Abstract
AML patients undergoing induction chemotherapy have significant decreases in alloimmune platelet refractoriness if they receive filter-leukoreduced or UV-B irradiated versus standard platelet transfusions (3% to 5% versus 13%, respectively, p≤0.03) with no differences among the treated platelet arms (TRAP Trial). Therefore, measuring antibody persistence might identify the best platelets for transfusion. Lymphocytotoxic (LCT) antibody duration was evaluated for association with patient age, sex, prior transfusion and pregnancy history, study assigned platelet transfusions, and percent LCT panel reactive antibodies (PRA). During the TRAP trial, 145 patients became antibody-positive, and 81 (56%) of them subsequently became antibody-negative. Using Kaplan-Meier estimates, projected antibody loss was 73% at one year. Major factors associated with antibody persistence were prior pregnancy and percent PRA positivity, while neither the assigned type of platelets transfused during the 8 weeks of the trial nor prior transfusion history were predictive. After 5 to 8 weeks, the number and type of blood products transfused had no effect on either antibody development or loss. A majority of AML patients who develop LCT antibodies during induction chemotherapy will lose their antibodies within 4 months regardless of the type or number of blood products they receive.
Keywords: Platelet Transfusions, UV-B Irradiation, Leukoreduction, Platelet Refractoriness, Platelet Alloimmunization, Lymphocytotoxic Antibodies
INTRODUCTION
The Trial to Reduce Alloimmunization to Platelets (TRAP Trial) was a multi-institutional trial to evaluate the effectiveness of modified platelet products for the prevention of alloimmune platelet refractoriness in patients with acute myelogenous leukemia (AML) undergoing induction chemotherapy.(1) The modified platelet products were filter leukoreduced or UV-B irradiated. The primary endpoint of the trial was the incidence of alloimmune platelet refractoriness. Secondary study endpoints were: 1) the development of alloantibodies; and 2) platelet refractoriness. The TRAP Trial showed statistically significant decreases in all study endpoints when the patients receiving modified platelet products were compared to patients receiving standard platelet products (control arm). However, there were no differences among the patients receiving any of the three types of modified platelet products.
Alloimmune platelet refractoriness occurred in 13% of patients in the control arm and between 3% to 5% of patients in the treated arms. Although the rates of alloimmune platelet refractoriness were very low, development of lymphocytotoxic (LCT) alloantibodies was much more common; i.e. 45% in the control arm ,and between 17 to 21% in the modified platelet arms.(1) Because the recovery of autologous platelet production may have preceded the development of alloantibodies, the relevance of HLA sensitization to platelet refractoriness was obscured. For example, 40% (50/124) of the patients who developed antibodies on study became antibody positive on or after the date of their last study transfusion. However, if the antibodies persist, they might cause platelet refractoriness during subsequent courses of consolidation chemotherapy.
This report focuses on the persistence of antibodies that developed during the initial 8 weeks of the TRAP Trial while patients were receiving their assigned platelet transfusions and during a follow-up period of 3 to 12 months when they received platelet and red cell transfusions as per local practice guidelines. Our primary objective was to determine if any of the variables recorded during the TRAP trial were associated with antibody persistence. Importantly, did one or more of the modified platelet transfusion arms result in a higher percentage of patients losing their antibodies over time? If so, this might indicate a preference for this transfusion strategy.
METHODS
Enrollment, Randomization, And Blood Product Support
Six hundred and three patients with previously untreated AML met the entry criteria for the TRAP Trial.(1) All study patients received filtered leukoreduced red cells, and patients were randomly assigned to receive either control standard pooled platelet concentrates (STD-PC), UV-B irradiated pooled platelet concentrates (UVB-PC), filtered leukoreduced pooled platelet concentrates (F-PC), or filter leukoreduced apheresis platelets (F-AP). All platelet transfusions a patient received during the first 8 weeks after their enrollment were expected to be prepared according to their randomization assignment. To be considered evaluable for the purposes of this report, a patient was required to have a negative baseline antibody test at study entry and become antibody positive during the 12 month study. Furthermore, patients must have received at least 2 platelet transfusions during the 8-week transfusion trial and have had at least 2 antibody samples tested during the total 12 months of the study.
After the 8-week study period, any additional platelet and RBC transfusions a patient received were provided following local practice guidelines. As post-trial transfusion therapy, 4 of the 7 trial sites gave non-filtered pooled random donor platelet concentrates, 1 gave non-filtered single donor apheresis platelets, and 2 sites gave filtered platelets (1 gave pooled random donor platelet concentrates, and 1 gave single donor apheresis platelets). For the 2 sites that gave filtered platelets, they also provided filtered RBCs; all the other sites gave non-filtered RBCs.
