Abstract
Objective
To determine if the combination of lifestyle(caloric restriction and exercise) and metformin(MET) would be superior to placebo and lifestyle(PBO) in improving PCOS phenotype.
Design
Double-blind randomized 6 month trial of MET vs PBO
Setting
Two academic medical centers
Patients
114 subjects
Interventions
Subjects collected urines daily for ovulation monitoring, had monthly monitoring of hormones/weight, and determination of body composition by DXA, glucose tolerance, and quality of life at baseline and completion.
Main outcome measures
Ovulation rates and testosterone levels
Results
Dropout rates were high. There was no significant difference in ovulation rates. Testosterone levels were significantly lower compared to baseline in the MET group at 3 mos but not at 6 mos. There were no differences in weight loss between groups, but MET showed a significant decline at 6 mos compared to baseline(−3.4 kg, 95% CI:(−5.3, −1.5)). We noted divergent effects of MET vs PBO on OGTT indices of insulin sensitivity (increased) and secretion (worsened). Total bone mineral density (BMD) increased significantly in MET. There were no differences in QOL measures between groups. MET had increased diarrhea and headache, but fewer bladder infections and musculoskeletal complaints.
Conclusions
The addition of metformin to lifestyle produced little reproductive or glycemic benefit in women with PCOS, though our study had limited power due to high dropout. It is not possible at baseline to identify women likely to drop out.
Keywords: ovarian function, insulin action, nutrition, exercise, androgen
INTRODUCTION
There are multiple treatments options for obese women with PCOS. Although PCOS is diagnosed primarily on reproductive abnormalities, i.e. chronic anovulation, hyperandrogenism and polycystic ovaries, PCOS is associated with metabolic disturbances including insulin resistance, dyslipidemia, and an increased risk for type 2 diabetes, that are exacerbated by the obesity.(1) Therapies used for the treatment and prevention of type 2 diabetes, including diabetic medications and lifestyle changes, are also used in PCOS.(2, 3) Diabetes prevention trials are often conducted in populations that overlap with obese PCOS women, and their results are often extrapolated to treatment of PCOS. Large scale trials are now testing the benefit of combination therapy for diabetes prevention.(4) The combination of lifestyle and metformin therapies has had additive benefits at preventing weight gain in other populations.(5)
Lifestyle modification in obese women with PCOS has become, at least based on expert opinion, the cornerstone of therapy for PCOS.(6, 7) The results of similar combination lifestyle/metformin trials, although on a smaller scale, have shown more modest results in PCOS often with minimal or mixed effects on the reproductive abnormalities of chronic anovulation and hyperandrogenism.(8–10) We hypothesized that the combination of lifestyle/metformin would be superior to lifestyle alone in improving ovulatory frequency and hyperandrogenism, as well as insulin sensitivity in women with PCOS.
MATERIALS AND METHODS
Subjects
The Institutional Review Boards of the Meharry Medical College and Penn Sate College of Medicine approved the study. Subjects were randomized between 2004–2007 and all gave written informed consent. We used the 1990 NIH/NICHD PCOS diagnostic criteria to identify subjects (11): chronic anovulation, defined as spontaneous intermenstrual periods of ≥45 days or a total of ≤8 menses per year, and hyperandrogenism defined as an elevated total testosterone (>50 ng/dL) or a free androgen index[ratio of testosterone/SHBG(100)] >1.5.(12) Other causes of anovulation and hyperandrogenism were excluded by appropriate tests. Subjects were age 21–39y, in good general health and currently off of confounding medications (e.g. hormonal contraceptives, diabetic medications, etc.).
Power Analysis
The primary outcome was the ovulation rate as determined by urinary progestin. We proposed an absolute difference of 25% between the two treatment arms assuming the number of observed ovulations follows a Poisson process and projected a 15% dropout rate for this trial. The ovulation rate in a double-blind, placebo controlled trial of troglitazone in PCOS was 32%.(13) Based on a projected ovulation rate of 30% in the lifestyle/placebo arm and 55% in the combined lifestyle/metformin arm, the study required a total of 58 subjects per treatment arm to have a two-sided test having 80% power with a type I error of 5%.
Subjects were randomized in a 1:1 allocation ratio to the two treatment arms each lasting 6 mos using a computer generated random number table using permuted blocks and stratified by center and prior metformin exposure status after a baseline visit. The block schedule was blinded to the investigators and research subjects. Subjects were advised to use barrier contraception and avoid pregnancy during the study in order to encourage study completion.
Study Interventions
(More complete details can be found in the Supplemental Text section) A combined intervention of diet and exercise was employed with the goal of achieving an average weight loss of at least 7% of initial body weight over six months with a prescription of 150 min/week of exercise combined with a low-calorie diet.(2)
Dietary Intervention
The number of calories to maintain current body weight was assessed by combining the analysis of three day diet records obtained during screening (Nutritionist V, First Databank, Sunnyvale, CA) with the results of the Harris Benedict Equation multiplied by an activity factor of 1.3. The target level of calorie intake was calculated as the daily calories required to maintain weight minus 500 kcal.
