Abstract
Background
A key challenge inhibiting the timely initiation of pediatric antiretroviral treatment is the loss to follow-up of mothers and their infants between the time of mothers' HIV diagnoses in pregnancy and return after delivery for early infant diagnosis (EID) of HIV. We sought to identify barriers to follow-up of HIV-exposed infants in rural Zambézia Province, Mozambique.
Methods
We determined follow-up rates for early infant diagnosis and age at first test in a retrospective cohort of 443 HIV-infected mothers and their infants. Multivariable logistic regression models were used to identify factors associated with successful follow-up.
Results
Of the 443 mother-infant pairs, 217 (49%) mothers enrolled in the adult HIV care clinic, and only 110 (25%) infants were brought for early infant diagnosis. The predictors of follow-up for EID were larger household size (OR=1.30; 95% CI, 1.09-1.53), independent maternal source of income (OR=10.8; 95% CI, 3.42-34.0), greater distance from the hospital (OR=2.14; 95% CI, 1.01-4.51) and maternal receipt of ART (OR=3.15; 95% CI, 1.02-9.73). The median age at first test among 105 infants was 5 months (interquartile range 2 to 7); 16% of the tested infants were infected.
Conclusions
Three of four HIV-infected women in rural Mozambique did not bring their children for early infant HIV diagnosis. Maternal receipt of ART has favorable implications for maternal health that will increase the likelihood of early infant diagnosis. We are working with local health authorities to improve the linkage of HIV-infected women to HIV care to maximize early infant diagnosis and care.
Keywords: HIV/AIDS, antiretroviral therapy, early infant diagnosis, prevention of mother-to-child transmission, Mozambique, Sub-Saharan Africa
Introduction
HIV-related child mortality rates remain high in sub-Saharan Africa despite growing access to antiretroviral therapy (ART).1-6 In resource-limited settings, up to 30% of untreated HIV-infected children die before their first birthday and more than 50% die before they reach 2 years of age.7 Early Infant Diagnosis (EID) is crucial to reduce morbidity and mortality in HIV-infected children through the timely initiation of antibiotic prophylaxis and ART.8 The Children with HIV Early Antiretroviral Therapy (CHER) study showed that mortality among HIV-infected infants randomized to ART within the first 12 weeks of life was significantly reduced compared to those receiving later ART based on clinical criteria as per prior WHO guidelines.9 With the development of dried blood spot (DBS) methods to simplify sample management and transport, polymerase chain reaction (PCR) testing of infants has become feasible in rural settings using centralized laboratories.10,11 The use of DBS-PCR methods in infants has been demonstrated to be both highly sensitive and specific, enabling EID in even resource-limited settings.10-14
Mozambique has one of the world's most severe HIV/AIDS epidemics. National HIV prevalence is 16%, with 1.5 million persons, including 100,000 children, estimated to be living with HIV within a population of 21 million.15,16 In 2008, <10% of children in need of treatment were receiving ART.16 Zambézia is the country's second most populated province and has the second highest estimated adult HIV prevalence (19%).16,17 While programs for the prevention of mother to child transmission of HIV (PMTCT) have been in place since the 1990s, coverage has been limited and resources required to address pediatric HIV infection have only been available since 2007. EID is offered in the “child at-risk” clinics within the maternal and child health (MCH) services at all district hospitals and some primary health centers.16 EID services include: 1) infant feeding advice, 2) counseling and support, 3) cotrimoxazole prophylaxis, and 4) HIV diagnosis for exposed infants <18 months using DBS-PCR testing methods and ≥18 months using rapid antibody testing. DBS samples are sent to one of two national referral laboratories for PCR analysis. Infants with positive HIV test results are referred to clinicians trained in pediatric care and ART use, while uninfected infants are followed in the “child at-risk” clinics for continued surveillance. One of the principal goals of the EID program is to increase ART coverage among eligible children.