Antibody Testing
Serum samples were obtained at baseline, weekly for 8 weeks, and then monthly for 1 year. The samples were tested for LCT antibodies in a central laboratory at the end of 8 weeks, and the 3 to 12 month samples for each patient were analyzed after completion of their 1 year follow-up.
Antibodies against Human Leukocyte Antigen (HLA) Class I antigens were detected with an anti-globulin-augmented complement-dependent lymphocytotoxicity assay.(2) Serum samples were tested against a panel of 30 to 60 HLA typed frozen cells. Serum samples were considered positive if they reproducibly caused at least 60% cytotoxicity with one or more cells in the panel or at least 40% cytotoxicity with two or more cells.(1) Panel reactivity (PRA) was calculated as a percentage (i.e., the number of positive panel cells divided by the number of panel cells × 100).
An antibody-positive patient was considered to have “lost” his/her antibody when a negative test result was obtained subsequent to one or more positive tests. For a patient who had had a positive antibody test and then later had a negative antibody test, the duration of the patient's antibody in weeks was calculated as the difference between the date of the negative antibody test and the date of their initial positive antibody test, divided by seven and rounded to the nearest integer. Baseline antibody positive patients were excluded from the analysis of antibody persistence as the time of onset of their antibodies was unknown. However, we did determine and correlate with their platelet randomization assignment the percentage of baseline antibody-positive patients who lost their antibodies during the initial 8 weeks of the study and then during the follow-up 3 to 12 month period.
Platelet Refractoriness
The definition of platelet refractoriness for the TRAP Trial was two sequential post-transfusion corrected platelet count increments (CCIs) of <5,000 and at least one of the sequential transfusions had to be ABO-compatible and stored for ≤48 hours.
Alloimmune platelet refractoriness was defined as a patient who became platelet refractory within two weeks before or after the development of an antibody. Post-transfusion platelet counts after the initial 8 weeks were not recorded so platelet refractoriness could not be assessed during the 3 to 12 month follow-up period.
Observed And Derived Study Variables Recorded During The Trial
The recorded variables included age, sex, prior antigen exposure by transfusion or pregnancy, whether transfusions were given within 2 weeks of study entry, study platelet transfusion assignment, number of study platelet transfusions received, whether study transfusions were γ-irradiated, number of weeks a patient was antibody positive during the third through the 12 month follow-up period, type of blood product support received during the follow-up period, baseline antibody status, PRA value of the first positive antibody test, and the maximum PRA value during the 12-month study.
During the three to 12 month follow-up period, no patient related factors such as additional chemotherapy, bone marrow transplantation, etc. that could have affected antibody persistence were recorded in the TRAP Trial data base.
Statistical Analysis
Rates of antibody disappearance (duration of antibodies) and the effects of prior transfusion, or pregnancy on antibody duration were compared between study arms using Kaplan-Meier methods. A multivariable analysis of antibody duration was performed using the Cox proportional hazards regression model. This provided a means of simultaneously evaluating the effects of the observed and derived study variables (listed above) while adjusting for covariates that may be associated with antibody duration. Comparisons of antibody duration during the follow-up period were made using the Mann-Whitney test.
RESULTS
Patients Who Became Antibody-Positive
Of the 603 patients who were enrolled in the TRAP Trial, 3 had no baseline antibody data. Among the remaining 600 patients, 45 (8%) were baseline LCT antibody positive, and another 16 were baseline platelet human platelet antigen antibody positive.(3) As platelet glycoprotein antibodies developed only at low rates (7% to 11% of patients), were equal among all study arms, and did not correlate with platelet refractoriness, persistence of these antibodies was not analyzed. Nine additional patients received fewer than 2 study transfusions and another 8 patients had only 1 antibody sample drawn while on study. The remaining 522 were candidates for evaluation of antibody duration. Among these, 145 (28%) had at least 1 positive antibody test at some time during the 12-month study. Antibody persistence was evaluated in these patients.
Of the 145 patients who developed antibodies, 61 (42%) were in the control group, 31 (21%) were in the UVB-PC group, 28 (19%) were in the F-PC group, and 25 (17%) were in the F-AP group. The relative frequencies among the modified platelet arms did not differ (p = 0.73), but the rates in all of the modified arms were significantly less than the control arm (p<0.001).(1)
Characteristics of the 145 antibody positive patients are given in Table 1. The majority of these patients had either been transfused and/or pregnant prior to entry on study, but less than half (64/145 = 44%) became platelet refractory during the 8 weeks of the trial (Table 1). Most of these patients (124 or 86%) became antibody positive before the end of the 8-week study transfusion period. The median time (25th, 75th percentiles) for the development of antibodies was 3 weeks (2, 5 weeks), and the time to development of antibodies did not differ among any of the study groups.
