Abstract
Glucose-stimulated insulin secretion [GSIS] involves a sequence of metabolic events leading to small G-protein [e.g., Rac1]-mediated cytoskeletal remodeling to promote granule mobilization toward the plasma membrane for fusion and release of insulin. Existing evidence supports a positive modulatory role for Rac1 in GSIS. Specific regulatory factors of Rac1 function, including the guanine nucleotide exchange factors [e.g., Tiam1] have also been identified and studied in the islet. Inhibition of Tiam1/Rac1 signaling axis attenuates GSIS suggesting its pivotal role in insulin secretion. In addition to its positive [i.e., friendly] roles in GSIS, Rac1 also plays “non-friendly” role[s] in the islet function. For example, it up-regulates the intracellular reactive oxygen species [ROS] levels via activation of phagocyte-like NADPH oxidase [Nox]. Despite the emerging evidence that a tonic increase in intracellular ROS is necessary for GSIS, experimental evidence also suggests that chronic exposure of β-cells to high glucose, palmitate or cytokines results in the onset of oxidative stress leading to reduction in mitochondrial membrane potential, cytosolic accumulation of cytochrome C and activation of caspase-3 leading to β-cell apoptosis. Pharmacological and molecular biological inhibition of Rac1 activation affords partial protection against Nox-induced oxidative stress and mitochondrial dysfunction induced by elevated glucose, lipids or cytokines. Herein, we overview the existing evidence to suggest positive as well as negative modulatory roles of Rac1 in islet function. Potential avenues for future research including development of inhibitors to halt the Rac1-Nox activation and generation of oxidative stress leading to the metabolic dysfunction of the β-cell are discussed.
Keywords: Rac1, Tiam1, NADPH-oxidase, Pancreatic islet, mitochondrial dysfunction and insulin secretion
1. Introduction
Insulin secretion from the pancreatic β-cell is regulated principally by the extracellular concentration of glucose. However, the molecular and cellular mechanisms underlying the stimulus-secretion coupling of glucose-stimulated insulin secretion [GSIS] remain only partially understood. It is widely accepted that GSIS is mediated largely via the generation of soluble second messengers, such as cyclic nucleotides and hydrolytic products synthesized by phospholipases A2, C and D [1, 2]. The principal signaling cascade involves the glucose-transporter protein [i.e., Glut-2]-mediated entry of glucose into the β-cell resulting in an increase in the intracellular ATP/ADP ratio as a consequence of glucose metabolism. Such an increase in ATP levels culminates in the closure of membrane-associated ATP-sensitive potassium channels resulting in membrane depolarization followed by influx of the extracellular calcium through the voltage-gated calcium channels on the plasma membrane. A net increase in the intracellular calcium that occurs via the influx of extracellular calcium into the cytosolic fraction of the stimulated β-cell, in addition to the mobilization of calcium from the intracellular storage compartments, has been shown to play critical roles in insulin secretion.
It is well established that small G-proteins [e.g., Cdc42 and Rac1] play a significant role in cytoskeletal remodeling thereby favoring mobilization of secretory granules to the plasma membrane for fusion and release of their cargo into circulation. Published evidence from multiple laboratories [recently reviewed in 3, 4] have clearly implicated regulatory roles of small G-proteins [e.g., Cdc42 and Rac1] in GSIS. In addition, recent investigations have also suggested novel regulatory roles for ADP-ribosylation factor 6 [Arf6] in insulin secretion [5, 6]. As will be discussed in the following sections specific regulatory factors for G-proteins [i.e., guanine nucleotide exchange factors; GEFs and guanine nucleotide dissociation inhibitors; GDIs] have also been identified and studied extensively in the islet β-cell [3, 4]. In addition to its positive modulatory role in insulin secretion, Rac1 has also been implicated in the metabolic dysregulation of the β-cell, specifically at the level of generation of reactive oxygen species [ROS] thereby creating oxidative stress and subsequent dysregulation of the β-cell. Thus, the overall objective of this commentary is to propose a model in favor of “friendly” and “unfriendly” roles of Rac1 in islet β-cell function.
2. Materials and Methods
2. 1 Materials
Glucose, palmitic acid and antibody for actin were from Sigma [St. Louis, MO]. Interleukin-1β, IFN-γ and TNF-α were from R&D Systems [Minneapolis, MN]. Rac1 activation assay kit was from Cytoskeleton Inc [Denver, CO]. Rac1-siRNA and scrambled siRNA were from Ambion [Foster City, CA]. p47phox antiserum was from Santa Cruz Biotechnology, Inc [Santa Cruz, CA]. HiPerFect transfection reagent was from Qiagen [Valencia, CA]. The rat insulin ELISA kit was from American Laboratory Products [Windham, NH]. C2-Ceramide, NSC23766, GGTI-2147 and Rac1 antisera were from Calbiochem [San Diego, CA].
2.2 Methods
2.2.1 Insulin release studies
INS 832/13 cells were transfected with either scrambled siRNA [negative control] or siRNA targeted against Rac1 [Rac1-siRNA] at a final concentration of 100 nM for 24 h. Extent of Rac1 knockdown, as determined by Western blot analysis, was found to be ~ 50%. At confluence [~80%], cells were cultured overnight in low serum low glucose media and then incubated with Krebs-Ringer bicarbonate buffer for 1 h prior to stimulation with low [2.5 mM] or high glucose [20 mM] for 30 min at 37°C. Insulin released into the medium was quantified by ELISA and expressed as ng/mL [6].
2.2.2 Effect of glucose, palmitate, C2-ceramide and cytomix on the expression profile of p47phox in pancreatic β-cells
INS-832/13 cells or normal rat islets were incubated with either low glucose [2.5 mM] or high glucose [20 mM] or C2-CER [30 μM] or PA [100 μM] or cytomix [IL-1β, TNFα, IFN-γ = 10 ng/mL] as indicated in the text. Cells were homogenized in mannitol-HEPES buffer (250 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EGTA, and 1 mM DTT) containing protease inhibitor cocktail. An equal amount of homogenate was used for detection of p47phox expression by Western blot analysis and probed with actin for equal loading.
2.2.3 Rac1 activation assay
INS 832/13 cells or normal rat islets were incubated overnight in low serum low glucose media in the presence of either diluent or NSC23766 [20 μM] or GGTI-2147 [10 μM]. Further cells were either treated with low glucose [5 mM] or high glucose [20 mM] or C2-CER [30 μM] or PA [100 μM] or cytomix [IL-1β, TNFα, IFN-γ = 10 ng/mL] as mentioned in the figure text in the continuous presence or absence of above mentioned inhibitors. Lysates [~ 500 μg protein] were clarified for 5 min at 4800 × g, and PAK-PBD [p21-activated kinase-binding domain] beads [20 μl] were added to the supernatant. The mixture was then rotated for 1 h at 4°C and centrifuged at 4000 × g for 3 min. The pellet obtained was washed with 25 mM Tris, pH 7.5 containing 30 mM MgCl2, 40 mM NaCl, and 150 mM EDTA. Proteins in the pellet were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and Western blotted for relative abundance of activated Rac1 [6].
3. What is the evidence for friendly roles of Rac1 in islet β-cell function?
In 1989, Didsbury et al first identified and implicated a novel class of Rac [ras-related C3 botulinum toxin substrate] proteins in cellular function [7]. They isolated two cDNAs encoding proteins with ~92% homology, which were designated as Rac1 and Rac2. Evidence was also provided to suggest that the Rac proteins vastly differed in their tissue distribution and biological function. Interestingly, transfection experiments in myeloid cells demonstrated that Rac1 and Rac2 undergo ADP ribosylation by C3 component of botulinum toxin leading to their membrane association. Based on these findings, these investigators proposed roles for Rac1 and Rac2 in secretory functions. A Pubmed search for Rac1 yielded nearly 4,000 publications, to substantiate the proposal that it is involved in the regulation of a variety of cellular functions, including cytoskeletal organization and remodeling, superoxide generation and secretion. The reader is referred to recent reviews on Rac1 for its roles in the regulation of cellular function [3, 4, 8, 9, 10]. At the outset we would like to state that both Rac1 and Rac2 have been shown to be expressed in insulin-secreting cells [3, 4, 6, 11]. Further, given the significant homology between Rac1 and Rac2, it is likely they both could play the friendly and non-friendly roles in islet function. However, only the regulatory roles of Rac1 will be discussed below due to the paucity of available data on roles of Rac2 in islet function.