While EID implementation guidelines and protocols have been established in many sub-Saharan African countries, few studies have evaluated the retention of mother-infant pairs from prenatal PMTCT to EID in “real world” field conditions. Published reports from pilot programs or urban areas may not reflect rural African realities.18-20 Reports from Malawi and northern Uganda, as well as our own experience in Mozambique, indicate substantial attrition of mother-infant pairs in PMTCT services.21,22 We characterize our experiences in the first year following the introduction of DBS-PCR services in a rural district hospital by describing follow-up rates for EID; we further identify predictors of successful follow-up and earlier age of infant testing.
Methods
Study Context
In Zambézia Province, all district hospitals and many primary health centers offer PMTCT services as part of routine prenatal care. Current Ministry of Health PMTCT protocols in Mozambique mandate that as part of their first prenatal visit all pregnant women should receive group HIV counseling and be offered an opt-out rapid HIV test as part of their first prenatal visit. HIV-seropositive women are offered antiretroviral (ARV) prophylaxis at 28 weeks gestational age or as soon thereafter as enrolled in prenatal care and are also are advised to bring their infants to the “child at-risk” clinics for follow-up care and diagnosis. Women are also given the recommendation to exclusively breastfeed unless they have a safe, accessible, and affordable source of artificial formula; 98% of women in the province breastfeed, only 41% exclusively.23 All HIV-infected women identified in PMTCT services are referred to the adult HIV clinic within the district hospital where further one-on-one counseling is provided, and a CD4+-cell count is performed. Women on combination ART (cART) are followed regularly in the adult HIV clinic. At the Alto Molócuè district hospital, all of these services are provided within the same hospital compound.
Study Design and Population
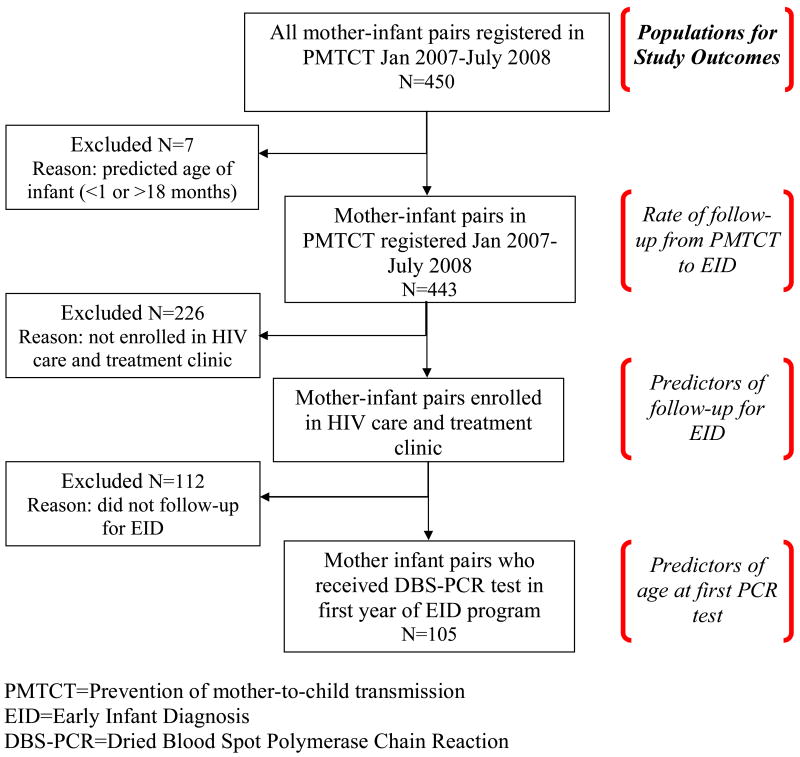
We conducted a retrospective cohort study of mother-infant pairs who were identified through record reviews from January 2007 to November 2008 at a single rural district-level hospital in Alto Molócuè, Zambézia Province after the first year of the EID program. All HIV-positive women who received PMTCT services at Alto Molócuè district hospital were included in the primary data analysis of retention for EID. Mother-infant pairs were excluded if the infants were ineligible for DBS-PCR testing due to their age (<1 month or ≥18 months) during the study period. Follow-up for EID was determined by review of PMTCT and “child at-risk” clinic registries. Mother/infant pairs from both registries were matched by maternal name and verified with infant date-of-birth, maternal age, and village. For the secondary data analysis designed to identify mother-infant characteristics predictive of follow-up for EID, only mothers from PMTCT services who enrolled in the adult HIV clinic were included because socio-demographic data collection occurs during enrollment at the adult HIV clinic. In the secondary analysis considering predictors of early testing, we included those infants born to eligible mothers who were registered and tested at the “child at-risk” clinic (Figure 1).