TABLE 1.
CHARACTERISTICS OF ANTIBODY POSITIVE PATIENTS
| Characteristic | Number | Percent |
|---|---|---|
| Antibody Positive Patients | 145 | 100 |
| Became Antibody Negative During The 12 Months of the Study | 81 | 56 |
| Female | 90 | 62 |
| Prior Transfusions | 108 | 75 |
| Prior Transfusion ≤2 Weeks Before Study Entry | 98 | 68 |
| Prior Pregnancy | 83 | 57 |
| Both Prior Pregnancy and Transfusion | 65 | 45 |
| Became Platelet Refractory While Receiving Study Platelet Transfusions (weeks 1-8) | 64 | 44 |
| Antibody Positive While Receiving Study Platelet Transfusions (Weeks 1-8) | 124 | 86 |
| Received All Gamma-Irradiated Platelet Transfusions (Weeks 1-8) | 71 | 49 |
| Received Leukoreduced Blood Products During Follow-up Period (3-12 Months) | 38 | 26 |
| Patients With At Least 1 Antibody Sample During the Follow-up Period (3-12 months) | 126 | 87 |
| Patients With An Antibody Sample At End Of Follow-up (12 Months) | 53 | 37 |
Among the 298 patients who were antibody negative at the end of 8 weeks and who had at least 1 antibody sample collected during the 3 to 12 month follow-up period, 275 patients received additional platelet and/or RBC transfusions, and 16 (5%) developed an antibody between 3 and 6 months and an additional 5 patients (2%) became antibody-positive between 6 and 12 months. Of these 21 patients, 7 had been given STD-PC during the first 8 weeks, 6 received UVB-PC, 5 received F-PC, and 3 received F-AP. Differences in rates of antibody development among the study arms in the follow-up period were not statistically significant. None of the 19 patients who were not transfused during follow-up became antibody positive (p=0.22 compared to the 21 of 275 who were transfused and became antibody positive). Of the 275 patients who were antibody negative during the initial 8 weeks of the trial and who received additional blood products afterwards, the percentage of patients who developed antibodies in the followup period did not differ between those patients who received leukoreduced blood products versus those who received non-leukoreduced blood products [6/21 (24%) patients versus 61/254 (24%) patients, respectively.]
Although data were recorded on the number of platelet and RBC transfusions a patient received after the initial eight weeks, this information should be considered approximate as it was often collected at a distant location. There were no differences in number or type of blood products transfused for patients previously assigned to any study arm (Table 2). Using a multivariate stepwise logistic regression analysis of the study recorded covariates, only the percentage of γ-irradiated platelet transfusions a patient received during the initial 8 weeks reduced the development of antibodies between 3 to 12 months. At 6 months, the percentage of γ-irradiated transfusions received was marginally associated with a decreased incidence of antibody development (p=0.06), becoming statistically significant at 12 months (p=0.004). However, these data may be confounded by the indication for γ-irradiation which may have suggested that the patient was receiving additional courses of immunosuppressive chemotherapy.
TABLE 2.
MEDIAN NUMBER OF TRANSFUSIONS RECEIVED BETWEEN 3 AND 12 MONTHS
| Type of Transfusion | PRIOR STUDY ASSIGNMENT* | |||
|---|---|---|---|---|
| STD-PC | F-PC | UVB-PC | F-AP | |
| Pooled Random Donor Platelets | 24 | 54 | 48 | 45 |
| Apheresis Platelets | 3 | 5 | 3 | 3 |
| RBC | 10 | 16 | 15 | 8 |
STD-PC (standard, unmodified pooled platelet concentrates), F-PC (filtered pooled platelet concentrates), UVB-PC (UVB-irradiated pooled platelet concentrates), and F-AP (single donor filtered apheresis platelets).
Sample Accrual For Antibody Testing
Among the 145 patients who became antibody positive during the year-long study, more than 92% had 8 weekly antibody samples, 126 patients (87%) had an 8-week sample, and 53 patients (37%) were still having antibody samples drawn at 12 months. There were progressively fewer patients for whom antibody samples were obtained over time. However, there were no consistent differences in the rate of decline in sample accrual among any of the study arms or between the antibody positive and negative patients.
Antibody Persistence
The event of interest, disappearance of an antibody, was observed in over half of the 145 evaluable patients; 81 patients (56%) lost their antibody before 12 months. The Kaplan-Meier estimate of the median time to antibody loss in the evaluable patients was 14 weeks (95% confidence limits: 12-19 weeks). From the Kaplan-Meier analysis, it was estimated that 73% of the patients (95% confidence limits: 62%-81%) would loose their antibodies within one year.