It is important to note that, in addition to the regulation by adenine nucleotides [e.g., ATP], several lines of evidence implicated positive modulatory roles for guanine nucleotides [e.g., GTP] in physiological insulin secretion. For example, using selective inhibitors of GTP biosynthetic pathway [e.g., mycophenolic acid], Metz et al first demonstrated a permissive role for GTP in GSIS [12]. Although the precise mechanisms underlying the roles of GTP in GSIS remain to be completely understood, it is now well established that it might involve activation of one [or more] G-proteins within the islet β-cell. At least two major groups of G-proteins have been identified and studied in the islet. The first group is comprised of trimeric G-proteins, which are involved in the coupling of various G-protein coupled receptors to their intracellular effector proteins [e.g., adenylate cyclases, phospholipases]. The second group is composed of small G-proteins, which are involved in protein sorting and actin cytoskeletal remodeling. The existing evidence clearly suggests that GSIS involves a well coordinated, small G-protein-mediated actin cytoskeletal rearrangements conducive for docking and fusion of insulin-laden secretory granules with the plasma membrane [3, 4].
3.1. Geranylgeranylation inhibitors as tools to study roles of Rac1 in islet function
The majority of small G-proteins and the γ-subunits of trimeric G-proteins are endowed with a unique C-terminal sequence [i.e., the CAAX motif], which makes them suitable for a sequence of post-translational modifications [4, 13, 14]. The first of these modifications involves attachment of either 15-carbon [i.e., farnesylation] or a 20-carbon [i.e., geranylgeranylation] derivative of mevalonic acid at the C-terminal cysteine via a thioester linkage. These reactions are catalyzed by farnesyltransferases [FTases] and geranylgeranyltransferases [GGTases], respectively. Examples of farnesylated proteins include Ras and nuclear lamins. Rho subfamily of G-proteins, including Cdc42 and Rac1 undergo geranylgeranylation. Following prenylation, the three terminal amino acids after the isoprenylated cysteine are cleaved by a protease, resulting in the exposure of the carboxylate anion of the prenylated cysteine residue. This site is then subjected to methylation by a prenyl cysteine methyltransferase, which neutralizes the carboxylate anion, thus making the candidate G-proteins more hydrophobic resulting in their translocation to the membranous sites for interaction with their effector proteins [4, 14].
In further support of the hypothesis that posttranslational geranylgeranylation of G-proteins [e.g., Rac1] is necessary for GSIS are our findings, which suggested that overexpression of an inactive mutant of the common α-subunit of FTase/GGTase markedly attenuated GSIS in INS 832/13 cells and normal rodent islets [15]. These molecular biological observations were further confirmed by the pharmacological observations, which demonstrated a significant reduction in GSIS by inhibitors of GGTase [e.g., GGTI-2368 and GGTI-2147]. Data from multiple laboratories have demonstrated a significant activation of Rac1 [i.e., the GTP-bound form of Rac1] by glucose in a variety of clonal β [INS-1, MIN6, β-TC3 and INS 832/13] cells, normal rat and mouse islets [3, 4, 16, 17, 18]. Such an increase in the activation of Rac1 was followed by its translocation to the membrane fraction [15]. Inhibition of prenylation [via overexpression of inactive mutant of FTase/GGTase α-subunit or pharmacological inhibitors] led to selective accumulation of Rac1 in the cytosolic fraction. Together, these data suggested that geranylgeranylation of Rac1 is necessary for its optimal activation and function.
3.2 Lessons learnt on potential Insulin for secretory abnormalities in Rac1-knockout models
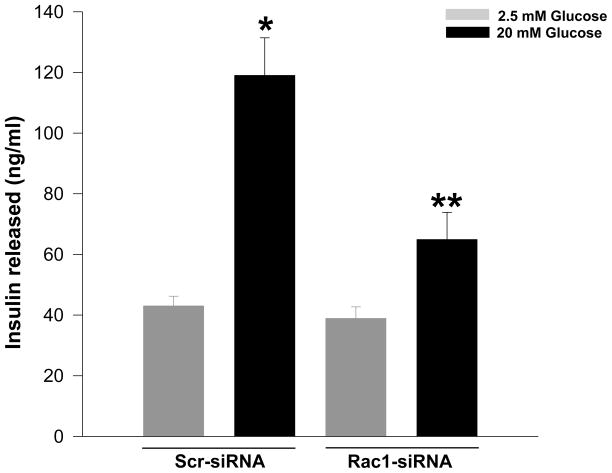
In an alternate approach to the above, Li and coworkers have demonstrated that overexpression of a dominant negative Rac1 mutant [N17Rac1] markedly reduced glucose- and forskolin-, but not KCl-induced insulin secretion in INS-1 cells [16]. N17Rac1 also induced significant morphological changes including the disappearance of F-actin. It was concluded that Rac1 activation may be necessary for the recruitment of secretory granules through actin cytoskeletal reorganization and remodeling [16]. In support of these observations are findings from our laboratory demonstrating a marked reduction in mastoparan [a global G-protein activator]-induced insulin secretion in βTC3 cells overexpressing the inactive N17Rac1 mutant [19]. Lastly, we also observed that siRNA-mediated knockdown of Rac1 in INS 832/13 cells [~ 50% knockdown] markedly attenuated GSIS, without affecting basal secretion [Figure 1], suggesting positive modulatory roles for Rac1 in insulin secretion.
Figure 1. siRNA-mediated knockdown of Rac1 markedly attenuates GSIS in INS 832/13 pancreatic β-cells.
INS 832/13 cells were transfected either with scrambled siRNA [Scr-siRNA] or Rac1-siRNA at a final concentration of 100 nM as indicated in the figure. Following 24 h culture in regular medium, cells were starved overnight and further stimulated with either low [2.5 mM] or high [20 mM] glucose for 30 min. Insulin released into the medium was quantitated by ELISA and expressed as ng/mL [see Methods for experimental details]. Data are mean ± SEM from three independent experiments. * represents p < 0.05 vs. Scr-siRNA transfected low glucose and ** p < 0.05 vs. Scr-siRNA transfected high glucose.
Additional support for a regulatory role for Rac1 in insulin secretion comes from the Rac1 knockout animal models. For example, Rac1-null [βRac1−/−] mice exhibited impaired glucose tolerance and hypoinsulinemia. Glucose-, but not KCl-induced insulin secretion, was markedly attenuated in islets from the Rac1 null mice. The β-cell mass or islet density remained unaltered in these mice. Based on these findings, it was concluded that Rac1 plays a key regulatory role in insulin secretion primarily through regulating cytoskeletal reorganization [20]. In another related study, Greiner et al demonstrated that Rac1 null mice exhibited marked alterations in islet morphogenesis [21]. The β-cell spreading and migration were significantly reduced in this model. Cell to cell contact of D-cadherin was also increased in Rac1-null mice. Actin remodeling and cell spreading induced by betacellulin was also not demonstrable in the transgenic islets. This is the first study to suggest a role for Rac1 in islet morphogenesis.
Taken together, the findings reviewed above provide significant support to the viewpoint that Rac1 plays a positive modulatory role in islet function including GSIS. It is also clear from the above findings that prenylation of Rac1 is necessary not only for its membrane translocation and association, but also to gain the GTP-bound active conformation. It is also important to note that a growing body of evidence appears to suggest that the activation [GTP-bound]-deactivation [GDP-bound] cycles of Rac1 are critical for GSIS to occur [3, 4]. Such signaling steps appear to be mediated via the intermediacy of specific regulatory factors. These include GDP-dissociation inhibitors [GDIs] and guanine nucleotide exchange factors [GEFs]. Following is a brief description of these factors/proteins, which might serve as potential therapeutic targets to halt Rac1-mediated metabolic dysfunction under conditions of chronic exposure of isolated β-cells to elevated glucose [i.e., glucotoxicity], saturated fatty acids [i.e., lipotoxicity] or a mixture of proinflammatory cytokines [i.e., IL-1β, TNFα and IFNγ].