Figure 1. Study population in Alto Molócuè district hospital, 2007-2008.
The National Committee of Bioethics for Health in Mozambique and the Institutional Review Board at Vanderbilt University School of Medicine approved this research, including collection of the data from hospital records.
Statistical Analysis
Socio-demographic and clinical characteristics of mother-infant pairs who obtained and did not obtain EID follow-up were compared using Wilcoxon rank sum and chi-squared tests. To identify those characteristics that were independently predictive of follow-up, we used a multivariable logistic regression model. At most, 5% of the data was missing for any predictor; thus we used single imputation to replace missing values with the median value of non-missing data. For the analysis of age-at-first PCR test, we used Kaplan-Meier estimates to compute time to first PCR test in HIV-exposed infants; we assessed for difference in PCR testing by HIV status using a log-rank test. We used linear regression to identify predictors of earlier testing. Distance from hospital was excluded from the model, as it correlated highly with birth location. There were no excessive missing data for predictors of age at testing; missing values were accounted for using multiple imputation techniques. In this technique, we used other patient characteristics to predict the missing value for each mother-infant pair, and then we performed regression on the imputed data. We repeated this task 10 times and took the average estimates from the multiple linear regressions.24 R-software 2.9.2 was used for data analysis.25
Results
Study Population Characteristics and Rates of Follow-up for EID
At Alto Molócuè district hospital, 443 HIV-positive women received PMTCT services during the study period; 217 (49%) of those women enrolled in the adult HIV clinic. Of the enrollees, 76% lived within 10 km of the hospital, 82% reported no source of income, 84% reported basic reading skills, 87% reported having a domestic partner, and 11% were on cART (Table 1).
Table 1. Characteristics by maternal/infant follow-up to EID services among women registered in PMTCT (n=217) in Alto Molócuè district hospital, 2007-2008.
| Total (n=217) |
Follow-up for EID (n=105) |
No follow-up for EID (N=112) |
P-Value | |
|---|---|---|---|---|
| Household size, median (IQR) | 6 (5 to 8) | 7 (5 to 8) | 6 (4 to 7) | <0.001 |
| Distance, n (%) | 0.003 | |||
| ≤10 km | 165 (76%) | 70 (67%) | 95 (85%) | |
| >10 km | 52 (24%) | 35 (33%) | 17 (15%) | |
| Maternal income, n (%) | <0.001 | |||
| No income | 179 (83%) | 71 (68%) | 108 (96%) | |
| Income from job or agriculture | 37 (17%) | 33 (32%) | 4 (4%) | |
| Maternal education, n (%) | 0.050 | |||
| None | 35 (16%) | 12 (11%) | 23 (21%) | |
| Primary School | 131 (60%) | 62 (59%) | 69 (62%) | |
| Secondary School | 51 (24%) | 31 (30%) | 20 (18%) | |
| Maternal literacy, n (%) | 0.101 | |||
| Cannot read at basic level | 35 (16%) | 12 (11%) | 23 (21%) | |
| Reads at least at basic level | 182 (84%) | 93 (89%) | 89 (79%) | |
| Maternal relationship status, n (%) | 0.757 | |||
| No partner | 28 (13%) | 15 (14%) | 13 (12%) | |
| Partner | 186 (87%) | 90 (86%) | 96 (88%) | |
| Mother on combined ART | 0.003 | |||
| No | 193 (89%) | 86 (82%) | 107 (96%) | |
| Yes | 24 (11%) | 19 (18%) | 5 (4%) |
NOTE: Of the 217 mother-infant pairs, missing values for characteristics included: household size (11), maternal income (1), and maternal civil status (3). Percentages are computed using the number of patients with non-missing value.