A similar rate of antibody loss was observed in the 45 baseline antibody positive patients; 24 patients (53%) lost their antibody. Loss of antibody in this group did not correlate with their platelet randomization assignment. Furthermore, in only 40% of these patients did their baseline percent PRA more than double. This result was evenly distributed among the arms; i.e., 5 patients in the STD-PC arm, 4 in the UVB-PC arm, 5 in the F-PC arm, and 4 in the F-AP arm. For over 50% of the patients, their baseline percent PRA was 95% or more of their maximum percent PRA.
Effects of Study Factors On Antibody Persistence When Considered Individually
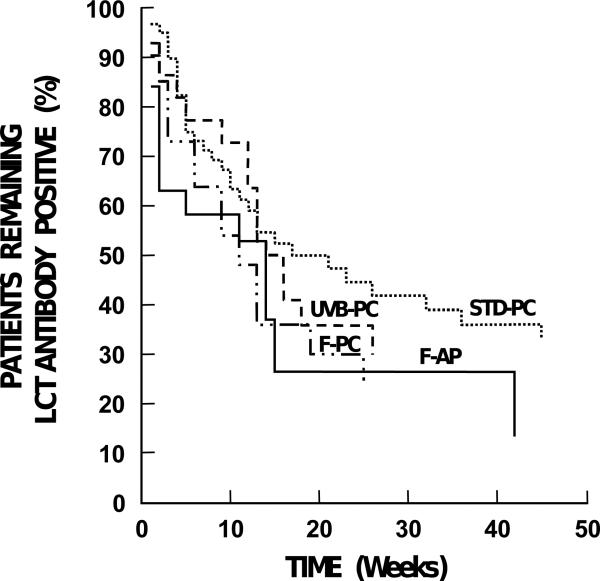
Table 3 provides a summary of the results of the Kaplan-Meier analysis of individual factors and their effects on antibody persistence. Unlike antibody development, there were no statistically significant differences with respect to antibody persistence between patients who received STD-PC and those who received modified platelet transfusions (Figure 1). During the study, the observed antibody loss rate was 32/61 (52%) in the control arm, 16/31 (52%) in the UVB-PC arm, 16/28 (57%) in the F-PC arm, and 17/25 (68%) in the F-AP arm. Of the 124 patients who became antibody positive within 8 weeks, 37 (30%) lost their antibody while on study transfusions; and among these patients, 10 (8%) had it reappear before the end of the 8-week study period.
TABLE 3.
RESULTS OF KAPLAN-MEIER ANALYSIS OF INDIVIDUAL STUDY FACTORS AND ANTIBODY DURATION
| Trial Factor | Log-Rank Statistic (d.f.)** | p-value |
|---|---|---|
| Treatment assignment (STD-PC, UVB-PC, F-PC, F-AP)* | 4.43 (3) | 0.22 |
| Prior transfusions | 0.04 (1) | 0.85 |
| Prior transfusion ≤2 weeks before study entry | 0.22 (1) | 0.64 |
| Sex | 7.95 (1) | 0.005 |
| Prior pregnancy | 6.88 (1) | 0.01 |
| Received all gamma irradiated platelet transfusions | 3.72 (1) | 0.05 |
STD-PC (standard, unmodified pooled platelet concentrates), F-PC (filtered pooled platelet concentrates), UVB-PC (UVB-irradiated pooled platelet concentrates), and F-AP (single donor filtered apheresis platelets).
(d.f.) = degrees of freedom associated with log-rank statistic.
Figure 1. Antibody Duration Based On Platelet Randomization Assignment.
There were no differences in duration of antibody positivity or in proportion of patients who became antibody-negative based on platelet randomization assignment. Data are plotted until the last patient in each group became antibody-negative. STD-PC (standard, unmodified pooled platelet concentrates), F-PC (filtered pooled platelet concentrates), UVB-PC (UVB-irradiated pooled platelet concentrates), and F-AP (single donor filtered apheresis platelets).
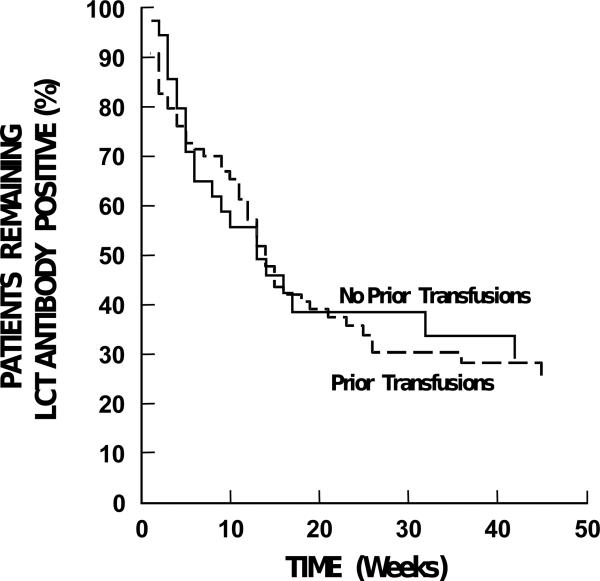
Transfusion history was represented by two factors: whether the patient had ever been previously transfused and whether the patient had been transfused in the 2 weeks before study entry. Neither of these factors was associated with antibody duration (Table 3, Figure 2).
