzamide derivatives (5a–5l) were prepared from the corresponding thiobenzoic acids (Scheme 4, see supporting information for details). We expected different substituents could affect the reaction rates of compounds 5 with cysteine, therefore regulating the rate of H2S generation.
Scheme 4.
Synthesis of N-(benzoylthio)benzamides.
Compounds 5a–5l proved to be stable in aqueous buffers. As shown in Scheme 5, they do not react with potential cellular nucloephiles such as –OH and –NH2 groups. However, in the presence of cysteine, we observed a time-dependent decomposition of the donors and H2S release. The formation of H2S was monitored by a 2-mm H2S-selective microelectrode (ISO-H2S-2; WPI) attached to an Apollo 1100 Free Radical Analyser (WPI). A typical H2S generation curve in pH 7.4 buffer is shown in Figure 1. In the presence of excess of cysteine, the concentration of H2S released from 5a reached a maximum value at 18 min (peaking time), and then started to drop, presumably due to oxidation by air. We also measured H2S generation under other pH including pH 5.5 and pH 9.0. Similar releasing curves were observed (see supporting information).
Scheme 5.
H2S generation from N-(benzoylthio)benzamides.
Figure 1.
H2S generation curves from 5a.
We believe peaking time and H2S concentration at peaking time are useful parameters to assess the rate of H2S generation from donors. Therefore, peaking time and H2S concentration of 5a–5l in pH 7.4 PBS buffer were measured and summarized in Table 1. In general, electron donating groups led to slow generation of H2S while electron withdrawing groups led to fast generation. These results proved that controllable H2S release can be achieved by structural modifications on donors.
Table 1.
H2S generation peaking time.
 | |||||
|---|---|---|---|---|---|
| donors | peaking time (min) |
peaking time [H2S] (µM) |
donors | peaking time (min) |
peaking time [H2S] (µM) |
 |
18 | 25.4 |  |
22 | 31.0 |
 |
16 | 35.2 | 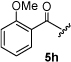 |
25 | 20.8 |
 |
13 | 35.6 |  |
30 | 24.2 |
 |
14 | 30.7 |  |
25 | 21.5 |
 |
25 | 26.1 |  |
50 | 23.0 |
 |
18 | 31.4 |  |
22 | 17.5 |
It is known that plasma could contain significant amount of free cysteine.8 We therefore measured H2S generation of 5 in plasma (containing ~500 µM cysteine) using a colorimetry method.9 We observed a similar time-dependent H2S release (Figure 2 illustrated an example using 5a) as the one shown in Figure 1. However, when plasma was first treated with N-methylmaleimide (NMM) to block free cysteine, no H2S generation was observed. These results demonstrated the capability of H2S release from N-(benzoylthio)benzamide-based donors in complex biological systems. It is also demonstrated that cysteine is the regulator of this type of donors.
Figure 2.
H2S generation from 5a in plasma.
Finally, to understand the mechanism of H2S generation from N-(benzoylthio)benzamides, we analyzed the reaction between 5a and cysteine (10 eq). As shown in Scheme 6, we confirmed the formation of N-acyl cysteine 7, benzamide 9, and cystine 11 in high yields. Based on the products observed, we proposed the following mechanism: this reaction is initiated by a reversible thiol exchange between 5a and cysteine to first generate a new thioester 6 and N-mercapto-benzamide 8. Compound 6 then undergoes a fast S to N acyl transfer to form amide 7. This process is similar to the well-known native chemical ligation. Meanwhile the reaction between 8 and excess cysteine should lead to benzamide 9 and cysteine perthiol 10. Finally the reaction between 10 and cysteine should complete the generation of H2S and provide cystine 11.
Scheme 6.
Proposed H2S generation mechanism.