3.3. Rac1 functions in the β-cell are modulated by specific regulatory factors
Published evidence describes the expression and regulation of GDIs in the pancreatic β-cell [17, 18, 22]. As reviewed recently in [4], GDIs have been shown to: [i] prevent dissociation of GDP from G-proteins; [ii] inhibit the intrinsic and GTPase-activating protein-catalyzed hydrolysis of GTP; [iii] sequester back specific G proteins [e.g., Rac1 and Cdc42] from their membranous sites, thereby inhibiting their interaction with their respective effector proteins; and [iv] regulate spatial determination in the actin cytoskeletal control. Using several complementary experimental approaches, a potential association between GDI and Rac1 in isolated β-cells was reported recently. In addition, overexpression of the wild-type GDI significantly inhibited GSIS in INS 832/13 cells. These findings were further supported by studies in which expression of endogenous GDI was knocked down by siRNA-GDI, which demonstrated that siRNA-mediated knockdown of endogenous GDI resulted in a significant increase in GSIS [17]. Together, these findings suggested an inhibitory role for GDI in the glucose metabolic signaling cascade, which may be relevant for GSIS. Based on the experimental evidence available up until now, it is proposed that GDIs play a negative modulatory role in the cascade of events leading to GSIS by retaining the candidate G-proteins [e.g., Rac1] in their inactive GDP-bound conformation.
In addition to GDIs, expression of GEFs in the islet β-cell was also reported. For example, novel regulatory roles of T-lymphoma invasion and metastasis1 [Tiam1], a GEF for Rac1, in GSIS from a variety of insulin-secreting cells have also been demonstrated [23]. NSC23766, a specific inhibitor of Tiam1-mediated activation of Rac1, but not Cdc42 or Rho, markedly attenuated glucose-induced, but not KCl-induced, insulin secretion in INS 832/13 cells and normal rat islets [23]. Furthermore, NSC23766 significantly reduced glucose-induced activation and membrane association of Rac1 in INS 832/13 cells and rat islets. The pharmacological data were further confirmed by molecular biological approaches in that the siRNA-mediated knockdown of Tiam1 markedly inhibited glucose-induced membrane trafficking and activation of Rac1 in INS 832/13 cells [23]. Together, glucose-mediated activation of Rac1 and associated insulin secretion appear to be under the fine control of Rho-GDI and Tiam1 in isolated β-cells.
In conclusion, data reviewed above support the viewpoint that Rac1 plays an essential role in physiological insulin secretion. Existing evidence suggests that this G-protein is involved in cytoskeletal rearrangements thereby providing conditions favorable for the translocation and fusion of insulin granules with the plasma membrane for the release of insulin into the circulation [3, 4]. The underlying mechanisms for cytoskeletal remodeling, vesicular transport and fusion might also include functional activation of scaffolding proteins such as IQGAP1 and gelsolin, which have been shown to regulate cytoskeletal reorganization [4, 24]. In the context of downstream effector proteins, emerging evidence suggests that phagocyte-like NADPH-oxidase [Nox] is involved in glucose- and palmitate-induced insulin secretion [25, 26, 27, 28, 29]. It is felt that a tonic increase in reactive oxygen species [ROS] generated by Nox is necessary for GSIS; this is confirmed by pharmacological and molecular biological approaches [25, 26, 29, 30]. The following section will highlight the existing evidence to further substantiate positive modulatory roles of Rac1 in insulin secretion via regulation of Nox activation, generation of ROS thereby facilitating insulin secretion under normal physiological conditions.
4. The glucose-Rac1-Nox-ROS connection in insulin release
Nox is a highly regulated membrane-associated protein complex that facilitates the one electron reduction of oxygen to superoxide anion involving oxidation of cytosolic NADPH [31, 32]. The Nox holoenzyme is comprised of membrane as well as cytosolic components. The membrane-associated catalytic core consists of gp91phox, p22phox, and the small G-protein Rap1. The cytosolic regulatory components include p47phox, p67phox, p40phox and the small G-protein Rac1 [or Rac2]. Following stimulation, the cytosolic components of Nox translocate to the membrane fraction for association with the catalytic core for the holoenzyme assembly. Existing evidence suggests that a protein kinase C-sensitive phosphorylation of p47phox leads to its translocation to the membrane fraction. It has also been shown that functional activation of Rac [Rac.GTP] is vital for the holoenzyme assembly and activation of Nox [31, 32, 33, 34, 35, 36].
Several recent studies have demonstrated localization and functional activation of the Nox in clonal β-cells, normal rat islets, and human islets [25, 26, 28, 29]. The reader is referred to recent reviews on contributory roles of Nox in pancreatic β-cell function and dysfunction [37, 38, 39, 40, 41]. In this context, three previous studies highlighted roles for Nox in insulin secretion. In the first study, Oliveira et al [26] provided a detailed description of localization of expression and functional regulation of Nox within the islet. Immunohistochemical approach was used to demonstrate activation of Nox by glucose. Diphenylene iodonium [DPI], a selective inhibitor of Nox inhibited the enzyme activity. Lastly, through the use of an activator [e.g., phorbol ester] or an inhibitor [e.g., GF109203X] these studies demonstrated that a protein kinase C-mediated mechanism might underlie glucose-mediated activation of Nox. Based on this evidence it was concluded that Nox activation by glucose may play an important role in the regulation of β-cell function. In a second study, Morgan and colleagues have demonstrated [25] that pharmacological [e.g., DPI] and molecular biological [e.g., p47phox antisense] inhibition of Nox holoenzyme resulted in significant inhibition of intracellular responses to calcium to glucose and GSIS under static as well as perifusion conditions. Further, reduction in the Nox activation culminated in inhibition of glucose oxidation, glucokinase and Glut-2 expression. In the third study, Graciano et al [29] demonstrated an essential role for Nox in palmitate-induced superoxide generation and insulin secretion in rat pancreatic islets. These studies specifically showed a significant increase in the superoxide generation by palmitate under short-term incubation conditions; this was attenuated by DPI, a Nox inhibitor and calphostin C, an inhibitor of protein kinase C. Further, Nox inhibition led to a significant reduction in palmitate-induced insulin secretion in the presence of high glucose. Together, the above three studies provide a compelling evidence in support of a positive modulatory role[s] for Nox signaling pathway in insulin secretion. In this context, we recently demonstrated that prenylation of Rac1 is necessary for glucose- and mitochondrial fuel-induced Nox-dependent ROS generation in INS 832/13 cells and rodent islets [30]. These findings are in accord with earlier observations of Gorzalczany and coworkers [42] suggesting that targeting of Rac1 to the membrane fraction is adequate for the activation of Nox and associated generation of ROS. They demonstrated that prenylated Rac1, but not unprenylated Rac1 or prenylated Cdc42Hs binds to the phagocyte membranes more efficiently, which is followed by recruitment of cytosolic p67phox to the membrane and generation of superoxides following addition of NADPH. Based on these observations, these investigators concluded that Rac1 functions in Nox activation might include recruitment of p67phox and promoting the association between p67phox and cytochrome b559. Along these lines, Pi and Collins overviewed the existing evidence in supporting “second-messenger” roles of ROS in physiological insulin secretion [43]. Thus, based on the above discussion it can be surmised that a tonic increase in the intracelluar ROS is critical for physiological insulin secretion and that Rac1 plays a friendly role in glucose-induced Nox activation and insulin secretion. It is, therefore, concluded that glucose-induced activation of Rac1 initiates subsequent signaling steps including activation of Nox and insulin release.