PMTCT=Prevention of mother-to-child transmission
EID=Early infant diagnosis
ART=antiretroviral therapy
Among the mother-infant pairs who accessed EID services (N=105), 88% of the infants were born at the hospital. Maternal self-report suggested that 91% of mothers and 87% of infants might have received ARV prophylaxis. By self-report, 74% of infants received combination nevirapine and zidovudine prophylaxis, 88% of infants were breastfed, 63% exclusively (Table 2).
Table 2. Characteristics of HIV-exposed infants tested with DBS-PCR tests in Alto Molócuè district hospital, 2007-2008.
| Tested Infants (n=105) | |
|---|---|
| Sex, n (%) | |
| Male | 48 (46%) |
| Female | 56 (54%) |
| Distance from Hospital, n (%) | |
| ≤10 km | 87 (84%) |
| >10 km | 16 (16%) |
| Birth Location, n (%) | |
| Home | 10 (12%) |
| Hospital | 7 (88%) |
| Type of Birth, n (%) | |
| Vaginal | 91 (97%) |
| C-section | 3 (3%) |
| A e at first DBS-PCR, median (IQR) | 5 months (2 to 7) |
| Age range at PCR test | 1-18 months |
| Maternal ARV prophylaxis, n (%) | |
| Combination ART | 10 (10%) |
| Zidovudine+Nevirapine | 37 (38%) |
| Nevirapine Only | 43 (44%) |
| None | 8 (8%) |
| Infant ARV Prophylaxis, n (%) | |
| Zidovudine+Nevirapine | 75 (74%) |
| Nevirapine Only | 13 (13%) |
| Yes, but regimen unknown | 1 (1%) |
| None | 13 (13%) |
| Infant Feeding, n (%) | |
| Breast feeding | 87 (89%) |
| Exclusive breast feeding | 61 (63%) |
| Mixed feeding (breast + formula or food) | 11 (11%) |
NOTE: Of the 105 infants, missing values for characteristics included: distance (2), birth location (19), type of birth (11), maternal ARV prophylaxis (7), infant ARV prophylaxis (3), and feeding (22). Percentages are computed using the number of patients with non-missing value.
DBS-PCR=Dried Blood Spot Polymerase Chain Reaction
ARV=antiretroviral
ART= antiretroviral therapy
Of the 443 HIV-positive mothers who received PMTCT services, 110 (25%) brought their infants for EID. Almost all of the mothers (95%) who accessed EID services with their infants were themselves enrolled in the adult HIV clinic.
Predictors of Follow-up for EID
In a multivariable logistic regression model, we found that an independent source of maternal income, larger household size, greater distance, and mothers on cART were all statistically significant predictors of follow-up for EID (Table 3). Independent maternal income, via formal employment or agriculture, markedly increased the odds of follow-up for EID (OR=10.8; 95% CI, 3.42-34.0). For each member increase in household size, the odds of mother-infant pairs accessing EID services increased (OR= 1.29; 95% CI, 1.09-1.53). Although 76% of the mother-infant pairs lived within 10 km of the hospital, there was an increased odds of accessing EID services for those who lived >10 km away (OR=2.14; 95% CI, 1.01-4.51). Maternal receipt of cART increased substantially the odds of mother-infant pairs accessing EID (OR=3.15; 95% CI, 1.02-9.73).