In summary, a series of new H2S donors have been developed based on the N-(benzoylthio)benzamide template. These compounds are stable in aqueous buffers. H2S generation from these compounds is regulated by cysteine. We have proved that H2S release rates from these compounds are controllable upon structural modifications. It should note that H2S release rates shown in Table 1 can only serve as a reference to predict H2S release capability of these donors. In complex biological systems the perthiol intermediate (i.e. compound 10-Scheme 6) may react with other redox active biomolecules. Therefore, actual H2S release rates in such systems might be quite different. In addition, in some biological systems, free cysteine might be lacking due to disulfide formation or bound to proteins. When these donors are applied in such systems, extra cysteine must be added together with the donors in order to produce H2S, which may compromise the redox balance of the system under study. Therefore, careful control experiments are needed to clarify the potential problem. Nevertheless, N-(benzoylthio)benzamides provide a new H2S donor option to the researchers and we expect them will be useful tools in H2S studies. Further development of N-mercapto based H2S donors and evaluation of their biological activities are currently undergoing in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
This work is supported in part by NIH (R01GM088226) and a CAREER award from NSF (0844931).
Footnotes
Supporting Information Available: Spectroscopic and analytical data and selected experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.(a) Calvert JW, Coetzee WA, Lefer DJ. Antioxid. Redox Signal. 2010;12:1203. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gadalla MM, Snyder SH. J. Neurochem. 2010;113:14. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kabil O, Banerjee R. J. Biol. Chem. 2010;285:21903. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Szabo C. Nat. Rev. 2007;6:917. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]; (e) Lowicka E, Beltowski J. Pharmaco. Rep. 2007;59:4. [PubMed] [Google Scholar]; (f) Blackstone E, Morrison M, Roth MB. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 2.(a) Caliendo G, Cirino G, Santagada V, Wallace JL. J. Med. Chem. 2010;53:6275. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]; (b) Jacob C, Anwar A, Burkholz T. Planta Medica. 2008;74:1580. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- 3.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Proc. Natl. Acad. Sci. USA. 2007;104:17977. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Li L, Salto-Tellex M, Tang CH, Whiteman M, Moore PK. Free Radic. Biol. Med. 2009;47:103. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]; (b) Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PL. Circulation. 2008;117:2351. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 5.Baskar R, Sparatore N, Del Soldato P, Moore PK. Eur. J. Pharm. 2008;594:1. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zheng Y, Ho CT. ACS Sym. Ser. 1994:138. Ch11. [Google Scholar]; (b) Morra MJ, Dick WA. Appl. Envir. Microbiol. 1991;57:1413. doi: 10.1128/aem.57.5.1413-1417.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wang H, Xian M. Angew Chem. Int. Ed. 2008;47:6598. doi: 10.1002/anie.200801654. [DOI] [PubMed] [Google Scholar]; (b) Zhang J, Wang H, Xian M. J. Am. Chem. Soc. 2009;131:3854. doi: 10.1021/ja900370y. [DOI] [PubMed] [Google Scholar]; (c) Zhang J, Li S, Zhang D, Wang H, Whorton AR, Xian M. Org. Lett. 2010;12:4208. doi: 10.1021/ol101863s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pan J, Xian M. Chem. Commun. in press. [Google Scholar]
- 8.(a) El-Khairy L, Ueland PM, Refsum H, Graham IM, Vollset SE. Circulation. 2001;103:2544. doi: 10.1161/01.cir.103.21.2544. [DOI] [PubMed] [Google Scholar]; (b) Ueland PM. Clin. Chem. 1995;41:340. [PubMed] [Google Scholar]; (c) Nakanishi T, Hasuike Y, Otaki Y, Hama Y, Nanami M, Miyagawa K, Moriguchi R, Nishikage H, Izumi M, Takamitsu Y. Kidney Int. 2003;63:1137. doi: 10.1046/j.1523-1755.2003.00808.x. [DOI] [PubMed] [Google Scholar]; (d) van den Brandhof WE, Haks K, Schouten EG, Verhoef P. Atherosclerosis. 2001;157:403. doi: 10.1016/s0021-9150(00)00724-3. [DOI] [PubMed] [Google Scholar]
- 9.Siegel LM. Anal. Biochem. 1965;11:126. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










