Synopsis
Blood Oxygen Level Dependent (BOLD) functional magnetic resonance imaging (fMRI) depicts changes in deoxyhemoglobin concentration consequent to task-induced or spontaneous modulation of neural metabolism. Since its inception in 1990, this method has been widely employed in thousands of studies of cognition for clinical applications such as surgical planning, for monitoring treatment outcomes, and as a biomarker in pharmacologic and training programs. Technical developments have solved most of the challenges of applying fMRI in practice. These challenges include low contrast to noise ratio of BOLD signals, image distortion, and signal dropout. More recently, attention is turning to the use of pattern classification and other statistical methods to draw increasingly complex inferences about cognitive brain states from fMRI data. This paper reviews the methods, some of the challenges and the future of fMRI.
Keywords: functional magnetic resonance imaging, fMRI
Introduction
Functional Magnetic Resonance Imaging (fMRI) is a class of imaging methods developed in order to demonstrate regional, time-varying changes in brain metabolism3,37,49. These metabolic changes can be consequent to task-induced cognitive state changes or the result of unregulated processes in the resting brain. Since its inception in 1990, fMRI has been used in an exceptionally large number of studies in the cognitive neurosciences, clinical psychiatry/psychology, and presurgical planning (between 100,000 and 250,000 entries in PubMed, depending on keywords). The popularity of fMRI derives from its widespread availability (can be performed on a clinical 1.5T scanner), non-invasive nature (does not require injection of a radioisotope or other pharmacologic agent), relatively low cost, and good spatial resolution. Increasingly, fMRI is being used as a biomarker for disease33,36, to monitor therapy54, or for studying pharmacological efficacy62. Thus, it is of interest to review the fMRI contrast mechanisms, the strengths and weaknesses, and evolutionary trends of this important tool.
Basis for fMRI
fMRI is of course based on MRI, which in turn uses Nuclear Magnetic Resonance coupled with gradients in magnetic field38 to create images that can incorporate many different types of contrast such as T1 weighting, T2 weighting, susceptibility, flow, etc.7 In order to understand the particular contrast mechanism predominantly used in fMRI it is necessary to first discuss brain metabolism.
All the processes of neural signaling in the brain, including formation and propagation of action potentials, binding of vesicles to the pre-synaptic junction, the release of neurotransmitters across the synaptic gap, their reception and regeneration of action potentials in the postsynaptic structures, scavenging of excess neurotransmitters, etc., require energy in the form of adenosine triphosphate (ATP)55. This nucleotide is produced principally by the mitochondria from glycolytic oxygenation of glucose, and its production results in carbon dioxide as a byproduct. When a region of the brain is up-regulated (i.e. activated) by a cognitive task such as finger tapping, the additional neural firing and other increased signaling processes result in a locally increased energy requirement, in turn resulting in up-regulated cerebral metabolic rate of oxygen (CMRO2) in the affected brain region12. As the local stores of oxygen in tissues adjacent to capillaries are transiently consumed by glycolysis and waste products build up, various chemical signals (CO2, NO, H+) cause a vasomotor reaction in arterial sphincters upstream of the capillary bed, causes dilation of these vessels. The increased blood flow acts to restore the local [O2] level required to overcome the transient deficit; however, for reasons that are still not fully understood more oxygen is delivered than is needed to offset the increase in CMRO2. As a result, neural up-regulation results initially in a build-up of deoxygenated hemoglobin [Hb] and a decrease in deoxygenated hemoglobin [HbO2] in the intra- and extravascular spaces, followed within a second or two by a vasodilatory response that reverses the situation to result in an increase in [HbO2] and decrease in [Hb] over that in the resting condition13,22 (see Fig. 1). This sequence of processes is described as the hemodynamic response to the neural event.
Figure 1.