Figure 2. Antibody Duration Based on Transfusion History.
There were no differences in duration of antibody positivity based on whether the patient had previously received any transfusions. Data are plotted until the last patient in each group became antibody-negative.
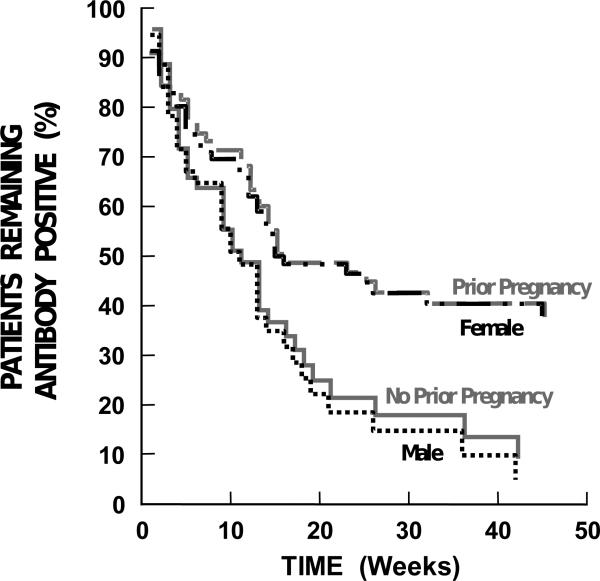
Females tended to have more persistent antibodies than males, p=0.005 (Table 3, Figure 3), with previously pregnant females having longer antibody duration than patients who had never been pregnant (female or male), p=0.01 (Table 3, Figure 3). However, these two factors are confounded, as all but nine of the women had been previously pregnant. There was no effect of number of prior pregnancies on antibody duration.
Figure 3. Antibody Duration Based on Gender And Prior Pregnancy.
There was a statistically-significant decrease in duration of antibody positivity between male and female patients (p=0.005). No prior pregnancy significantly decreased the duration of antibody positivity compared to having been previously pregnant (p=0.01). However, there was no effect based on the number of prior pregnancies. The data are plotted until the last patient in each group became antibody-negative.
Whether the patient received all gamma irradiated platelet transfusions on study showed a mild association with antibody duration (Table 3). Those patients whose platelet transfusions were all gamma irradiated tended to have slightly longer antibody duration (p=0.05).
Ninety-four of the 145 evaluable patients were antibody positive for 1 or more weeks during the 3 to 12 month follow-up period, and 88 received additional non-study transfusions, 5 did not, and, for 1, there was no information. The median number of weeks of antibody positivity for the transfused and non-transfused patients was 11 and 12, respectively (p = 0.57, Mann-Whitney). Thirty patients received leukoreduced blood products , and 58 did not, and antibodies persisted for 14 and 10 weeks, respectively (p=0.27, Mann-Whitney).
Effects Of Study Factors When Assessed By Multivariable Analysis
Kaplan-Meier analysis of an association between study arm and duration of antibody indicated that the type of platelet product (modified or standard) received did not influence the duration of the patient's antibody. To determine if there was an effect of study arm after adjusting for the effects of other study variables, a Cox proportional hazards (PH) regression model was fit to the antibody duration data that included study arm, age, sex, prior transfusion history, prior pregnancy (Yes/No), week of first positive antibody test, percent PRA of the first positive antibody test, highest PRA on study, and whether all study transfusions were gamma irradiated as covariates. After adjusting for these covariates, there was still no evidence that study arm influenced antibody duration (Table 4).
TABLE 4.
ESTIMATES FROM A COX PROPORTIONAL HAZARDS REGRESSION ANALYSIS OF ANTIBODY DURATION
| Covariate | Coef* | se (coef)/** | p-value |
|---|---|---|---|
| Percent PRA of 1st Positive Antibody Test and Previously Pregnant | -0.020 | 0.009 | <0.025 |
| Maximum Percent PRA, all samples and Previously Pregnant | -0.020 | 0.008 | <0.017 |
| All Gamma Irradiated Platelet Transfusions | -0.41 | 0.23 | 0.08 |
| UVB-PC† | -0.078 | 0.063 | 0.22 |
| F-PC† | 0.004 | 0.114 | 0.97 |
| F-AP† | -0.068 | 0.184 | 0.71 |
Coefficients of study arms are given as log (relative risk) compared to Standard arm.