5. What are the non-friendly roles of Rac1 in islet function ?
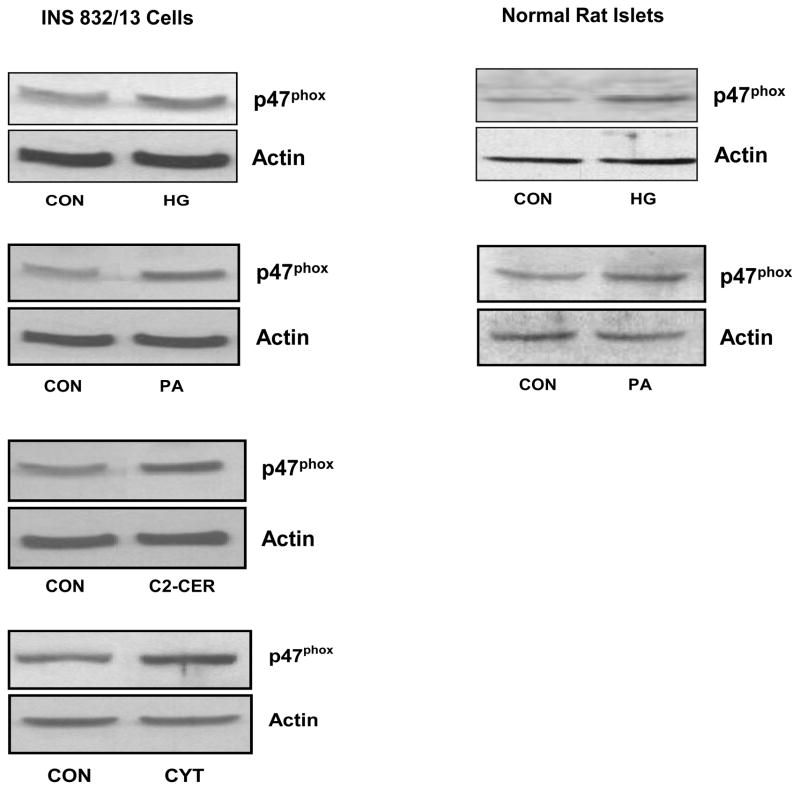
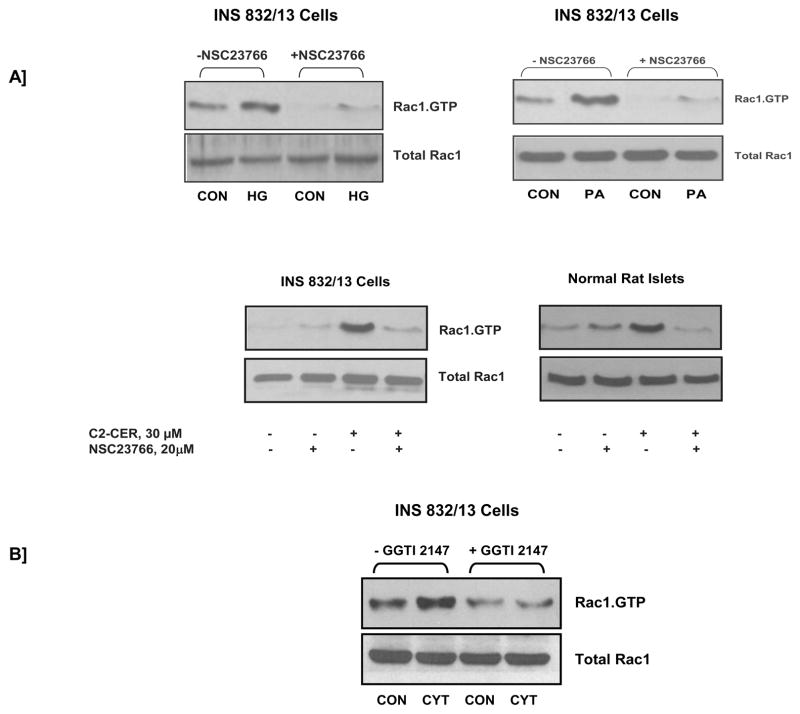
In addition to its positive modulatory roles, accumulating evidence implicates negative modulatory [i.e., non-friendly] roles for Rac1 in the metabolic dysfunction of the islet β-cell, specifically at the level of its participatory role[s] in the regulation of Nox activation. Emerging evidence from recent studies seems to implicate Nox in the constitutive generation of ROS following long-term exposure of β-cells to various stimuli known to cause metabolic dysregulation [40, 44, 45]. Some of these stimuli include, but are not limited to, high glucose, saturated fatty acids, and proinflammatory cytokines. For example, in a recent study Morgan and coworkers [40] have demonstrated a significant increase in Nox activity and associated increase in ROS production in normal rat islets and clonal BRIN BD11 cells following incubation with glucose, palmitate or proinflammatory cytokines. Coprovision of DPI or p47phox antisense oligonucleotides markedly attenuated these effects [40]. Together, these findings suggest regulatory roles for Nox in normal physiology of the islet β-cell. They also implicate Nox in the onset of oxidative stress under specific conditions, including glucolipotoxicity. More recent investigations from our laboratory have suggested roles of Tiam1/Rac1/Nox signaling axis in β-cell dysregulation induced by these stimuli [44, 45]. For example, data shown in Figure 2 demonstrated that incubation of INS 832/13 cells or normal rodent islets to high glucose [see Methods for experimental details] or palmitate led to a significant increase in the expression of p47phox subunit [45]. Under these conditions, we have also observed a significant increase in the ROS generation and lipid peroxidation, which was inhibited by DPI [44]. In addition, exposure of INS 832/13 cells to either a cell-permeable ceramide [i.e., C2-ceramide; C2-CER] or a mixture of cytokines [cytomix; i.e., IL-1β, TNFα and IFNγ] also led to increased expression of p47phox, Nox activation and ROS generation [Figure 2; ref. 44]. DPI, apocynin or siRNA-mediated depletion of p47phox reduced cytomix-induced Nox activation. Together, these data suggested that constitutive activation of Nox by high glucose, palmitate, C2-CER or cytokines results in increased p47phox expression, Rac1 activation and Nox-mediated ROS generation culminating in the metabolic dysfunction of the β-cell induced by the above stimuli. It should be noted that inhibition of Tiam1/Rac1 signaling axis by NSC23766 significantly attenuated Nox activity and ROS generation in INS 832/13 cells under the duress of cytomix or palmitate [44, 45]. Data shown in Figure 3 demonstrated that chronic exposure of insulin-secreting cells to high glucose, palmitate, C2-CER or cytomix [as described under Figure 2] results in activation of Rac1 and that coprovision of these cells with NSC23766, a selective inhibitor of Tiam1/Rac1 signaling pathway, markedly attenuated the activation of Rac1 seen under those conditions. Lastly, in a separate set of investigations we have demonstrated that cytomix induced significant defects in mitochondrial function in INS 832/13 cells as evidenced by a marked loss of mitochondrial membrane potential and upregulated caspase 3 activity [46]. In addition, GGTI-2147 and NSC23766, known Rac1 inhibitors, not only attenuated cytomix-induced Rac1 activation but also significantly prevented loss of mitochondrial membrane potential [NSC23766 > GGTI-2147; ref. 44]. Together, our experimental evidence [Figures 2 and 3] suggests that Tiam1/Rac1/Nox signaling cascade contributes to metabolic dysfunction in the β-cell, which is seen in the presence of these noxious stimuli. In addition, a regulatory role for Rac1 in the induction of Nox-mediated oxidative stress has been demonstrated in other cells as well. For example, studies in cultured retinal pericytes have demonstrated a role for Nox in palmitate-induced apoptosis [47]. A significant increase in Nox activity, oxidative stress, and caspase-3 activity was demonstrable in cells exposed to palmitate. Overexpression of dominant-negative mutants of p67phox and Rac1 [N17Rac1] markedly inhibited the observed increase in caspase-3 activation. Furthermore, overexpression of an active mutant of Rac1 [V12Rac1] increased caspase-3 activity, suggesting that constitutive activation of Rac1 results in Nox activation culminating in the generation of oxidative stress and metabolic dysfunction.
Figure 2. High glucose, palmitate, C2-CER or cytomix increases the expression of p47phox in INS 832/13 cells and normal rat islets.
INS 832/13 cells or normal rat islets were treated with either low glucose [CON; 2.5 mM for 6 h]; high glucose [HG; 20 mM for 6 h]; palmitate [PA; 100 μM for 6 h]; cell-permeable C2-CER [30 μM for 6 h] or a mixture of cytokines [CYT; IL-1β, TNFα, IFN-γ = 10 ng/mL each for 24 h] as indicated in the figure. Expression profiles of p47phox were determined in total lysates by Western blotting [44, 45]. Please see Methods for experimental details.
Figure 3. Inhibitors of Rac1 activation attenuate high glucose-, palmitate-, C2-CER- or cytomix-induced Rac1 activation in INS 832/13 cells and normal rat islets.
Panel A: NSC23766, a selective inhibitor of Tiam1-mediated activation of Rac1, markedly attenuates high glucose-, palmitate-, and C2-CER-induced Rac1 activation in INS 832/13 cells and normal rat islets. INS 832/13 cells or normal rat islets were incubated overnight with either diluent alone or NSC23766 [20 μM] in low serum low glucose media. The cells were then incubated further with either low glucose [CON; 5 mM for 30 min]; high glucose [HG; 20 mM for 30 min]; palmitate [PA; 100 μM for 3 h]; C2-CER [30 μM for 30 min in INS 832/13 cells and 3 h in normal rat islets] in the continuous presence of the diluent or NSC23766. The degree of Rac1 activation was determined by PAK-PBD pull down assay [44, 45].