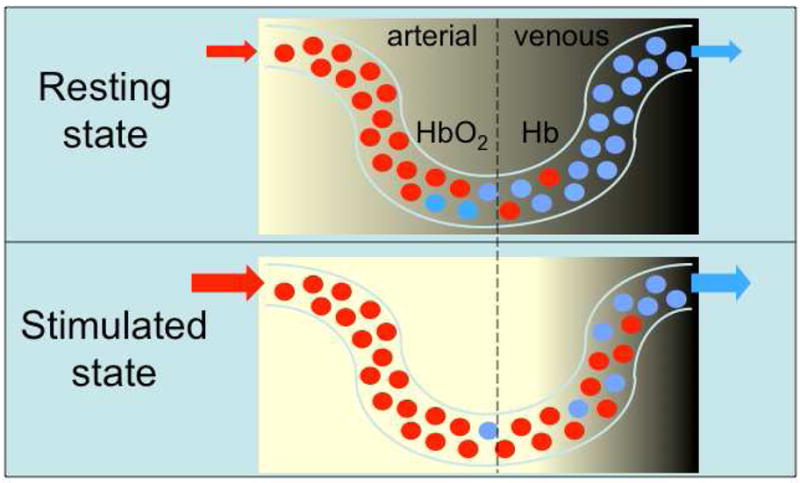
Sketch of brain tissue containing a capillary during rest (top) and activation (bottom). Red and blue circles represent red blood cells that are fully oxygenated (HbO2) and fully deoxygenated (Hb), respectively. The MRI signal is depressed in the venous side of the capillary due to the paramagnetic susceptibility of the HB acting as an endogenous contrast agent (shown darker). In the stimulated condition, increased blood flow causes the Hb to be swept out and replaced by HbO2, causing a BOLD signal increase.
Thus, there are two primary consequences of increased neural activity, and both can be detected by MRI: increased local cerebral blood flow (CBF) and changes in oxygenation concentration (Blood Oxygen Level Dependent, or BOLD contrast). The change in CBF can be observed using an injected contrast agent and perfusion weighted MRI, first demonstrated by Belliveau6, or non-invasively by arterial spin labeling (ASL)25. However, ASL suffers from reduced sensitivity, increased acquisition time and increased sensitivity to motion compared with the BOLD contrast method, and its use has therefore centered on obtaining quantitative measurements of baseline cerebral blood flow (CBF) for studies modeling the neurobiological mechanisms of activation12,22 or calibration of vasoreactivity4, rather than in routine mapping of brain function.
The second mechanism, termed Blood Oxygenation Level Dependent (BOLD) contrast, was first demonstrated in rats48,50 and later in humans3,37,49,52, and is the contrast that is used in virtually all conventional fMRI experiments. BOLD contrast results from the change in magnetic field surrounding the red blood cells depending on the oxygen state of the hemoglobin. When fully oxygenated, HbO2 is diamagnetic and is magnetically indistinguishable from brain tissue. However, fully deoxygenated Hb has 4 unpaired electrons and is highly paramagnetic59. This paramagnetism results in local gradients in magnetic field whose strength depends on the [Hb] concentration. These endogenous gradients in turn modulate the intra- and extra-vascular blood’s T2 and T2* relaxation times through diffusion and intravoxel dephasing, respectively. Using a gradient refocused echo (GRE) MRI pulse sequence7, the acquisition is made sensitive to T2* and T2. At 1.5T and 3T, the T2* contrast is predominant and is largest in venules61, while at higher field strength the diffusion-weighted contrast of T2 relaxation becomes more important and, because signals are generated preferentially in capillaries and tissue with spin-echo acquisitions, provides greater spatial specificity57,64. Since most fMRI is currently performed at 3 Tesla or below, BOLD fMRI utilizes primarily GRE methods because of the increased T2* contrast10.
Task activation fMRI studies seek to induce different neural states in the brain as the visual, auditory or other stimulus is manipulated during the scan, and activation maps are obtained by comparing the signals recorded during the different states. Therefore, it is important to collect each image in a snapshot mode to avoid head motion and physiological processes of respiration8 and cardiovascular functions15,16 from injecting noise signals unrelated to the neural processing being interrogated. Generally, most fMRI is performed using an Echo Planar Imaging (EPI) method43, which can collect data for a two dimensional image in approximately 60 ms at typical resolutions (3.4 × 3.4 × 4 mm3 voxel size). Typically, whole brain scans with ~ 32 2D slices are acquired with a repetition time (TR) of 2s/volume. Each voxel in the resulting scan produces a time series that is subsequently analyzed in accordance to the task design.