Exercise Training: Supervised
The exercise program consisted of supervised and non-supervised components to maximize flexibility and acceptance of the intervention. Subjects were given the opportunity to attend at least 2 sessions per week at a fitness facility run by the research team.
Unsupervised
Because logistics precluded frequent visits to the training facility, subjects were responsible for performing aerobic activity on their own to achieve a total exercise time of 150 minutes/week (including supervised sessions). Subjects completed a daily physical activity log once per month.
Medication Arm
Metformin hydrochloride was obtained as a powder (Spectrum Chemical Manufacturing, New Brunswick, NJ) and formulated with the appropriate dose of drug into capsules with identically appearing placebo capsules.(9) Drug and placebo were packaged and labeled according to subject number by the Investigational Pharmacies in a double-blind fashion. Medication was initiated in a step-up fashion every 5 days, from one tablet a day to four. This dose was maintained as tolerated throughout the remainder of the study.
Study Procedures
Urine collections (daily)
Subjects collected a first morning void urine specimen (10 ml) and refrigerated the specimen. Pregnanediol-3 alpha-Glucuronide(PdG) was determined in an aliquot of diluted human urine by competitive EIA (Immunometrics(UK)Ltd, London UK). Results were normalized to urine creatinine.
Physical Exam (monthly)
Hirsutism was assessed by trained study personnel using the modified Ferriman-Galwey score.(14) Facial lesions counts of open and closed comedones (noninflammatory lesions) were obtained from the forehead, left, and right cheeks, nose, and chin by trained study personnel.(15)
Exercise Testing (monthly)
Prior to beginning the exercise program and at each monthly visit during the study, subjects underwent a submaximal test of aerobic capacity to determine fitness levels.(16)
DXA Scan (baseline and end of study)
Body composition data were determined by dual energy x-ray absorptiometry (DXA) using a Hologic QDR-4500W (Hologic Inc., Bedford, MA). Subregion analysis of visceral and central abdominal fat were modeled.(17)
Serum Reproductive Hormones (baseline, at 3 mos, and at end of study)
LH, FSH, DHEAS, and SHBG were determined by chemi-luminescence using the Siemens (Los Angeles, CA) Immulite platform. Testosterone was measured by RIA (18) and non-SHBG testosterone(uT) by ammonium sulfate precipitation.(19)
Serum Metabolic Hormones (baseline and at end of study)
A 75-g oral glucose tolerance test with glucose and insulin levels obtained at 0, 30, 60, 90, and 120 min post-challenge was performed after an overnight fast.(20) Fasting glucose (FBG), insulin (FI), and lipids were determined as previously reported.(18) The insulinogenic index, a measure of early phase insulin secretion, was defined as (30 min. glucose–FBG) divided by (30 min. insulin–FI).(21) The insulin sensitivity index was calculated using the formula (10,000/the square root of [(FBG)(FI)][(mean OGTT glucose)(mean OGTT insulin)].(22)
Ultrasound Scan (baseline and end of study)
A transvaginal or transabdominal(in adolescents) ultrasound of the pelvis was performed.(18) Volume of the ovary was calculated using the formula for a prolate ellipsoid (length × height × width × (π/6)).(18)
PCOS quality of life survey (baseline and end of study)
The validated PCOS health related Quality of Life(HR-QOL) questionnaire includes five domains: emotional, body hair, infertility, weight, and menstrual problems.(23) Each domain score is graded on a scale of 1(poorest function) to 7(optimal) with a change of 0.5 approximating the minimal important difference, the smallest change in score that women feel is important in their daily lives.
Determination of the number of Ovulations
A modified version of the Kassam et al urinary PdG ratio algorithm was used to estimate the number of ovulations. (24). For this study, the modifications presented by O’Connor et al to the Kassam algorithm were sampling every other day with a baseline calculated using three-day rather than a five-day running average and the threshold of ratio >3 exceeded for two, not three, days(25)
Data Analysis
As there were a large number of women with zero ovulations in this study, a zero-altered negative binomial model was fit to compare the number of ovulations.(26) Linear mixed-effects models were fit to continuous outcomes to compare metformin to placebo with respect to the change from baseline measurement.(27) Adverse event rates were compared between the two treatment arms using Possion regression models.
All analyses followed the intention to treat principle whereby data from all participants were analyzed according to the assigned treatment group. All analyses were adjusted for center, prior metformin use, baseline age, and baseline BMI. For change from baseline outcomes, the models also adjusted for the baseline value of the outcome. No adjustments for multiple hypothesis testing were performed as all outcomes other than the primary are considered exploratory. All statistical tests are two-sided and all analyses were performed using SAS software (version 9.1, SAS Institute Inc., Cary, NC) with graphics constructed using S-plus (version 8.0, TIBCO Software Inc., Palo Alto, CA).