Table 3. Multivariable model of predictors of follow-up to early infant diagnosis in Alto Molócuè district hospital, 2007-2008.
| Odds Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|
| Household size | |||
| Per member increase | 1.29 | 1.09-1.53 | 0.003 |
| Distance from hospital | |||
| >10 km vs. ≤10 km | 2.14 | 1.01-4.51 | 0.046 |
| Maternal income | |||
| Job or agriculture vs. no income | 10.79 | 3.42-34.02 | <0.001 |
| Maternal literacy | |||
| Can read vs. cannot read | 1.88 | 0.78-4.51 | 0.159 |
| Maternal relationship status | |||
| Partner vs. no partner | 0.92 | 0.35-2.44 | 0.870 |
| Mother on combination antiretroviral therapy | 3.15 | 1.02-9.73 | 0.047 |
Note: Of the 217 mother-infant pairs, missing values for predictors singly imputed included: household size (11), and income (1).
Age at First Test and Predictors of Early PCR Testing
The median age of HIV-exposed infants at first DBS-PCR test in the first year of the EID program was 5 months (IQR 2 to 7). Ten percent of infants were tested at the target of 1 month of age. In a linear regression model of age at first test, an independent source of maternal income and maternal receipt of cART were statistically significant predictors of timing of first PCR test (Table 5). A woman having an independent source of income decreased age at first test by almost 2 months (effect=-1.67; 95% CI, -3.12 to -0.22) compared to women with no independent income. In contrast, maternal receipt of cART delayed age at first test by >2 months (effect=2.63; 95% CI, 0.26-5.00) in comparison to those not on ART.
EID Program Outcomes
Among the 105 mother-infant pairs included in the analysis, 16% of the infants tested positive with the HIV DBS-PCR test. HIV infection varied by age at test and ARV prophylaxis (data not shown). The median turn-around time for DBS results was 12.6 weeks (IQR 10.9 TO 16.4).
Discussion
Our study found that low uptake was the norm in the first year of the EID program in rural Mozambique; only 25% of HIV-positive mothers who received PMTCT services returned for infant EID. Furthermore, a majority of the mother-infant pairs that did successfully return for EID did so with months of delay that the CHER study documents have negative clinical implications.9 While most of the predictors of successful follow-up for EID in this study were structural (e.g., economic, societal), maternal receipt of cART was a strong independent predictor of follow-up that could be improved with targeted interventions. Mothers on cART may be more likely to access EID services because of increased understanding of their HIV status, more frequent medical visits, increased opportunities for providers to identify at-risk infants, and the up-front selection of women who at least intermittently secure transportation to the hospital. While maternal receipt of cART increased the likelihood of infant EID, it also was associated with a later age at testing. We need to further investigate the source of delays in seeking infant care; for instance, are women delaying care with a more or less advanced burden of disease?
The high attrition between PMTCT and EID services in our study is consistent with other published estimates from PMTCT services across sub-Saharan Africa.20-22,26-32 The strong association in our study between follow-up for EID and familial socio-demographic factors such as maternal income and household size has also been observed in other resource-limited settings.32-36 The paradoxical association between greater distance and higher EID follow-up likelihood in our study may reflect a selection bias of women who have already overcome the challenge of distance as well as decreased fear of social stigma when seeking HIV care outside one's own community. A Zambian study reported that adult cART adherence was unrelated to distance traveled; similar interactions may have applied.37 There is growing evidence that expanding eligibility criteria for receipt of ART to all peripartum HIV-infected women may be an effective strategy to improve both maternal HIV outcomes and diminish maternal to child HIV transmission (MTCT); results from this study suggest the additional benefit of improved infant retention.21,38,39
Although this study was not designed to examine factors contributing to MTCT rates, it is notable that there is a large discrepancy between the relatively high self-reported coverage of ARV prophylaxis for mother-infant pairs (>80%) and the high rate of infant infection in our study. Work from Zambia and confirmed from the multinational PEARL study supports the finding that self-report of receipt of ARV prophylaxis is an unreliable measure of coverage.40,18 There are several programmatic explanations that may contribute to the high rate of MTCT observed during the first year of our program; all are amenable to intervention with quality improvement efforts at all steps of the “prevention cascade”.41,42