The fMRI experiment
The typical fMRI task activation experiment utilizes visual, auditory or other stimuli to alternately induce two or more different cognitive states in the subject, while collecting MRI volumes continuously as described above. With a two-condition design, one state is called the experimental condition, while the other is denoted the control condition, and the goal is to test the hypothesis that the signals differ between the two states. Using a block design, the trials are arranged to alternate between the experimental and control conditions, as shown in Fig. 2, with each block typically being a few tens of seconds long. The block design is optimum for detecting activation, but a jittered event-related (ER) design is superior when characterization of the amplitude or timing of the hemodynamic response is desired 11,41. In the ER design, task events are relatively brief and occur at non-constant inter-trial intervals with longer periods of control condition, which allows the hemodynamic response to return more fully to baseline. Jittering the timing serves to sample the hemodynamic response with higher temporal frequency in the overall time series, but may also be used to induce a desired cognitive strategy, e.g., to avoid an anticipatory response or maintain attention.
Figure 2.
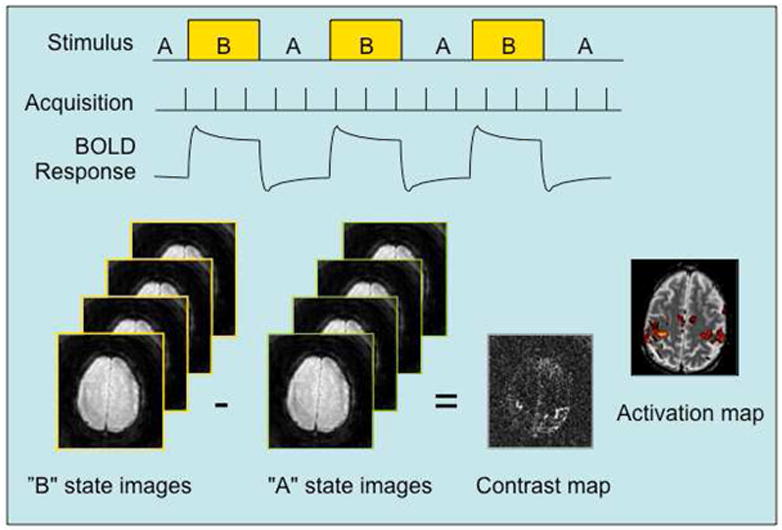
Block design fMRI experiment. A neural response to the state change from A to B in the stimulus is accompanied by a hemodynamic response (as shown in Fig. 1) that is detected by the rapid and continuous acquisition of MR images sensitized to BOLD signal changes. Using single- or multi-variate time series analysis methods, the average signal difference between the two states is computed for the scan and a contrast map generated. A statistical activation map is finally obtained using a suitable threshold for the difference; the map depicts the probability that a voxel is activated given the uncertainty due to noise and the small BOLD signal differences.
The degree to which valid inferences can be drawn from the measured time series data depends in large part on careful design of the task. The investigator must take care that only the effect of interest changes between experimental and control conditions, while confounding effects such as attention and valence are maintained constant or irrelevant. In some studies this is straightforward, such as the use of a sensory task for presurgical mapping, wherein the goal is only to localize activation so that important brain functions can be maintained after surgery. In this case the signal intensity is of minor interest as long as it is adequate to characterize the functional substrates to be preserved during surgical intervention. In many other cases, however, comparative inferences are desired such as parametric studies of the influence of task difficulty on a cognitive process, and thus control of such factors as learning, adaptation, and salience must be considered.