RESULTS
Baseline Characteristics and Subject Retention
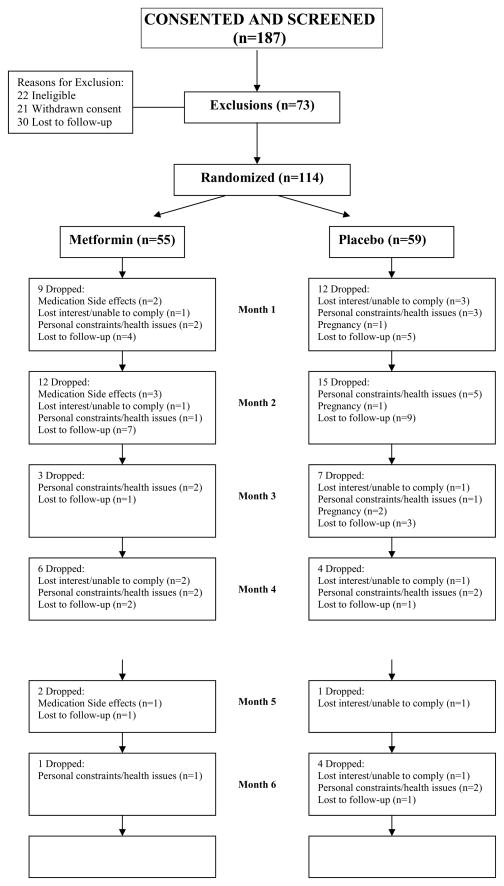
The two treatment groups were similar at baseline.(Table 1). We experienced a high drop out rate without group differences(MET: N=33(60%) vs. PBO: N=43(73%); P=0.14)(Figure 1). The most common reason for subject dropout was lost to follow up(MET: N=15 and PBO: N=19). The drop out rate for Blacks was 76% vs. 62% for Whites(P=0.14). We performed a stratification by baseline variables into study completers(N=38), late dropouts completing 3 to 6 mos of the study (N=28), and early dropouts completing 1–3 months of the study(N=48) to look for factors that would predict dropouts. We found no significant trends in baseline variables.(Supplemental Table 1).
TABLE 1.
Baseline Characteristics of treatment groups.
| Units (), Normal range or cutoff [] | Metformin (n=55) | Placebo (n=59) | ||
|---|---|---|---|---|
| Race | n | % | n | % |
| Black | 20 | 36% | 21 | 36% |
| White | 33 | 60% | 38 | 64% |
| Other | 2 | 4% | 0 | 0% |
| Biometric | Mean (SD) | Mean (SD) | ||
| Age (years) | 55 | 29.0 (4.5) | 59 | 28.8 (4.6) |
| Weight (kg) | 55 | 102.7 (22.7) | 59 | 104.1 (23.2) |
| BMI (kg/m2) [<25] | 55 | 38.0 (7.8) | 59 | 38.3 (8.0) |
| Waist (inches) [<35] | 54 | 42.7 (6.9) | 59 | 42.2 (7.3) |
| Ferriman-Gallwey Score [< 6] | 55 | 17.7 (8.3) | 59 | 19.1 (8.9) |
| Total Acne Lesion Count | 43 | 4.4 (7.4) | 46 | 2.6 (4.1) |
| Systolic Blood Pressure (mmHg) [<130] | 55 | 122.1 (17.6) | 59 | 120.0 (14.9) |
| Diastolic Blood Pressure (mmHg) [<85] | 55 | 79.5 (11.7) | 59 | 76.8 (10.2) |
| Hormones | ||||
| Testosterone (ng/dL) [< 50] | 54 | 73.6 (36.9) | 59 | 77.3 (36.9) |
| SHBG (nmol/L) [20 – 130] | 54 | 28.1 (25.4) | 56 | 26.5 (12.9) |
| Free Androgen Index [<1.5] | 53 | 13.7 (14.5) | 56 | 12.5 (8.8) |
| Estradiol (pg/ml) [20–350] | 50 | 45.7 (33.5) | 57 | 45.2 (32.2) |
| LH (mIU/ml) [0.6 – 16.3] | 54 | 8.7 (4.0) | 58 | 9.4 (8.3) |
| FSH (mIU/ml) [1.09 – 10.0] | 53 | 5.0 (1.9) | 58 | 5.0 (2.0) |
| Lipid Parameters | ||||
| Cholesterol (mg/dl) [<200] | 53 | 187.4 (55.4) | 55 | 173.0 (32.6) |
| HDL (mg/dl) [>50] | 53 | 37.1 (15.8) | 55 | 36.7 (13.2) |
| LDL (mg/dl) [<100] | 52 | 123.9 (45.7) | 55 | 114.8 (32.2) |
| Triglycerides (mg/dl) [<150] | 54 | 128.5 (87.4) | 55 | 107.8 (48.1) |
| Glycemic Parameters from OGTT | ||||
| Fasting Glucose (mg/dl) [< 100] | 55 | 89.4 (11.0) | 55 | 88.4 (8.6) |
| Fasting Insulin (μU/ml) [<35] | 51 | 12.9 (9.4) | 49 | 16.6 (13.9) |