Panel B: GGTI-2147, a selective inhibitor of geranylgeranylation of Rac1, markedly attenuates cytomix-induced Rac1 activation in INS 832/13 cells. INS 832/13 cells were incubated overnight with either diluent alone or GGTI-2147 [10 μM] in low serum low glucose media. The cells were then incubated further with either low glucose [CON; 5 mM for 15 min] or a mixture of cytokines [CYT; IL-1β, TNFα, IFN-γ = 10 ng/mL each for 15 min] in the continuous presence of the diluent or GGTI-2147. The degree of Rac1 activation was determined by PAK-PBD pull down assay [50, 51]. Please see Methods for experimental details.
One logical question is how specific are palmitate effects on β-cells in inducing oxidative stress? Recent studies of Yuan and associates [48] suggested that, in addition to palmitate, incubation of insulin-secreting clonal β-[NIT-1] cells with oleate results in a significant activation of Nox and associated increase in the ROS generation and subsequent demise of the β-cell. These studies have demonstrated that palmitate- or oleate-induced apoptosis of the β-cell by activating the JNK signaling axis leading to inhibition of Akt culminating in the decreased phosphorylation of FOXO1. Evidence was also presented in these studies that palmitate or oleate promotes apoptosis of the β-cell through p38 MAPK and p53 signaling mechanisms. Lastly, the fatty acid-induced metabolic defects and apoptosis were restored in these cells to a large degree via siRNA-mediated inactivation of Nox. Together, these studies provide further supporting evidence for the overall hypothesis that Nox plays a negative modulatory role by inducing oxidative stress and the apoptosis of the β-cell under the duress of fatty acids, such as palmitate and oleate. Furthermore, these data identify additional downstream signaling pathways for Nox-mediated ROS generation, which might be contributing toward metabolic dysfunction of the β-cell.
Lastly, functional activation of Nox has also been demonstrated in islets from the diabetic animal models [db/db mice and Otsuka Long-Evans Tokushima Fatty rat; OLETF rat; 49]. Interestingly, treatment of these animals with angiotensin II type-1 receptor antagonists significantly reduced the expression of Nox subunits and the associated oxidative stress in islets derived from these animal models. These data implicate a significant contributory role for Nox in the metabolic dysfunction of the beta-cell under conditions of oxidative stress [49]. Potential beneficial effects of Rac1 inhibitors [e.g., NSC23766] in the normalization of oxidative stress need to be investigated in such animal models.
6. A working model
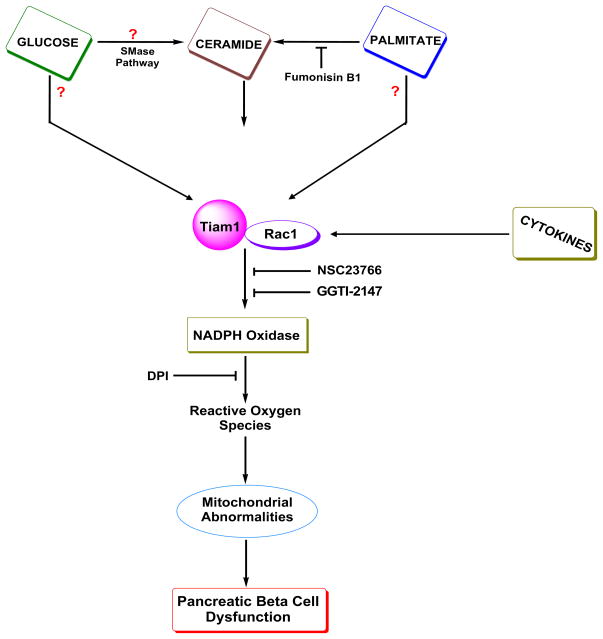
Based on the above discussion, which is supported to a large degree, by experimental evidence, we propose the following model for the involvement of Tiam1/Rac1 signaling axis in Nox-induced oxidative stress in isolated β-cells under the duress of long-term exposure to glucose, palmitate, ceramide and cytokines [Figure 4]. We propose that chronic exposure of β-cells to glucolipotoxic conditions leads to the activation of Tiam1/Rac1 signaling pathway. Palmitate-induced Rac1 activation may be mediated by CER accumulation via the de novo pathway since fumonisin B1, a selective inhibitor of CER biosynthesis from palmitate attenuated the effects of palmitate on ROS generation [45]. It is likely that glucose mediated activation of Tiam1/Rac1 is direct rather than mediated via CER; however it needs to be verified experimentally. Alternatively, glucose could mediate intracellular accumulation of CER either via activation of islet endogenous sphingomyelinases or via the endoplasmic stress route. Exposure of isolated β-cells to cytokines also leads to activation of Tiam1/Rac1 signaling pathway. Potential roles of CER in this signaling cascade[s] need to be verified as well. Tiam1/Rac1 activation leads to activation of Nox enzyme culminating in the accumulation of ROS and onset of oxidative stress within the β-cell. This, in turn, affects mitochondrial function including generation of oxidative stress within the mitochondria, reduction in the mitochondrial membrane permeability, cytochrome C release, caspase 3 activation and loss in metabolic cell viability leading to the demise of the β-cell. Coprovision of inhibitors of Nox [e.g., apocynin and DPI], inhibitors of Rac1 [e.g., GGTI-2147 and NSC23766] or siRNA-mediated knockdown of p47phox leads to inhibition of Nox activation and at least a partial restoration of mitochondrial dysfunction. It should be noted that the proposed model does not include potential participatory roles of other GEFs and effector proteins for Rac1. For example, using Ra2 microglia cell line Roepstroff and coworkers have demonstrated [50] key regulatory roles for PAK1, an effector protein of Rac1, in formyl-methionyl-leucyl-phenylalanine-or phorbolester-induced Nox activation. They also demonstrated key roles for Rac1 and VAV1, a known GEF for Rac1 in this signaling cascade. Their findings further demonstrated that PAK-1-mediates phosphorylation of p47phox, which is necessary for its membrane association and Nox activation. In summary, it is likely that Rac1 could mediate Nox activation through the intermediacy of signaling steps involving other GEFs [e.g., VAV1] and effector proteins [e.g., PAK-1]. These remain to be investigated in the islet β-cell [50]. In addition, not included in our current model are other known mechanisms whereby chronic hyperglycemia exerts damaging effects on β-cells, presumably not involving the activation of Nox [51]. These include oxidative phosphorylation, sorbitol metabolism, hexosamine metabolism, protein kinase C activation by DAG etc. A recent review by Poitout and Robertson highlights such mechanisms, which are involved in the generation of oxidative stress in the β-cell under the duress of glucolipotoxic conditions [51].
Figure 4. A model depicting potential involvement of Rac1 in the metabolic dysfunction of the islet β-cell induced by high glucose, palmitate, ceramide or cytokines.
We propose that exposure of pancreatic β-cells to high glucose, palmitate or cytokines leads to activation of Tiam1/Rac1 signaling axis culminating in the activation of Nox. This, in turn, results in generation of ROS, which adversely affect mitochondrial functions leading to loss in membrane potential thus favoring cytochrome C release into the cytosolic compartment. The latter promotes the activation of pro-apoptotic caspase 3 and associated cleavage of substrate proteins leading to the apoptotic demise of the β-cell. Inhibition of Nox by DPI appears to prevent such metabolic defects. Potential involvement of Taim1/Rac1 signaling cascade was verified by the use of NSC23766, a selective inhibitor of this pathway. It also appears that intracellularly-generated CER via the de novo pathway from palmitate mediates activation of Tiam1/Rac1 and Nox activation steps since fumonisin B1, a selective inhibitor of the ceramide biosynthesis from palmitate largely restored palmitate effects [45]. Furthermore, C2-CER, a cell permeable analogue of CER, mimicked palmitate effects [45]. It is likely that exposure of isolated β-cells to glucose also leads to intracellular accumulation of CER via activation of sphingomyelinases endogenous to the β-cell. In addition, glucose can directly activate Tiam1/Rac1 signaling pathway independent of its effects on intracellular CER levels. Our recently published evidence indicates that exposure of isolated β-cells to a mixture of cytokines [i.e., IL-1β, TNFα and IFNγ] also leads to increase in the expression of p47phox, Tiam1/Rac1 activation and metabolic dysfunction [44]. Together, these data appear to implicate critical regulatory roles of Tiam1/Rac1/Nox signaling steps in the metabolic dysfunction of the β-cell under the duress of various noxious stimuli.