Coef = Coefficient.
se (coef) = Standard error of the coefficient.
STD-PC (standard, unmodified pooled platelet concentrates)
F-PC (filtered pooled platelet concentrates)
UVB-PC (UVB-irradiated pooled platelet concentrates)
F-AP (single donor filtered apheresis platelets)
This multivariable analysis indicated that duration of a previously pregnant patient's antibody tended to increase as the percent PRA of the first positive antibody test increased (p<0.025). A similar association was noted when the patients’ maximum percent PRA values were substituted for the percent PRA of their first positive antibody test in previously-pregnant women (p<0.017). In never-pregnant patients, antibody persistence versus loss occurred at about equal rates over a wide range of PRA values. In previously pregnant women, antibodies became more durable as the maximum percent PRA increased. There was also a weak association between longer antibody duration and gamma irradiation of all blood products compared to patients whose blood products were not all gamma irradiated (p=0.08). Table 4 gives the estimated coefficients from this regression model and their associated p values.
DISCUSSION
The TRAP Trial is the largest trial that sought to determine whether modified platelets - either filtered leukoreduced or UV-B irradiated given for up to 8 weeks - would reduce rates of alloimmunization and alloimmune platelet refractoriness in 603 AML patients receiving induction chemotherapy.(1) In the subset of 522 patients evaluated in the current study who received at least two study platelet transfusions so that refractoriness could be evaluated, 64 of the 145 patients (44%) who developed antibodies became platelet-refractory versus 26% for the patients who remained antibody-negative (p<0.001). Although antibody positivity correlated with platelet refractoriness, 56% of the antibody-positive patients did not become platelet-refractory. Previous studies have also demonstrated that platelet refractoriness occurs in only about 30% of patients who develop HLA antibodies.(4,5) However, if antibodies persist, they might adversely affect the response to platelet transfusions during subsequent episodes of thrombocytopenia following consolidation chemotherapy. To determine antibody persistence, antibody samples that were collected weekly for 8 weeks and monthly for 3 to 12 months were analyzed.
As might have been anticipated in a year-long study, sample accrual was highest at study initiation and declined over time. Of the 145 patients who became antibody positive and were evaluable for antibody persistence, 92% had 8 weekly samples, 87% had an 8-week sample, and 37% had a 12-month sample. Importantly, there were no differences in sample accrual in patients assigned to any of the study arms or between patients who became antibody positive and those who remained antibody negative.
Antibodies progressively decreased over time at the same rate and to the same degree in all study patients, regardless of the platelet product they received during the 8 weeks of the TRAP Trial (Figure 1). Among the 145 patients who became antibody-positive, 81 (56%) were documented to have become antibody-negative by 1 year. Based on the Kaplan-Meier estimate, if all patients were followed for 12 months, antibody loss would be 73% (95% confidence interval: 62% to 81%). These results are consistent with the average 52% of patients whose antibodies were not durable in previous studies.(reviewed in (6,7) This compares to antibody loss in 53% of the 45 patients who were already antibody positive at study entry, and antibody loss in these patients was the same regardless of the platelet arm to which they were assigned (p=0.80).
Median time for documented antibody loss in the patients who developed antibodies during the course of the study was 14 weeks from their first positive antibody sample (95% confidence interval of 12 weeks to 19 weeks). Among the 298 patients who were still antibody negative at 8 weeks, only 21 additional patients (7%) developed antibodies. These 21 patients had all been transfused after the 8 weeks of the TRAP Trial, but antibody development was not affected by their prior platelet randomization assignment nor by the number or type (filter-leukoreduced or standard) of blood products they subsequently received. These data suggest there will be little benefit in preventing alloimmune platelet refractoriness from continuing modified blood product transfusions beyond 8 weeks and maybe even beyond 5 weeks, which is the time when 75% of the patients would have developed an antibody. It has previously been documented that 92% of AML patients who do not become immunized to platelets during induction chemotherapy never become immunized in spite of multiple additional transfusions.(8) Furthermore, 73% of patients with a PRA of <60% and 58% of patients with a PRA of ≥60% will respond to HLA one-antigen mismatched donor transfusions, suggesting that continued platelet support for alloimmunized patients may not require fully HLA-matched donors.(9)
In a multivariable analysis, the factors that reduced the incidence of developing alloantibodies during the TRAP Trial were no prior pregnancy (p<0.001), transfusions given within 2 weeks of study entry (p=0.02), and assignment to receive any of the modified platelet transfusions (p<0.001 compared to patients who received standard platelets).(1) The factors that most affected the persistence of antibodies were prior pregnancy (Figure 3) and the percent positivity of the PRA (Figure 4). Thus, prior pregnancies increased both the development and persistence of LCT antibodies. In contrast, although receiving transfusions within two weeks of study entry lowered the incidence of antibody development, any prior transfusions - regardless of the timing of the transfusions - had no affect on antibody persistence. Interestingly, γ-irradiation of blood products had disparate effects; i.e., it reduced the incidence of late developing antibodies that occurred in the 3-12 month follow-up period, while increasing the persistence of antibodies that developed within the first 8 weeks.