| AUC Glucose (μU · min/mL) | 54 | 15,808 (4,089) | 55 | 15,754 (3,354) |
| AUC Insulin (mg · min/dL) | 51 | 11,242 (6,395) | 50 | 11,030 (6,565) |
| Insulinogenic Index | 50 | 2.2 (2.0) | 43 | 2.0 (3.2) |
| Insulin Sensitivity Index | 50 | 4.9 (4.5) | 44 | 5.5 (5.6) |
| Ultrasound Parameters | ||||
| Left Ovarian Volume (cm3) [< 10] | 54 | 10.3 (4.8) | 53 | 11.7 (5.7) |
| Right Ovarian Volume (cm3) [< 10] | 53 | 12.0 (6.6) | 54 | 13.0 (7.8) |
| Largest Follicle Diameter (mm) | 50 | 8.2 (3.9) | 52 | 9.8 (5.9) |
| DXA Parameters | ||||
| BMD (g/cm2) [1.00–1.17] | 49 | 1.22 (0.12) | 53 | 1.21 (0.12) |
| Total Lean (g) | 49 | 55,867 (8,340) | 53 | 56,455 (8,122) |
| Total Fat (g) | 49 | 40,923 (12,082) | 53 | 40,165 (11,523) |
| Central Abdominal Fat (g) | 47 | 3,188 (1,061) | 52 | 3,051 (952) |
| Abdominal Fat (g) | 47 | 7,208 (2,299) | 52 | 6,985 (2,072) |
| Central-to-total body fat ratio | 47 | 0.08 (0.01) | 52 | 0.08 (0.01) |
| % Body fat [25–31] | 49 | 41.4 (6.0) | 53 | 40.8 (5.8) |
| Submaximal Exercise Testing | ||||
| Estimated VO2 max ml/kg/min [31–43] | 49 | 24.3 (4.7) | 54 | 23.8 (5.8) |
| PCOS QOL Parameters | ||||
| Domain: Emotion Mean Score | 55 | 4.30 (1.25) | 59 | 3.63 (1.17) |
| Domain: Body Hair Mean Score | 55 | 3.47 (1.87) | 59 | 2.98 (1.55) |
| Domain: Weight Mean Score | 55 | 2.32 (1.43) | 59 | 1.98 (1.16) |
| Domain: Menstrual Problems Mean Score | 55 | 3.69 (1.07) | 59 | 3.36 (1.08) |
| Overall Physical Well-Being | 55 | 3.75 (1.48) | 59 | 3.46 (1.33) |
| Overall Emotional Well-Being | 55 | 4.16 (1.58) | 59 | 3.61 (1.33) |
| Overall General Well-Being | 55 | 4.31 (1.33) | 59 | 3.88 (1.22) |
Figure 1.
Flow Chart of Study Participants
Primary and Secondary Outcomes
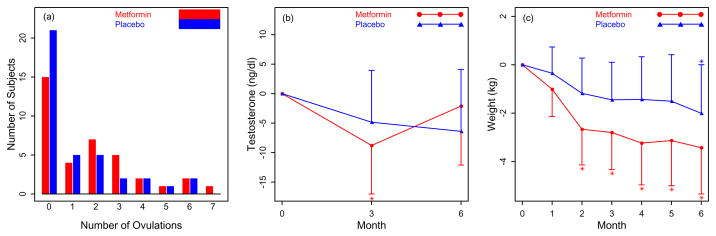
There was no difference in ovulation rates between groups(Figure 2a). The ovulation rate ratio comparing MET to PBO was 2.5(95% CI: (0.9, 6.6); P = 0.07) with respect to whether a woman ovulated or not; whereas, the ovulation rate ratio comparing MET to PBO was 1.2(95% CI: (0.7, 1.9); P = 0.51) with respect to the number of ovulations provided a woman actually had ovulated. Testosterone levels, however, were only significantly less at 3 mos in MET compared to baseline(Figure 2b) Weight declined significantly from baseline at all points after the first month in MET, but the decrease was not significantly different compared to PBO(Figure 2c).
Figure 2.