Abbreviations used in this figure are: SMase: sphingomyelinase; Tiam1: T-lymphoma invasion and metastasis1; DPI: diphenyleneiodonium chloride.
7. What are our options then in the discovery and development of therapeutics to relieve Nox-induced oxidative stress?
It is evident from the above discussion that members of the Nox holoenzyme could represent novel drug targets for halting the generation of excess ROS generation and oxidative stress under conditions of glucolipotoxicity, exposure to cytokines or C2-CER. In a recent article, El-Benna and colleagues [52] have provided an excellent description of developing peptide-based inhibitors for Nox. Based on in an in-depth analysis, they concluded that gp91phox, p47phox and p67phox might serve as potential drug targets due to their “exclusive membership” in the Nox holoenzyme complex, but not other NADPH oxidases. In contrast, peptide inhibitors for Rac1/2 and p47phox may not be beneficial since they are active members of other NADPH oxidase holoenzyme complexes as well.
It is likely that CER synthesis inhibitors [e.g., fumonisin, cycloserine and myriocin] might turn out to be valuable in halting the toxic effects of CER, which is formed from palmitate via the de novo pathway. Indeed, recent studies have clearly demonstrated beneficial effects of CER biosynthesis inhibitors in relieving detrimental effects of CER in animal models of obesity and diabetes [53]. Additional studies are needed to further examine the effects of such a therapy to prevent mitochondrial defects arising from Nox-mediated, CER-sensitive signaling mechanisms.
Along the lines of potential modulators of Nox activity, Mizrahi and coworkers recently developed and tested the efficacy of p47phox-p67phox-Rac1 chimera as the quintessential single molecule activator of Nox [54]. They also provided evidence to suggest that prenylation of Rac1 plays critical regulatory roles in the membrane association and functional activation of the enzyme. These findings support our observations described above to suggest marked inhibition of glucose-induced, Nox-derived generation of ROS in cells incubated with selective inhibitors of protein prenylation [30]. Albeit, studies by Mizrahi and coworkers [54] described methods and provided tools to study their effects on Nox activation, approaches such as these might be valuable in the development of novel therapeutics to inhibit Nox-derived oxidative stress in β-cells in obesity and diabetes.
Other known approaches to neutralize or detoxify the oxidative environment in the cell include antioxidant therapy and overexpression of antioxidant enzymes. Indeed, such modalities have been shown to work efficiently. The outcomes of such therapy are more predictable given the fact that the islet β-cell carries extremely poor antioxidant capacity. Quantitation of gene expression profiles of antioxidant enzymes in rodent islets yielded very low values compared to their respective counterparts in the liver. For example, relative abundance of CuZn superoxide dismutase, Mn-superoxide dismutase and glutathione peroxidase corresponded only to 38%, 30% and 15% of the liver values, respectively. Catalase activity was undetectable in islets [55 and references therein]. Furthermore, studies by Modak and coworkers [56] have demonstrated extremely poor repair of oxidatively damaged DNA in β-cells compared to other cell types [e.g., liver cells] under conditions of oxidative stress. In addition, studies from Robertson’s laboratory have yielded valuable insights into beneficial effects of overexpression of anti-oxidant enzymes [e.g., glutathione peroxidase] against the damaging effects of oxidative stress [57, 58]. Indeed, recent data from the studies of Xiao and associates in humans [59] have also suggested beneficial effects of antioxidant therapy [e.g., taurine], which affectively restored lipid-induced reductions in plasma biomarkers of oxidative stress, insulin sensitivity and β-cell function. A recent review by Giacca and coworkers [60] provides additional advances in the area of lipid-induced pancreatic β-cell dysfunction, specifically focusing on in vivo studies. Together, these studies further emphasize antioxidant therapy as one of the viable options in lowering the oxidative stress in the islet under conditions of glucolipotoxicity.
Emerging evidence suggests regulation by adiponectin and leptin of Nox protein expression in multiple cells. For examples, studies by Tao and colleagues have demonstrated increased expression on Nox in the heart from the adiponectin knockout mice [61]. Similar increase in the expression of Nox was also reported in the kidney from the adiponectin knockout animal models compared to their wild-type counterparts, suggesting potential downregulation of Nox expression by adiponectin [62]. In addition, studies by Dong and coworkers reported significant increase in the expression Nox protein by leptin in murine caridiomyocytes [63]. In this context, recent studies by Décordé et al [64] have reported a significant beneficial effects of polyphenolic grape seed extract [GSE] against high-fat diet induced obesity, adiponectin-leptin imbalance and oxidative stress markers in hamsters. Specifically, these studies demonstrated a marked reduction in high-fat-induced abdominal fat, plasma glucose, triglycerides and insulin resistance in these animal model following GSE therapy. Furthermore, plasma levels of adiponectin and leptin were normalized in GSE-treated high-fat-fed animals, under which conditions increased cardiac production of superoxides and Nox expression were normalized to a large degree. Together, these observations [64 and studies reported therein] implicate regulatory roles of adiponectin and leptin in the regulation of Nox activity. Further, they provide evidence for the therapeutic efficacy of grape phenolics in the prevention of Nox-mediated effects on cellular functions. Potential roles of Tiam1/Rac1 axis in this signaling cascade remains to be examined.
As discussed above, Nakyama et al have reported a significant improvement in islet function in diabetic OLETF rats and db/db mice following treatment with angiotensin1 receptor antagonist [49]. They demonstrated attenuation in the increased expression of gp91phox and p22phox and the associated oxidative stress in islets derived from these models following treatment with Valsartan, a known angiotensin1 receptor antagonist. Based on these findings, the authors concluded that angiotensin-related increase in the activation of Nox could potentially be contributing to the metabolic dysfunction of the islet β-cell in type 2 diabetes. These findings are compatible with in vitro observations by Hirata and colleagues demonstrating a significant activation of Nox-mediated and associated generation of superoxides in rat pancreatic islets following exposure to angiotensin II [65]. Evidence was also presented to suggest a significant increase in the expression of p47phox and gp91phox at both mRNA and protein levels. Together, these data are suggestive of novel cytoprotective effects of angiotensin receptor antagonists against cell damage induced by glycolipotoxicity or proinflammatory cytokines.
Lastly, it may be germane to point out that several recent investigations have successfully utilized NSC23766, a selective inhibitor of Tiam1/Rac1 signaling axis to correct Nox-mediated effects on cellular function in vitro and in vivo [44, 45, 66]. Of immediate relevance to the current topic is a study that merits discussion here. Using streptozotocin diabetic mouse model, Shen and coworkers have demonstrated a regulatory role for Rac1 in hyperglycemia-induced apoptosis in cadiomyocytes [66]. Specifically, they were able to demonstrate upregulation of Rac1, Nox activity, increased ROS generation leading to apoptosis of cardiomyocytes under the duress of hyperglycemia. Such conditions significantly increased Rac1 and Nox activation in in vitro culture conditions, which were attenuated by pharmacological inhibition of Nox, overexpression of inactive mutant of Rac1 or gene knockdown of gp91phox or p47phox. Treatment of diabetic db/db mice with NSC23766 significantly inhibited Nox activity and cell apoptosis. In further support of a role for Rac1 in the onset of myocardial remodeling in type 1 diabetes, Li and coworkers have demonstrated that Rac1 knockout or apocynin-treatment significantly attenuated Nox subunit expression and activation, ROS production and cardiac collagen deposition. Rac1 deficiency also led to reduction in myocardial fibrosis and hypertrophy and improved myocardial function [67]. These studies provide adequate evidence in support of the hypothesis that Tiam1/Rac1 signaling axis plays a critical role in Nox-mediated cell dysfunction in diabetes. They also raise the potential need for the development of more specific therapeutic modalities to specifically inhibit this signaling pathway. However, as discussed above, at least in the context of the islet β-cell, this strategy may not be ideal since the Tiam1/Rac1/Nox signaling pathway is also implicated in the signaling cascade leading to physiological insulin secretion, including actin remodeling, granule mobilization and tonic increase in ROS [4, 30]. Alternatively, it is likely that a tonic level of attenuation of the Tiam1/Rac1 signaling pathway might prove beneficial to the islet function. This needs experimental verification. Further, as suggested recently by Bosco and associates [9], Rac1 regulates various cellular functions including microtubule stability [via IQGAPs], actin organization [via IRSp53/WAVE and PAK-1], transcription [via PAK-1], superoxide generation [via NOX] and nuclear signaling [via MLK2/3]. Together, the above mentioned positive modulatory roles of Rac1 in normal cell function implicate Rac1 as low priority target protein for therapeutic development.