Analysis methods
Once the images have been acquired, the time series data must be processed to obtain maps of brain activation. Because the BOLD contrast is small (< 1% in many studies of higher cognitive processes)35, simply averaging images over the experimental and control conditions and then subtracting (as sketched in Fig. 2) is inadequate to reliably determine differences because noise will compete to render false positives and negatives. The noise results from thermal sources in the subject and electronics, bulk motion of the head, cardiac and respiratory-induced noise, and variations in baseline neural metabolism. Because the noise can sometimes be larger than the signal of interest, fMRI analyses compare the signal difference between the states using a statistical test. These tests result in an activation map that is a function of the probability that the brain states differ. The statistical test for activation can utilize a general linear model (GLM)27,63, cross-correlation with a modeled regressor2, or one of several data-driven approaches such as independent components analysis (ICA)14. The models against which the acquired data are tested include the experimental design of interest as well as “nuisance regressors” of no interest such as signal drift, motion, and noise reflected in global or white-matter signals. In all cases, the activation testing is preceded by a series of preprocessing steps.
The steps in pre-processing can include all or some of the following: 1) time-slice correction, to eliminate differences between the time of acquisition of each slice in the volume; 2) motion coregistration, in which affine head motion is detected and the time series of volumes is resampled to register each time frame to a reference frame, such as the first or middle time series point; 3) correction for physiological noise from breathing and cardiovascular function8,15, low pass and/or high pass temporal filtering to improve the statistics while removing spectral components of no interest; 4) spatial smoothing to improve the signal to noise ratio (SNR) and improve the normality of the noise distribution; 5) pre-whitening to correct for autocorrelation in the time series35. The analysis of fMRI data continues to be a subject of intense research at this time, and is one about which numerous books have been written, to which the reader is referred for further information (e.g. Sarty56).
Comparisons with other functional imaging modalities
fMRI can be compared to other imaging methods used to obtained functional assessment of brain metabolism in terms of spatial and temporal resolution and availability. The primary alternatives are Positron Emission Tomography (PET), Near Infrared Spectroscopy (NIRS), ElectroEncephalography (EEG) and MagnetoEncephalography (MEG)35.
1) Spatial resolution
Resolution in fMRI is limited primarily by SNR because of the necessity for rapid acquisition of time series information. For MRI, , where p is the pixel size, w the slice thickness, Tacq is the k-space readout time and N is the number of time frames. Thus, as Tacq is reduced for single shot imaging (typically 20–30ms) the pixel size must be increased over that for conventional anatomic imaging to maintain an acceptable SNR. Accordingly, the typical fMRI pixel size is 3–4 mm, although with higher field magnets (7T) a pixel size of 500 microns or less may be readily achieved57. The resolution of PET is limited by the size of the gamma ray detectors as well as the positron-electron annihilation range, and is typically ≥5–10 mm. NIRS resolution is low (10–20 mm) and limited predominantly by the strong scatter and attenuation of IR photons (which also limits the depth of cortex that can be imaged within a banana-shaped region connecting optodes), the modest density of optodes and the ill-conditioned inverse problem of reconstructing 3D maps of [Hb] from scalp recordings20. The resolution in EEG and MEG is similarly limited to > 10–20 mm by the fact that a unique reconstruction of dipoles is not possible from scalp-based measurements of electrical or magnetic distributions and models and regularization must be employed for model estimation. Unlike EEG, MEG does not have the confound that scalp recordings may be spatially distorted by heterogeneous electrical conduction paths within the brain/skull.
2) Temporal resolution
fMRI’s temporal resolution is limited by hemodynamic response time; typically the BOLD response has a width of ~3s and a peak occurring ~5–6s after the onset of a brief neural stimulus. This is much slower than the underlying neural processes, and temporal information is thereby heavily blurred. Nevertheless, by jittering event-related stimuli and using appropriate analysis methods 11 temporal inferences in the 100ms resolution range can be achieved51. PET scans require minutes to complete because of the low count rates of injected radio nuclides, so changes in neural processes can only be studied by repeated scanning. Like BOLD, NIRS reports changes in blood oxygenation and, exacerbated by low SNR of NIR photons in the brain, has temporal limitations similar to those of fMRI. EEG and MEG, on the other hand, have millisecond temporal resolution and can easily capture the dynamics of evoked responses that last a few ms to several hundred ms. Multimodal approaches combining fMRI and EEG use fMRI maps as spatial priors to reconstruct high temporal resolution electrophysiology, thereby gaining resolution in both dimensions21.