Primary Outcome A Frequency of the number of ovulations by Urinary PdG per treatment arm. There was no difference between groups. This model adjusted for the covariates of prior metformin use, center, baseline age, and baseline body mass index in addition to use of the logarithm of the number of days on the trial as on offset to help account for subject dropout. Secondary Outcomes B. Change in testosterone levels at baseline, 3 mos and study completion at 6 mos per treatment arm. C. Change in weight (kg) by month per treatment arm. * P < 0. 05 for the change from baseline within the metformin group.
Other Outcomes at 6 months
Exercise parameters were not significantly different between treatment groups (Supplemental Text). There was a significant decrease in systolic blood pressure at 6 mos compared to baseline for PBO only, but no change in diastolic blood pressure or hirsutism or acne scores within either group.(Table 2) There was a significant improvement in the area under the curve(AUC) glucose in PBO compared to baseline and to MET(P<0.001 and P=0.002, respectively)(Supplemental Figure 1). Although there were favorable changes compared to baseline in individual domains in the QOL questionnaire such as for emotion and weight within each treatment group, there were no significant differences in the overall well-being scores between treatment arms at 6 mos. There were also no changes in ovarian volume or size of the largest follicle compared to baseline or between treatment groups (data not shown).
TABLE 2.
Mean change in key parameters in the PCOS phenotype compared to baseline (first two columns) and difference between treatment groups (last column on right).
| Metformin | Placebo | Difference (Metformin-Placebo) | ||||
|---|---|---|---|---|---|---|
| Mean Change (95% CI) | P-value | Mean Change (95% CI) | P- value | Mean Change (95% CI) | P-value | |
| Biometric | ||||||
| Waist (inches) | −2.1 (−3.0,−1.2) | <.001 | −1.6 (−2.6,−0.7) | 0.001 | −0.5 (−1.8,0.8) | 0.47 |
| Ferriman-Gallwey Score | 1.2 (−1.1,3.6) | 0.30 | −0.2 (−2.8,2.3) | 0.86 | 1.5 (−2.0,4.9) | 0.40 |
| Total Acne Lesion Count | −1.1 (−2.5,0.4) | 0.14 | −0.7 (−2.1,0.8) | 0.38 | −0.4 (−2.4,1.6) | 0.68 |
| Systolic Blood Pressure (mmHg) | −2.9 (−6.7,0.8) | 0.12 | −4.1 (−8.2,−0.02) | 0.05 | 1.2 (−4.3,6.7) | 0.66 |
| Diastolic Blood Pressure (mmHg) | −1.2 (−4.6,2.1) | 0.47 | −3.4 (−7.1,0.4) | 0.08 | 2.2 (−2.8,7.1) | 0.39 |
| Hormones | ||||||
| Testosterone (ng/dl) | −2.1 (−12.1,7.9) | 0.67 | −6.4 (−16.9,4.1) | 0.23 | 4.3 (−9.9,18.4) | 0.54 |
| SHBG (nmol/L) | 1.7 (−1.9,5.2) | 0.35 | 3.8 (−0.3,7.8) | 0.07 | −2.1 (−7.4,3.2) | 0.43 |
| Free Androgen Index | −2.3 (−5.1,0.6) | 0.12 | −1.6 (−4.9,1.6) | 0.32 | −0.6 (−4.8,3.6) | 0.76 |
| Estradiol (pg/ml) | 8.1 (−17.1,33.4) | 0.52 | 15.6 (−10.3,41.5) | 0.23 | −7.5 (−43.4,28.5) | 0.68 |
| LH (mIU/ml) | 1.0 (−1.3,3.4) | 0.37 | −0.5 (−3.2,2.3) | 0.73 | 1.5 (−2.0,5.0) | 0.38 |
| FSH (mIU/ml) | −0.2 (−1.0,0.7) | 0.70 | −0.1 (−1.1,0.9) | 0.88 | −0.1 (−1.4,1.2) | 0.89 |
| Lipid Parameters | ||||||
| Cholesterol (mg/dl) | −7.8 (−19.0,3.3) | 0.16 | −4.2 (−16.8,8.4) | 0.50 | −3.7 (−20.0,12.6) | 0.65 |
| HDL (mg/dl) | 5.2 (1.7,8.7) | 0.005 | 7.4 (3.5,11.4) | <.001 | −2.2 (−7.1,2.6) | 0.35 |
| LDL (mg/dl) | −7.5 (−17.7,2.6) | 0.14 | −8.1 (−19.4,3.3) | 0.16 | 0.5 (−14.2,15.3) | 0.94 |
| Triglycerides (mg/dl) | −14.0 (−31.1,3.0) | 0.10 | −3.9 (−23.5,15.7) | 0.69 | −10.2 (−35.0,14.7) | 0.41 |
| Glycemic Parameters from OGTT | ||||||