Higher percent PRA values were associated with antibodies of longer duration only in previously-pregnant women. This was true whether percent PRA was based on the results of the first positive sample or the maximum PRA value attained during the study. The estimated coefficient from the Cox PH model suggests that, for a 30 percentage point increase in the PRA, the relative risk of retaining an antibody would be expected to increase by a factor of 1.67. The effect of percent PRA positivity on antibody persistence has been previously reported.(5)
The type of platelet transfusions a patient receives, whether standard or modified, does not affect either the rate of antibody loss or the percentage of patients who lose antibodies. Even though a patient has previously required HLA-matched donor platelet transfusions because of the development of alloimmune platelet refractoriness, patients should be periodically either re-tested for HLA antibodies and/or given random donor platelet transfusions to determine if they remain platelet refractory. This strategy is particularly relevant for patients whose PRA is at a low level as these restricted HLA antibodies are often not durable as found in our study and by others.(5)
Principles relevant to the platelet transfusion support of AML patients based on data from the TRAP Trial are: 1) patients who are antibody positive prior to induction chemotherapy do not benefit from modified blood products; 2) modified transfusions to prevent platelet alloimmunization should be discontinued if alloantibodies develop during induction chemotherapy and also if antibodies have not developed after 5 to 8 weeks of transfusions; and 3) patients do not require modified blood products during consolidation chemotherapy as antibody development is very low. However, there are clearly other reasons to give leukoreduced blood products besides reducing the incidence of platelet alloimmunization which should be considered by physicians; e.g., prevention of CMV transmission by transfusion(17) and avoiding leukocyte-mediated transfusion reactions.(18)
Finally, the cost-effectiveness of providing modified platelet and red cell transfusions for AML patients undergoing induction chemotherapy to prevent platelet alloimmunization could be questioned for the following reasons: 1) the current major causes of platelet refractoriness in thrombocytopenic patients are not related to alloimmunization. Rather, refractoriness is usually associated with the patient's clinical condition, to the drugs they are receiving, or to the storage age of their transfused platelets; 2) the late development of HLA antibodies that often occurs after platelet support is no longer needed; 3) the low frequency of alloimmune platelet refractoriness during induction chemotherapy; and 4) the loss of antibodies over time.(10-16)
Because this study was done some time ago, one could question the relevance of this data today. However, the therapy for AML patients undergoing induction chemotherapy during the intervening years has not changed substantially. Current antibody assays may be more sensitive than the lymphocytotoxic assay used in this study, (19) but it is unlikely that there would be a differential effect among the randomized groups in either the development or loss of antibodies. However, there may be a higher percent of the refractory patients who would be found to be alloimmune platelet refractory, but many of the patients in this study who were antibody positive did not become refractory. The relevance of the newer antibody tests to development and loss of antibodies and their association with platelet refractoriness will have to be determined in future studies.
ACKNOWLEDGMENTS
The authors wish to acknowledge the excellent administrative support for the development of this manuscript provided by Ginny Knight, Puget Sound Blood Center, Seattle, WA.
Supported By: National Heart, Lung and Blood Institute, National Institutes of Health grants: U01 HL42799, U01 HL42802, U01 HL42810, U01 HL42811, U01 HL42815, U01 HL42824, U01 HL42832.
Biographies
Dr. S.J. Slichter is the primary author of the manuscript, worked with the biostatistician on data analysis, was involved in the design and implementation of the trial, enrolled patients in the trial, and ensured data collection on all enrolled patients.
Mr. D. Bolgiano reviewed all of the data, generated the tables and figures, and provided the statistical analyses of the data.
Drs. K.-J. Kao, T. Kickler, J. McFarland, J. McCullough, and R. Woodson were involved in the design and implementation of the trial, enrolled patients in the trial and ensured data collection on all enrolled patients, reviewed drafts of the manuscript, and contributed to writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: None.
Conflict of Interest: None.
Contributor Information
Sherrill J. Slichter, Puget Sound Blood Center and University of Washington School of Medicine; Seattle, WA.
Douglas Bolgiano, Puget Sound Blood Center; Seattle, WA.
Kuo-Jang Kao, University of Florida; Miami, FL.
Thomas S. Kickler, Johns Hopkins University; Baltimore, MD.