Strengths and weaknesses of fMRI
From the discussion above, a primary strength of fMRI is its relatively high spatial resolution and availability. In addition, it is readily available to both clinical and academic researchers, is noninvasive, and can provide high resolution anatomic scans in the same session to use for localization, vessel identification,45 or development of maps of white matter connectivity through the use of diffusion tensor imaging (DTI)5.
Because BOLD contrast derives from the sluggish hemodynamic response to metabolic changes, a significant weakness is its low temporal resolution. Another problem is signal dropout and/or spatial distortion in frontal orbital and lateral parietal regions, caused by the ~9 ppm difference in magnetic susceptibility at interfaces between air and brain tissue18. This can result in erroneous lack of BOLD signal in ventral, temporal and PFC regions important in many cognitive studies. Many methods have been developed to diminish these susceptibility losses, although most involve some tradeoff of SNR in magnetically uniform brain regions18,19,31,34,58,60,65. Other weaknesses include the scanner’s loud noise associated with switched magnetic fields, which can cause confounds in studies of audition28,29 and resting state networks30; however, methods using interleaved scanning/stimulus delivery epochs can avoid these problems, albeit with some loss of flexibility in experimental design26,28. Finally, the high magnetic fields require customized stimulus delivery and subject response systems, again limiting flexibility and complicating multimodal experiments such as concurrent EEG recording.
Future of fMRI
For the most part, the MRI physics and technology development behind BOLD fMRI acquisitions are mature, and the tradeoffs between acquisition speed, resolution, SNR, signal dropout and contrast are well understood. Over the years, a number of investigators have attempted to develop alternatives to BOLD contrast using direct neural current detection9, although by now it is understood42 that the weak size of the neural current signal relative to physiological noise makes a breakthrough unlikely. Another alternative is the use of diffusion weighted imaging to demonstrate activation-related changes in populations of bound vs. free water distributions39,40. A potential advantage is that such diffusion related changes may have more rapid responses than BOLD methods. However, again the signals are weaker than BOLD contrast and their biophysical origin is still unclear46. Other experiments have reported the use of spin echo rather than gradient echo acquisitions of BOLD contrast, especially at higher fields where T2* is foreshortened57.
While a modest research effort will continue in improving acquisition technology, the bulk of research in the development of fMRI has shifted to its application to answering more complex questions in cognitive neuroscience. One promising area is that of using activation maps as input to classification and state change algorithms to predict or classify cognitive behavior, such as predicting brain states44,53 (also see, e.g. Norman for a review47). Other emerging uses of fMRI include the development of quantitative measures, i.e. biomarkers for disease or monitoring behavioral modification such as reading disorders. A cautionary note, however, is that because of the small BOLD responses typical of cognitive processes, most studies are limited to employing group statistics to make inferences about populations rather than about individuals. Thus fMRI’s use in quantifying individual characteristics may continue to be limited to those tasks for which relatively strong BOLD responses are observed, such as primary sensory systems. Resting state networks and their modification by disease conditions such as Alzheimer’s, depression and other psychiatric disorders32 are gaining attention. However, there is growing awareness that these networks may be much more complex in their spatio-temporal dynamics than previously thought17, and much more work is indicated to understand their role and utility in predicting individual behavior/physiology. Finally, feedback derived from real-time fMRI has been shown to allow subjects to learn pain-reduction strategies24, enhance sensorimotor control23 and to control relevant brain regions in mood disorder experiments53. The reader is also referred to Bandettini1 for additional considerations regarding the future of fMRI.
Conclusions
Functional MRI has enjoyed an exciting development course with an exponential growth in published studies since its inception in the early 90’s, and it has become commonplace for clinical uses such as presurgical planning, fundamental cognitive neuroscience investigations, behavior modification and training. Informed by fMRI, more sophisticated modeling of brain networks is certain to lead to new levels of understanding of the human brain.