| Fasting Glucose (mg/dl) | −2.9 (−7.8,2.1) | 0.25 | 2.8 (−3.1,8.7) | 0.34 | −5.7 (−13.0,1.6) | 0.12 |
| Fasting Insulin (μU/ml) | 2.7 (−6.0,11.4) | 0.52 | 5.1 (−6.5,16.7) | 0.38 | −2.3 (−17.0,12.3) | 0.75 |
| AUC Glucose (μU · min/mL) | −105 (−1110,899) | 0.83 | −2109 (−3293,−925) | 0.001 | 2004 (566,3441) | 0.008 |
| AUC Insulin (mg · min/dL) | −626 (−2433,1182) | 0.48 | 725 (−1551,3002) | 0.52 | −1351 (−4121,1418) | 0.32 |
| Insulinogenic Index | −0.5 (−0.9,−0.1) | 0.01 | 0.8 (0.3,1.2) | 0.003 | −1.3 (−1.9,−0.7) | <.001 |
| Insulin Sensitivity Index | 1.9 (−1.0,4.8) | 0.19 | −2.7 (−6.2,0.9) | 0.13 | 4.6 (0.1,9.0) | 0.05 |
| DXA Parameters | ||||||
| Total BMD (g/cm2) | 0.016 (0.005,0.027) | 0.007 | −0.008 (−0.019,0.004) | 0.17 | 0.023 (0.009,0.037) | 0.002 |
| Total Lean (g) | −431 (−1732,870) | 0.50 | −53 (−1463,1358) | 0.94 | −378 (−2106,1349) | 0.65 |
| Total Fat (g) | −3603 (−5879,−1326) | 0.003 | −1369 (−3742,1004) | 0.24 | −2234 (−5195,727) | 0.13 |
| Central Abdominal Fat (g) | −201 (−451,50) | 0.11 | −307 (−606,−8) | 0.04 | 106 (−232,444) | 0.52 |
| Abdominal Fat (g) | −255 (−773,263) | 0.32 | −579 (−1180,22) | 0.06 | 324 (−361,1009) | 0.34 |
| Central-to-total body fat ratio | 0.001 (−0.002,0.005) | 0.34 | −0.005 (−0.009,−0.001) | 0.02 | 0.006 (0.002,0.011) | 0.007 |
| % Body fat | −2.2 (−3.3,−1.0) | <.001 | −0.8 (−2.0,0.4) | 0.18 | −1.4 (−2.9,0.1) | 0.07 |
| PCOS QOL Parameters | ||||||
| Domain: Emotion Mean Score | 0.41 (−0.06,0.87) | 0.09 | 0.75 (0.22,1.29) | 0.008 | −0.35 (−1.03,0.33) | 0.31 |
| Domain: Body Hair Mean Score | 0.41 (−0.25,1.08) | 0.21 | 0.64 (−0.11,1.38) | 0.09 | −0.23 (−1.16,0.71) | 0.62 |
| Domain: Weight Mean Score | 0.74 (−0.04,1.51) | 0.06 | 1.42 (0.56,2.27) | 0.002 | −0.68 (−1.75,0.39) | 0.21 |
| Domain: Menstrual Problems Mean Score | 0.41 (−0.07,0.90) | 0.09 | 0.32 (−0.22,0.85) | 0.23 | 0.10 (−0.58,0.77) | 0.77 |
| Overall Physical Well-Being | 0.33 (−0.25,0.90) | 0.26 | 0.61 (−0.03,1.24) | 0.06 | −0.28 (−1.08,0.51) | 0.47 |
| Overall Emotional Well-Being | −0.13 (−0.82,0.57) | 0.71 | 0.01 (−0.74,0.77) | 0.97 | −0.14 (−1.10,0.82) | 0.77 |
| Overall General Well-Being | 0.09 (−0.46,0.65) | 0.73 | 0.62 (0.01,1.23) | 0.05 | −0.53 (−1.30,0.25) | 0.18 |
Side Effects and Adverse Events
There were 6 dropouts in MET for medication side effects and none in PBO. In MET diarrhea and headaches were significantly more common, while bladder infections and musculoskeletal complaints were less common (Supplemental Table 2). There were no differences in the average percent days of vaginal bleeding during the study, 15±11% for MET vs 11±11% for PBO. There were four pregnancies, all in PBO. These resulted in one first trimester miscarriage, one elective abortion, and one full term birth of a healthy male without maternal or fetal pregnancy complications. The fourth pregnancy was lost to follow up.
DISCUSSION
We found that the addition of metformin to lifestyle had no significant benefit on ovulation and circulating androgen levels,. However due to high dropout, our study had limited power to detect differences between the treatment arms. Nevertheless we noted beneficial effects of the addition of metformin to lifestyle including improved insulin sensitivity and increased bone mineral density. There was no benefit to either treatment on QOL or skin parameters, i.e. acne or hirsutism, though our trial may have been too short. With the exception of increased diarrhea and fewer bladder infections in MET, the therapies were equally well tolerated and safe.