Lastly, a recent review by Sawada et al [68] provides additional insights into friendly and non-friendly roles of Rac1 in the cardiovascular system, but such a model could be applicable to other model systems, including the islet β-cell. Based on the existing evidence these investigators proposed a “context-dependent, Janus-faced functions of Rac1” in various signaling steps in endothelial cells, which are controlled precisely by specific stimuli and GFEs to regulate the function of Rac1. Further, they overviewed evidence in support of various downstream effector proteins of Rac1 [i.e., IQGAP and PAK-1] as determinants of endothelial barrier properties. Based on this discussion, it seems likely that positive and modulatory roles of Rac1 in a given cell type depends on the stimulus, the GEF protein [E.g., Tiam1, VaV etc] and the downstream effector proteins [i.e., IQGAP]. While considerable evidence implicates PAK-1 as one the effector proteins [3, 4], regulatory roles of Rac1/IQGAP signaling in the islet β-cell remains only partially understood [4, 69, 70, 71]. Based on the existing data in the islet, and in line with the proposal by Sawada and associates, we suggest that the future efforts must be made to develop novel pharmacological probes not based on Rac1 itself, but based on its upstream regulatory and downstream effector proteins.
8. Conclusions and future directions
GSIS involves a sequence of metabolic events and interplay between distinct signaling pathways leading to the transport of insulin granules to the plasma membrane for fusion and release of insulin by exocytosis. Convincing evidence is available now to implicate small G-proteins [e.g., Rac1 and Cdc42] in physiological insulin secretion, specifically at the level of vesicular transport and cytoskeletal remodeling. From the above discussion it is evident that Tiam1 serves the role of a GEF for Rac1 and that Tiam1/Rac1 signaling axis is necessary for GSIS to occur. Among various effector proteins known to exist for Rac1, Nox appears to require Tiam1/Rac1 signaling step for its holoenzyme assembly and catalytic activation. Also, it is evident that Tiam1/Rac1-mediated Nox-derived ROS generation is necessary for glucose-and fatty acid-stimulated insulin secretion. In addition, it is becoming increasingly clear that chronic exposure of isolated β-cells to high glucose, palmitate, cytokines or C2-CER leads to the generation of “excess” ROS culminating in oxidative stress leading to mitochondrial dysfunction of the effete β-cell. Despite the available evidence, which identifies Nox as one of the sources of oxidative stress under the duress of noxious stimuli, potential loci for the action of generated ROS within the β-cell remains to be determined. They might include, but not limited to, cytosolic ROS-induced oxidative stress within the mitochondria thereby triggering downstream signaling events leading to cellular dysregulation [Figure 4]. While there is substantial evidence to support this viewpoint, some aspects remain speculative and need to be confirmed experimentally. Further, precise mechanisms underlying the translocation of each of the three cytosolic components of Nox, namely Rac1, p67phox and p47phox remain to be fully defined. At least in the context of GSIS, published evidence from our laboratory [72] suggested novel roles for biologically-active phospholipids in the dissociation of Rac1/GDI complexes, which is a requisite for activation and translocation of Rac1 to the membrane fraction; this signaling step is under the fine control of Tiam1. Lastly, it is evident that post-translational geranylgeranylation of Rac1 is necessary for full activation of Nox as evidenced by inhibition of Nox-mediated ROS generation by GGTI-2147. However, it must be kept in mind that Rap1, which represents one of the components of Nox also, is geranylgeranylated and such a modification step could be potentially inhibited by GGTI-2147. A review of literature clearly indicates Rac1 activation as a critical step for Nox regulation, very little is understood with regard to potential regulatory roles of membrane-associated Rap1 in the Nox activation step. This could potentially be under the control of various GEFs and related mechanisms. Finally, data from the in vitro experiments are in agreement with those accrued in islets derived from diabetic animal models as evidenced by increased Nox-derived ROS generation in the diabetic islet. Functional relevance for Tiam1/Rac1 signaling axis in the hyper-activation of Nox in the diabetic islet requires further investigation.
Acknowledgments
The research work summarized in this article is supported by the National Institutes of Health [RO1 74921] and the Department of VA MERIT Review award. AK is also the recipient of a Senior Research Career Scientist Award from the Department of VA. The author would like to thank Mr. Ismail Syed, Ms. Bhavaani Jayaram, Dr. Wasanthi Subasinghe and Dr. Chandrashekara Kyathanahalli for their contributions summarized in this Commentary.
Non-standard abbreviations
- CER
ceramide
- DPI
diphenyleneiodonium chloride
- FTase
farnesyltransferase
- FTI
farnesyltransferase inhibitor
- GDI
GDP-dissociation inhibitor
- GEF
guanine nucleotide exchange factors
- GGTase
geranylgeranyltransferase
- GGT1
geranylgeranyltransferase inhibitor
- GSE
grape seed extract
- GSIS
glucose-stimulated insulin secretion
- Nox
phagocyte-NADPH-oxidase
- OLETF rat
Otsuka Long-Evans Tokushima fatty rat
- Rac1
Ras-related C3 botulinum toxin substrate 1
- ROS
reactive oxygen species
- Tiam1
T-lymphoma invasion and metastasis1
Footnotes
Financial interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDonald MJ. Elusive proximal signals of β-cells for insulin secretion. Diabetes. 1990;39(12):1461–66. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Matschinsky FM. Calcium, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67(4):1185–248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122(pt7):893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31(1):52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence JT, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2003;100(23):13320–5. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A. Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 β-cells and rat islets. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. rac, a Novel ras-related Family of Proteins That Are Botulinum Toxin Substrates. J Biol Chemistry. 1989;264(28):16378–82. [PubMed] [Google Scholar]
- 8.Bustelo XR, Suazeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29(4):356–70. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Curtis Functions of Rac GTPases during neuronal development. Dev Neurosci. 2008;30(1–3):47–58. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- 10.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: A “Rac” of all trades. Cell Mol Life Sci. 2009;66(3):370–4. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowluru A, Rabaglia ME, Muse KE, Metz SA. Subcellular localization and kinetic characterization of guanine nucleotide binding proteins in normal rat and human pancreatic islets and transformed beta cells. Biochim Biophys Acta. 1994;1222(3):348–359. doi: 10.1016/0167-4889(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 12.Metz SA, Rabaglia ME, Pintar TJ. Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J Biol Chem. 1992;267(18):12517–27. [PubMed] [Google Scholar]
- 13.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 14.Kowluru A. Protein prenylation in glucose-induced insulin secretion from the pancreatic islet β-cell: a perspective. J Cell Mol Med. 2008;12(1):164–73. doi: 10.1111/j.1582-4934.2007.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes. 2007;56(1):204–10. doi: 10.2337/db06-0668. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose-and forskolin-induced insulin secretion in INS-1 beta cell. Am J Physiol Endocrinol Metab. 2004;286(5):E818–27. doi: 10.1152/ajpendo.00307.2003. [DOI] [PubMed] [Google Scholar]
- 17.Kowluru A, Veluthakal R. Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes. 2005;54(12):3523–9. doi: 10.2337/diabetes.54.12.3523. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Thurmond DC. Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion. J Biol Chem. 2010;285(9):6186–197. doi: 10.1074/jbc.M109.072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin RH, Chen HQ, Veluthakal R, Solver RB, LIJ, Li G, et al. Mastoparan-induced insulin secretion from insulin secreting betaTC3 and INS-1 cells: Evidence for its regulation by Rho subfamily of G-proteins. Endocrinology. 2003;144(10):4508–18. doi: 10.1210/en.2003-0106. [DOI] [PubMed] [Google Scholar]
- 20.Asahara A, Kido Y, Shogeyama Y, Matsuda T, Takeda A, Inoue T, et al. Rac1 regulates glucose induced insulin secretion through modulation of cytoskeletal organization in beta cells. Diabetes. 2008;57(supplement 1):A55. [Google Scholar]
- 21.Greiner TU, Kesavan G, Stahlberg A, Semb H. Rac1 regulates pancreatic islet morphogenesis. BMC Dev Biol. 2009;9:2. doi: 10.1186/1471-213X-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regazzi R, Kikuchi A, Takai Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267(25):17512–9. [PubMed] [Google Scholar]