Acknowledgments
The author is indebted to C. E. Chang for suggestions on the manuscript.
Supported by NH Grant P41-RR009874
Footnotes
The author has no conflicts to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandettini PA, Jesmanowicz A, Wong EC, et al. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 3.Bandettini PA, Wong EC, Hinks RS, et al. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 4.Bangen KJ, Restom K, Liu TT, et al. Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol Aging. 2009;30:1276. doi: 10.1016/j.neurobiolaging.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Belliveau JW, Kennedy DJ, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein MA, King KF, Zhou XJ. Handbook of MRI Pulse sequences. New York: Elsevier Press; 2004. [Google Scholar]
- 8.Birn RM, Smith MA, Jones TB, et al. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodurka J, Bandettini PA. Toward direct mapping of neuronal activity: MRI detection of ultraweak, transient magnetic field changes. Magn Reson Med. 2002;47:1052. doi: 10.1002/mrm.10159. [DOI] [PubMed] [Google Scholar]
- 10.Boxerman JL, Bandettini PA, Kwong KK, et al. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med. 1995;34:4. doi: 10.1002/mrm.1910340103. [DOI] [PubMed] [Google Scholar]
- 11.Buckner RL, Bandettini PA, O’Craven KM, et al. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14878. doi: 10.1073/pnas.93.25.14878. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buxton R, Frank L. A model for the coupling between cerebral blood flow and oxyen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magnetic Resonance in Medicine. 1998;39:855. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- 14.Calhoun VD, Adali T, Pearlson GD, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang C, Glover GH. Relationship between respiration, end-tidal CO(2), and BOLD signals in resting-state fMRI. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho ZH, Ro YM. Reduction of susceptibility artifact in gradient-echo imaging. Magn Reson Med. 1992;23:193. doi: 10.1002/mrm.1910230120. [DOI] [PubMed] [Google Scholar]
- 19.Constable R, Spencer D. Composite image formation in Z-shimmed functional MR imaging. Magn Reson Med. 1999;42:110. doi: 10.1002/(sici)1522-2594(199907)42:1<110::aid-mrm15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Cui X, Bray S, Bryant DM, et al. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.10.069. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol. 2001;11:202. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 22.Davis TL, Kwong KK, Weisskoff RM, et al. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.deCharms RC, Christoff K, Glover GH, et al. Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage. 2004;21:436. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 24.deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med. 1992;23:37. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 26.Edmister WB, Talavage TM, Ledden PJ, et al. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7:89. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 28.Gaab N, Gabrieli JD, Glover GH. Assessing the influence of scanner background noise on auditory processing. I. An fMRI study comparing three experimental designs with varying degrees of scanner noise. Hum Brain Mapp. 2007;28:703. doi: 10.1002/hbm.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaab N, Gabrieli JD, Glover GH. Assessing the influence of scanner background noise on auditory processing. II. An fMRI study comparing auditory processing in the absence and presence of recorded scanner noise using a sparse design. Hum Brain Mapp. 2007;28:721. doi: 10.1002/hbm.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaab N, Gabrieli JD, Glover GH. Resting in peace or noise: scanner background noise suppresses default-mode network. Hum Brain Mapp. 2008;29:858. doi: 10.1002/hbm.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 32.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 33.Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu JJ, Glover GH. Mitigation of susceptibility-induced signal loss in neuroimaging using localized shim coils. Magn Reson Med. 2005;53:243. doi: 10.1002/mrm.20365. [DOI] [PubMed] [Google Scholar]
- 35.Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland: Sinauer Associates, Inc; 2004. [Google Scholar]