The strengths of our study include the relatively large numbers of subjects recruited into a combination lifestyle study, their racial and socioeconomic diversity, and the detailed study of reproductive and metabolic effects of treatment, including QOL measures. (29) We found no improvement in our primary outcome of ovulation rates by adding metformin to lifestyle., similar to the larger study of Tang et al,(8) which reported change in self reported menstrual frequency and did not quantify ovulation. Similarly a smaller study in PCOS adolescents showed no change in ovulation, based on urinary PdG levels, following lifestyle/metformin therapy.(10) These studies also did not detect any additional benefit of lifestyle/metformin.
Our study demonstrated a significant benefit in weight loss with metformin, at least compared to baseline(~3 kg). This is consistent with the largest trial of metformin alone(with no lifestyle modification) in PCOS, where the six month weight loss was ~2 kg,(30) and with the Diabetes Prevention Program where metformin was compared to placebo(~2 kg at 6 mos). Other studies have shown similar modest decreases in weight with combination or lifestyle therapy.(8–10) However there are studies of lifestyle therapy alone in women with PCOS, which have shown significantly more weight loss over a comparable period(as much as 8–10 kg).(31)
Metformin may have multiple metabolic benefits in women with PCOS. (32) Metformin significantly decreased body fat and also raised circulating HDL cholesterol. Fasting or glucose challenged glucose levels and the insulinogenic index were unaltered.(33) Improvements in the insulinogenic index lower diabetes risk, so the relative benefit of lifestyle alone on this parameter is probably small. The relative small impact of combination therapy on glycemic parameters is supported by other studies in women with PCOS with metformin added to oral contraceptives(34) or to lifestyle(9, 10). Given the absence of glycemic improvement, weight loss may be the primary metabolic benefit of metformin in our study.(33)
Increased BMD in the metformin group, after such a short period. has been seen after prolonged metformin treatment in girls with premature pubarche,(35) perhaps due to favorable effects on circulating sex steroids. The impact of this finding in our older group is probably minor. We noted no effect of treatment on the hip, and women with PCOS have generally been noted to have normal or increased bone mineral density, without increased risk for Osteoporosis and hip fractures. (36) Though pregnancy was considered an adverse event, we note that all pregnancies occurred in the placebo arm, further questioning the reproductive benefits of metformin,(30) and supporting lifestyle alone as a treatment for infertility in these women.
Our study has limitations. Recruitment was slow and dropout was high. It is possible that with more subjects and a higher retention, we would have demonstrated more benefit with metformin. However other lifestyle trials in PCOS have had high dropout, in adolescents,(10) and in an Australian trial of adults.(31) Our project included an urban minority medical center that focused on recruiting Blacks. The difficulty in recruiting and retaining minority women into clinical trials is well known (37),. Our study completion rate of 47% in Whites was comparable to that in the Australian trial (50%) that lasted 20 weeks versus our longer 24 week trial. We further examined factors at baseline and could not identify factors that predicted dropout. We conclude that it is difficult to individualize lifestyle therapy to those likely to complete all or part of it.
Our trial also enrolled many subjects who were severely or morbidly obese, subjects with the most difficulty complying and responding to lifestyle changes. Because of these drawbacks, some lifestyle studies in PCOS exclude subjects in this BMI range.(38) Our subject’s predicted VO2 max did not change during the trial, indicating that even the subjects who stayed in the protocol did not increase their aerobic capacity. Similar aerobic capacity and muscle strength has been noted in PCOS as weight matched controls, such that PCOS per se in unlikely to limit physical activity.(39)
Overall although the effects of lifestyle/metformin, alone or in combination, are generally beneficial, the absolute changes are quite modest. Many women with PCOS are unwilling to participate in such interventions or lose motivation and dropout early in the process. Thus the external validity of such studies, given the low rate of study completion, is questionable. These results bring into doubt routine recommendations that lifestyle is effective therapy for severely obese women with PCOS.(6, 7) More meaningful reproductive and metabolic changes in women with PCOS may be achieved in this weight group with massive weight loss, such as from bariatric surgery.(40)
Supplementary Material
Glucose and insulin levels during 2h oral glucose tolerance test at baseline and study completion per treatment arm. There were no differences between groups at any timepoints.
Acknowledgments
We wish to acknowledge the excellent coordination and oversight to this long term study provided by Barbara Dailey and Joy Vassel at Meharry and Patsy Rawa at Penn State College of Medicine, and the nursing staff of the GCRC at Penn State Hershey Medical Center who were all part of the Meharry-Penn State U54 Reproductive Center. We wish to also acknowledge the contributions to the analyses of Christy Stetter in the Dept of Public Health Sciences at Penn State. Finally we are grateful to the subjects who choose to participate in our study.