Janice McFarland, Blood Center of Southeastern Wisconsin; Milwaukee, WI.
Jeffrey McCullough, University of Minnesota; St. Paul, MN.
Robert Woodson, University of Wisconsin; Madison, WI.
REFERENCES
- 1.The Trial To Reduce Alloimmunization To Platelets Study Group Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 2.Fuller TC, Phelan D, Gebel HM, et al. Antigenic specificity of antibody reactive in the antiglobulin-augmented lymphocytotoxicity test. Transplantation. 1982;34:24–29. doi: 10.1097/00007890-198207000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Visentin GP, Wolfmeyer K, Newman PJ, et al. Detection of drug-dependent, platelet-reactive antibodies by antigen-capture ELISA and flow cytometry. Transfusion. 1990;30:694–700. doi: 10.1046/j.1537-2995.1990.30891020326.x. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MF, Waters AH. Platelet transfusions: The problem of refractoriness. Blood Rev. 1990;4:16–24. doi: 10.1016/0268-960x(90)90013-i. [DOI] [PubMed] [Google Scholar]
- 5.Brand A, Claas FH, Voogt PJ, et al. Alloimmunization after leukocyte-depleted multiple random donor platelet transfusions. Vox Sang. 1988;54:160–166. doi: 10.1111/j.1423-0410.1988.tb03892.x. [DOI] [PubMed] [Google Scholar]
- 6.Slichter SJ. Alloimmune refractoriness to transfused platelets. In: Garratty G, editor. Immunology of Transfusion Medicine. Dekker, Inc.; New York: 1994. pp. 597–627. [Google Scholar]
- 7.McFarland JG. Alloimmunization and platelet transfusion. Semin Hematol. 1996;33:315–328. [PubMed] [Google Scholar]
- 8.Dutcher JP, Schiffer CA, Aisner J, et al. Long-term follow-up of patients with leukemia receiving platelet transfusions: Identification of a large group of patients who do not become alloimmunized. Blood. 1981;58:1007–1011. [PubMed] [Google Scholar]
- 9.Hussein MA, Lee EJ, Fletcher R, et al. The effect of lymphocytotoxic antibody reactivity on the results of single antigen mismatched platelet transfusions to alloimmunized patients. Blood. 1996;87:3959–3962. [PubMed] [Google Scholar]
- 10.Slichter SJ. Controversies in platelet transfusion therapy. Ann Rev Med. 1980;31:509–540. doi: 10.1146/annurev.me.31.020180.002453. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg RC, Donnelly SF, Boyd JC, et al. Clinical and blood bank factors in the management of platelet refractoriness and alloimmunization. Blood. 1993;81:3428–3434. [PubMed] [Google Scholar]
- 12.Doughty HA, Murphy MF, Metcalfe P, et al. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang. 1994;66:200–205. doi: 10.1111/j.1423-0410.1994.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 13.Alcorta I, Pereira A, Ordinas A. Clinical and laboratory factors associated with platelet transfusion refractoriness: A case-control study. Br J Haematol. 1996;93:220–224. doi: 10.1046/j.1365-2141.1996.447982.x. [DOI] [PubMed] [Google Scholar]
- 14.Legler TJ, Fischer I, Dittmann J, et al. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol. 1997;74:185–189. doi: 10.1007/s002770050280. [DOI] [PubMed] [Google Scholar]
- 15.Levin M-D, de Veld JC, van der Holt B, et al. Immune and nonimmune causes of low recovery from leukodepleted platelet transfusions: A prospective study. Ann Hematol. 2003;82:357–362. doi: 10.1007/s00277-003-0648-7. [DOI] [PubMed] [Google Scholar]
- 16.Slichter SJ, Davis K, Enright H, et al. Factors affecting post-transfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden RA, Slichter SJ, Sayers M, Weisdorf D, Cays M, Schoch G, Banaji M, Haake R, Welk K, Fisher L, McCullough J, Miller W. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplantation. Blood. 1995;86:3598–3603. [PubMed] [Google Scholar]
- 18.Heddle NM, Blajchman MA, Meyer RM, Lipton JH, Walker IR, Sher GD, Constantini LA, Patterson B, Roberts RS, Thorpe KE, Levine MN. A randomized controlled trial comparing the frequency of acute reactions to plasma-removed platelets and prestorage WBC-reduced platelets. Transfusion. 2002;42:556–566. doi: 10.1046/j.1537-2995.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 19.Pei R, Wang G, Tarsitani C, Rojo S, Chen T, Takemura S, Liu A, Lee J. Simultaneous HLA Class I and Class II antibody screening with flow cytometry. Hum Immunol. 1998;59:313–322. doi: 10.1016/s0198-8859(98)00020-2. [DOI] [PubMed] [Google Scholar]