- 36.Kim DI, Sui J, Rachakonda S, et al. Identification of Imaging Biomarkers in Schizophrenia: A Coefficient-constrained Independent Component Analysis of the Mind Multi-site Schizophrenia Study. Neuroinformatics. 2010 doi: 10.1007/s12021-010-9077-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauterbur PC. Image formation by induced local interactions. Examples employing nuclear magnetic resonance. Nature. 1973;242:190. [PubMed] [Google Scholar]
- 39.Le Bihan D, Urayama S, Aso T, et al. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci U S A. 2006;103:8263. doi: 10.1073/pnas.0600644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T, Song AW. Fast functional brain signal changes detected by diffusion weighted fMRI. Magn Reson Imaging. 2003;21:829. doi: 10.1016/s0730-725x(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 41.Liu TT, Frank LR. Efficiency, power, and entropy in event-related FMRI with multiple trial types. Part I: theory. Neuroimage. 2004;21:387. doi: 10.1016/j.neuroimage.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Luo Q, Gao JH. Modeling magnitude and phase neuronal current MRI signal dependence on echo time. Magn Reson Med. 2010 doi: 10.1002/mrm.22569. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansfield P. Multi-planar image formation using NMR spin echoes. J Phys Chem C. 1977;10:L55. [Google Scholar]
- 44.Martinez-Ramon M, Koltchinskii V, Heileman GL, et al. fMRI pattern classification using neuroanatomically constrained boosting. Neuroimage. 2006;31:1129. doi: 10.1016/j.neuroimage.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Menon RS. Postacquisition suppression of large-vessel BOLD signals in high-resolution fMRI. Magn Reson Med. 2002;47:1. doi: 10.1002/mrm.10041. [DOI] [PubMed] [Google Scholar]
- 46.Miller KL, Bulte DP, Devlin H, et al. Evidence for a vascular contribution to diffusion FMRI at high b value. Proc Natl Acad Sci U S A. 2007;104:20967. doi: 10.1073/pnas.0707257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norman KA, Polyn SM, Detre GJ, et al. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990;16:9. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa S, Lee TM, Nayak AS, et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa S, Lee TM, Stepnoski R, et al. An approach to probe some neural systems interaction by functional MRI at neural time scale down to milliseconds. Proc Natl Acad Sci U S A. 2000;97:11026. doi: 10.1073/pnas.97.20.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phan KL, Fitzgerald DA, Gao K, et al. Real-time fMRI of cortico-limbic brain activity during emotional processing. Neuroreport. 2004;15:527. doi: 10.1097/00001756-200403010-00029. [DOI] [PubMed] [Google Scholar]
- 54.Richards TL, Berninger VW. Abnormal fMRI Connectivity in Children with Dyslexia During a Phoneme Task: Before But Not After Treatment 1. J Neurolinguistics. 2008;21:294. doi: 10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roland PE. Brain Activation. New York: John Wiley & Sons; 1993. [Google Scholar]
- 56.Sarty GE. Computing Brain Activity Maps from fMRI Teime Series Images. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 57.Shmuel A, Yacoub E, Chaimow D, et al. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage. 2007;35:539. doi: 10.1016/j.neuroimage.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenger VA, Boada FE, Noll DC. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T2*-weighted functional MRI. Magnetic Resonance in Medicine. 2000;44:525. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thulborn KR, Waterton JC, Matthews PM, et al. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 60.Weiger M, Pruessmann KP, Osterbauer R, et al. Sensitivity-encoded single-shot spiral imaging for reduced susceptibility artifacts in BOLD fMRI. Magn Reson Med. 2002;48:860. doi: 10.1002/mrm.10286. [DOI] [PubMed] [Google Scholar]
- 61.Weisskoff RM, Zuo CS, Boxerman JL, et al. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med. 1994;31:601. doi: 10.1002/mrm.1910310605. [DOI] [PubMed] [Google Scholar]
- 62.Wise RG, Preston C. What is the value of human FMRI in CNS drug development? Drug Discov Today. doi: 10.1016/j.drudis.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- 64.Yacoub E, Van De Moortele PF, Shmuel A, et al. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. Neuroimage. 2005;24:738. doi: 10.1016/j.neuroimage.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Yang QX, Dardzinski BJ, Li S, et al. Multi-gradient echo with susceptibility inhomogeneity compensation (MGESIC): demonstration of fMRI in the olfactory cortex at 3.0 T. Magn Reson Med. 1997;37:331. doi: 10.1002/mrm.1910370304. [DOI] [PubMed] [Google Scholar]