- 23.Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A. Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol. 2009;77(1):101–13. doi: 10.1016/j.bcp.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomas A, Yermen B, Min L, Pessin JE, Halban PA. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signaling pathway. J Cell Sci. 2006;119(Pt 10):2156–67. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 25.Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, et al. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology. 2009;150(5):2197–201. doi: 10.1210/en.2008-1149. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52(6):1457–63. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- 27.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56(7):1783–91. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 28.Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H, et al. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006;80(2):133–39. doi: 10.1016/j.lfs.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Graciano MF, Santos LR, Curi R, Carpinelli AR. NAD(P)H oxidase participates in the palmitate-induced superoxide production and insulin secretion by rat pancreatic islets. J Cell Physiol. 2010 doi: 10.1002/jcp.22432. [DOI] [PubMed] [Google Scholar]
- 30.Syed I, Kyathanahalli CN, Kowluru A. Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: Role of protein prenylation. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00786.2010. in press PMID: 21228337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babior BM. NADPH oxidase: an update. Blood. 1999;93(5):1464–76. [PubMed] [Google Scholar]
- 32.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71(2):289–99. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Borregaard N, Tauber AI. Subcellular localization of the human neutrophil NADPH oxidase b-cytochrome and associated flavoprotein. J Biol Chem. 1984;259(1):47–52. [PubMed] [Google Scholar]
- 34.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small-GTP binding protein p21rac1. Nature. 1991;353(6345):668–70. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 35.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254(5037):1512–5. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 36.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98(4):453–62. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 37.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, et al. Diabetes associated cell stress and dysfunction: role of mitochondrial and non mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoguchi T, Nawata H. NAD(P)H oxidase activation: a potential target mechanism for diabetes vascular complications, progressive beta-cell dysfunction and metabolic syndrome. Curr Drug Targets. 2005;6(4):495–501. doi: 10.2174/1389450054021927. [DOI] [PubMed] [Google Scholar]
- 39.Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans. 2008;36(Pt 5):920–29. doi: 10.1042/BST0360920. [DOI] [PubMed] [Google Scholar]
- 40.Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2007;50(2):359–69. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 41.Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, et al. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia. 2009;52(12):2489–98. doi: 10.1007/s00125-009-1536-z. [DOI] [PubMed] [Google Scholar]
- 42.Gorzalczany Y, Sigal N, Itan M, Lotan O, Pick E. Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly. J Biol Chem. 2000;275(1):40073–40081. doi: 10.1074/jbc.M006013200. [DOI] [PubMed] [Google Scholar]
- 43.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diab Obes and Metab. 2010;12 (suppl 2):141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 44.Subasinghe W, Syed I, Kowluru A. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic beta-cells: Evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol. 2011;300 (1):R12–20. doi: 10.1152/ajpregu.00421.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol. 2010;80(6):874–83. doi: 10.1016/j.bcp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 2005;10(4):841–50. doi: 10.1007/s10495-005-0431-4. [DOI] [PubMed] [Google Scholar]
- 47.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54(6):1838–45. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 48.Yuan H, Zhang X, Huang X, Lu Y, Tang W, Man Y, Wang S, Xi J, Li J. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of β-cells via JNK, p38 MAPK and p53 pathways. PloS ONE. 2010;5(12):1–9. doi: 10.1371/journal.pone.0015726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, et al. Increased expression of NAD(P)H oxidase in islets of animal models of Type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun. 2005;332(4):927–33. doi: 10.1016/j.bbrc.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 50.Roepstorff K, Rasmussen I, Sawada M, Cudre-Maroux C, Salmon P, Bokock G, et al. Stimulus-dependent regulation of the phagocyte NADPH oxidase by VAV1, Rac1, and PAK1 signaling axis. J Biol Chem. 2008;283:7983–7993. doi: 10.1074/jbc.M708281200. [DOI] [PubMed] [Google Scholar]
- 51.Poitout V, Robertson RP. Glucolipotoxicity: Fuel excess and β-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Benna J, Dang PM, Périanin A. Peptide-based inhibitors of the phagocyte NADPH oxidase. Biochem Pharmacol. 2010;80(6):778–85. doi: 10.1016/j.bcp.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation and sphingolipid metabolism. Endocr Rev. 2008;29(4):381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizrahi A, Berdichevsky Y, Casey PJ, Pick E. The quintessential NADPH oxidase activator: a prenylated p47PHOX-p67PHOX-Rac1 chimera - membrane association and functional capacity. J Biol Chem. 2010;285(33):25485–99. doi: 10.1074/jbc.M110.113779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acharya JD, Ghaskabi SS. Islets and their antioxidant defense. Islets. 2010;2:225–35. doi: 10.4161/isl.2.4.12219. [DOI] [PubMed] [Google Scholar]
- 56.Modak M, Parab P, Ghaskadbi S. Pancrearic islets are very poor in rectifying oxidative DNA damage. Pancreas. 2009;38:23–9. doi: 10.1097/MPA.0b013e318181da4e. [DOI] [PubMed] [Google Scholar]
- 57.Harmon JS, Bogdani M, Parazzoli SD, Mak SS, Oseid EA, Berghmans M, et al. Beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150(11):4855–62. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robertson RP, Harmon JS. Pancreatic islet beta-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett. 2007;581(19):3743–8. doi: 10.1016/j.febslet.2007.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non diabetic men. Diabetologia. 2008;51:139–46. doi: 10.1007/s00125-007-0859-x. [DOI] [PubMed] [Google Scholar]
- 60.Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am j Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00416.2010. in press. PMID: 21119027. [DOI] [PubMed] [Google Scholar]
- 61.Tao L, Gao E, Jiao X, Yuan Y, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 62.Ohashi K, Iwatani H, Kihara S, Nakagawa Y, et al. Exacerbation of albuminurea and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Atheroscler Thromb Vasc Biol. 2007;27:1910–17. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 63.Dong F, Zhang X, Renx J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH-oxidase pathway. Hypertension. 2006;47:222–29. doi: 10.1161/01.HYP.0000198555.51645.f1. [DOI] [PubMed] [Google Scholar]
- 64.Decorde K, Teissedre P-L, Sutra T, Ventura E, Cristo J-P, Rouanet J-M. Chardonnay grape seed procyaidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res. 2009;53:659–66. doi: 10.1002/mnfr.200800165. [DOI] [PubMed] [Google Scholar]
- 65.Hirata AE, Morgan D, Oliveira-EmilIo HR, Rocha MS, Carvalho CR, Curi R, et al. Angiotensin II induces superoxide generation via NAD(P)H oxidase activation in isolated rat pancreatic islets. Regul Pet. 2009;153(1–3):1–6. doi: 10.1016/j.regpep.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Shen E, Li Y, Li Y, Shan L, Zhu H, Feng Q, et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58(10):2386–95. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Zhu H, Shen E, Wan L, Arnold JMO, Peng T. Deficiency of Rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawada N, Li Y, Liao JK. Novel aspects of the roles of Rac1 GTPase in the cardiovascular system. Curr Opin Pharmacol. 2010;10:116–21. doi: 10.1016/j.coph.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nauert JB, Rigas JD, Lester LB. Identification of IQGAP/AKA79 complex in β-cells. J Cell Biochem. 2003;90:97–108. doi: 10.1002/jcb.10604. [DOI] [PubMed] [Google Scholar]
- 70.Rittmeyer EN, Daniel S, Hsu SC, Osman MA. A dual role for IQGAP in regulating exocytosis. J Cell Sci. 2008;121:391–403. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- 71.Yan J, Yang Y, Zhang H, King C, Kan HM, Cai Y, Yuan CX, Bloom GS, Hua X. Menin interacts with IQGAP1 to enhance intercellular adhesion of β-cells. Oncogene. 2009;28:973–982. doi: 10.1038/onc.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDonald P, Veluthakal R, Kaur H, Kowluru A. Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS832/13 cells. Am J Physiol Cell Physiol. 2007;292(3):C1216–20. doi: 10.1152/ajpcell.00467.2006. [DOI] [PubMed] [Google Scholar]