The Alto Molócuè hospital has a very modest infrastructure and faces serious human resource constraints that are difficult to overcome even with support from programs supported by the President's Emergency Plan for AIDS Relief (PEPFAR).43 EID was implemented after a single MCH nurse attended a week-long seminar in EID, with on-site training of the additional 3-4 MCH nurses, all of whom were already responsible for all routine perinatal services to also provide all PMTCT and EID services. Expanded PMTCT programs in the context of already stressed MCH programs may create substantial stress on the parent systems.44 The role of peer educators and community health workers are well-known strategies to improve adherence and retention in HIV programs, particularly when there are few trained health workers.45,46 In Alto Molócuè, social workers and peer educators are integral in services in the adult HIV clinic, but during the study period, there was only one part-time social worker for all MCH services. At the time of the study, two different non-governmental organizations were supporting HIV-related services in the hospital: one with antiretroviral treatment program and the other with PMTCT services. While all services are offered in the same hospital compound, weak linkages between programs, particularly between PMTCT and the adult HIV clinic likely contributed to loss to follow-up.
The principal study strength was its “real world” rural setting, in contrast to more optimistic findings that might emerge from model programs with substantial resources added for research and/or in urban settings. The design of this study has limitations, including the potential for misclassification and selection biases. While factors included in our multivariate model were each potentially important predictors of follow-up, we cannot exclude the possibility that our results were influenced by unmeasured confounders. Participant mortality and migration have been shown in other studies to be outcomes misclassified as lost-to-follow-up; our study was not designed to measure these outcomes, or to evaluate barriers to follow-up from the patient perspective.21,32 Finally, our relatively small sample size from a single site during the first program year limits ability to generalize to other areas with more mature EID programs.
Early experience from an EID program in a rural resource-limited setting provides an important baseline to inform future interventions aimed at improving retention of mother-infant pairs between PMTCT and EID. Our findings suggest that the timely initiation of cART in all pregnant women who are eligible is important not only for maternal health and reducing mother-to-child transmission, but may also be instrumental in improving follow-up of HIV-exposed infants. This adds further urgency to implement the World Health Organization's most recent guidelines recommending earlier antiretroviral therapy for a larger group of HIV-positive pregnant women.47 The findings from our study imply that improving linkages between PMTCT, adult HIV and post post-partum care may be a key modifiable factor to improve retention of HIV-exposed infants. We are conducting a pilot quality improvement intervention study to increase linkages between PMTCT and EID services; preliminary results suggest considerable promise in improving infant retention through systems improvement.
Supplementary Material
Table 4. Multivariable model of predictors of age at first test in Alto Molócuè district hospital, 2007-2008.
| Effect | 95% Confidence Interval | P-value | |
|---|---|---|---|
| Household size | |||
| Per member increase | -0.084 | -0.48 to 0.31 | 0.675 |
| Birth location | |||
| Hospital vs. home | 0.004 | -2.45 to 2.52 | 0.978 |
| Maternal Income | |||
| Job or agriculture vs. no income | -1.67 | -3.12 to -0.22 | 0.027 |
| Maternal Literacy | |||
| Can read vs. cannot read | 0.425 | -1.99 to 2.86 | 0.726 |
| Maternal relationship status | |||
| Partner vs. no partner | -0.14 | -2.37 to 2.09 | 0.902 |
| Mother on combination antiretroviral therapy | 2.63 | 0.26 to 5.00 | 0.032 |
Note: Of the 105 infants, missing values for predictors multiply imputed included: household size (3), birth location (19), and income (1).
Acknowledgments
We thank the mothers and staff from Alto Molócuè hospital and Friends in Global Health for their participation. Meredith Bortz at the Vanderbilt Institute for Global Health assisted with editing and references.