Funding/Support: This work was supported by PHS U54 HD044315 The Meharry Medical College/Penn State Cooperative Reproductive Science Center and a GCRC grant MO1 RR 10732, and GCRC construction grant C06 RR016499 to Pennsylvania State University.
Footnotes
Financial Disclosure: Allen Kunselman reports ownership of Merck stock. Richard Legro reports a paid lecture fee from Serono.
Author Contributions All authors researched data, contributed to discussion, and reviewed/edited data.
Previous Presentation: Portions of this article were presented at the 92nd Annual Meeting of the Endocrine Society, San Diego, CA, June 18-22nd, 2010.
Trial Registration: Clinical trials.gov NCT00151411
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Harris SB, Gerstein HC, Young TK, Raboud JM, Neuman J, et al. Preventing type 2 diabetes using combination therapy: design and methods of the CAnadian Normoglycaemia Outcomes Evaluation (CANOE) trial. Diabetes Obes Metab. 2006;8:531–7. doi: 10.1111/j.1463-1326.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–93. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 6.Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–22. doi: 10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92:1966–82. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21:80–9. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- 9.Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82:421–9. doi: 10.1016/j.fertnstert.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 10.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome; towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 12.Katcher HI, Kunselman AR, Dmitrovic R, Demers LM, Gnatuk CL, Kris-Etherton PM, et al. Comparison of hormonal and metabolic markers after a high-fat, Western meal versus a low-fat, high-fiber meal in women with polycystic ovary syndrome. Fertil Steril. 2009;91:1175–82. doi: 10.1016/j.fertnstert.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001 Apr;86:1626–32. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 14.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–30. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 15.Thiboutot D, Zaenglein A, Weiss J, Webster G, Calvarese B, Chen D. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792–800. doi: 10.1016/j.jaad.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Golding LA, Myers CR, Sinning WE. Y’s Way to Fitness: The complete guide to fitness testing and instruction. Champaign IL: Human Kinetics; 1989. [Google Scholar]
- 17.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–8. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 18.Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab. 2005;90:2571–9. doi: 10.1210/jc.2004-0219. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999 Oct;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 20.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 21.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion [see comments] Diabet Med. 1994;11:286–92. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 23.Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS) J Clin Endocrinol Metab. 1998;83:1976–87. doi: 10.1210/jcem.83.6.4990. [DOI] [PubMed] [Google Scholar]
- 24.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environmental Health Perspectives. 1996;104:408–13. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor KA, Brindle E, Miller RC, Shofer JB, Ferrell RJ, Klein NA, et al. Ovulation detection methods for urinary hormones: precision, daily and intermittent sampling and a combined hierarchical method. Hum Reprod. 2006;21:1442–52. doi: 10.1093/humrep/dei497. [DOI] [PubMed] [Google Scholar]
- 26.Hilbe JM. Negative Binomial Regression. New York: Cambridge University Press; 2007. [Google Scholar]
- 27.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Archives of Internal Medicine. 2001;161:1581–6. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 28.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–8. [PubMed] [Google Scholar]
- 29.Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2008;14:15–25. doi: 10.1093/humupd/dmm030. [DOI] [PubMed] [Google Scholar]
- 30.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 31.Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3373–80. doi: 10.1210/jc.2008-0751. [DOI] [PubMed] [Google Scholar]
- 32.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 33.Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes. 2007;56:1153–9. doi: 10.2337/db06-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4649–54. doi: 10.1210/jc.2002-021688. [DOI] [PubMed] [Google Scholar]
- 35.Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. Metformin treatment for four years to reduce total and visceral fat in low birth weight girls with precocious pubarche. J Clin Endocrinol Metab. 2008;93:1841–5. doi: 10.1210/jc.2008-0013. [DOI] [PubMed] [Google Scholar]
- 36.Zborowski JV, Cauley JA, Talbott EO, Guzick DS, Winters SJ. Clinical review 116: bone mineral density, androgens, and the polycystic ovary: the complex and controversial issue of androgenic influence in female bone. [Review] [113 refs] J Clin Endocrinol Metab. 2000 Oct;85:3496–506. doi: 10.1210/jcem.85.10.6902. [DOI] [PubMed] [Google Scholar]
- 37.Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol. 2000;10:S13–21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 38.Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23:642–50. doi: 10.1093/humrep/dem391. [DOI] [PubMed] [Google Scholar]
- 39.Thomson RL, Buckley JD, Moran LJ, Noakes M, Clifton PM, Norman RJ, et al. Comparison of aerobic exercise capacity and muscle strength in overweight women with and without polycystic ovary syndrome. Bjog. 2009;116:1242–50. doi: 10.1111/j.1471-0528.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 40.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millan JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90:6364–9. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glucose and insulin levels during 2h oral glucose tolerance test at baseline and study completion per treatment arm. There were no differences between groups at any timepoints.