Funding: The HIV/AIDS Treatment and Care program in Zambézia is supported by PEPFAR through the CDC Global AIDS Program (grant U2GPS000631), with training supported in part by the Fogarty-sponsored Vanderbilt-CIDRZ AIDS International Training and Research Program (NIH grant D43TW001035). Travel and other research expenses for this study were supported by the Office of the Dean, Vanderbilt University School of Medicine (the Emphasis Program). Funders had no role in study design, data collection, analysis, decision to publish or preparation of this manuscript.
Footnotes
Presented in part at the Global Health Council Conference; May 26-30, 2009 (Thursday Poster 34); Washington DC, USA; and at the International AIDS Conference; July 18-23, 2010 (#10928); Vienna, Austria.
References
- 1.Spira R, Lepage P, Msellati P, et al. Natural history of human immunodeficiency virus type 1 infection in children: a five-year prospective study in Rwanda. Mother-to-Child HIV-1 Transmission Study Group. Pediatrics. 1999;104:e56. doi: 10.1542/peds.104.5.e56. [DOI] [PubMed] [Google Scholar]
- 2.Obimbo EM, Mbori-Ngacha DA, Ochieng JO, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected African children. Pediatr Infect Dis J. 2004;23:536–43. doi: 10.1097/01.inf.0000129692.42964.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillay T, Adhikari M, Mokili J, et al. Severe, rapidly progressive human immunodeficiency virus type 1 disease in newborns with coinfections. Pediatr Infect Dis J. 2001;20:404–10. doi: 10.1097/00006454-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 5.Stringer JSA, Sinkala M, Stout JP, et al. Comparison of two strategies for administering nevirapine to prevent perinatal HIV transmission in high-prevalence, resource-poor settings. J Acquir Immune Defic Syndr. 2003;32:506–13. doi: 10.1097/00126334-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leyenaar JK, Novosad PM, Ferrer KT, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in Lesotho. Pediatr Infect Dis J. 2009;29:340–345. doi: 10.1097/INF.0b013e3181bf8ecb. [DOI] [PubMed] [Google Scholar]
- 7.Newell M, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 8.Aledort JE, Ronald A, Le Blancq SM, et al. Reducing the burden of HIV/AIDS in infants: the contribution of improved diagnostics. Nature. 2006;444 1:19–28. doi: 10.1038/nature05443. [DOI] [PubMed] [Google Scholar]
- 9.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creek T, Tanuri A, Smith M, et al. Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana's national program for prevention of mother-to-child transmission. Pediatr Infect Dis J. 2008;27:22–26. doi: 10.1097/INF.0b013e3181469050. [DOI] [PubMed] [Google Scholar]
- 11.Sherman GG, Stevens G, Jones SA, et al. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38:615–17. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 12.Stevens W, Sherman G, Downing R, et al. Role of the laboratory in ensuring global access to ARV treatment for HIV-infected children: consensus statement on the performance of laboratory assays for early infant diagnosis. Open AIDS J. 2008;2:17–25. doi: 10.2174/1874613600802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman GG, Cooper PA, Coovadia AH, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24(11):993–97. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 14.Ngo-Giang-Huong N, Khamduang W, Leurent B, et al. Early HIV-1 diagnosis using in-house real-time PCR amplification on dried blood spots for infants in remote and resource-limited settings. J Acquir Immune Defic Syndr. 2008;49:465–471. doi: 10.1097/QAI.0b013e31818e2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Country Profile: Mozambique. [June 28, 2009];2008 Available at: http://2006-2009.pepfar.gov/press/81612.htm.
- 16.HIV/SIDA / Home - Ministério da Saúde. [June 28, 2009]; Available at: http://www.misau.gov.mz/pt/hiv_sida.
- 17.Moon TD, Burlison JR, Sidat M, et al. Lessons learned while implementing an HIV/AIDS care and treatment program in rural Mozambique. Retrovirology: Research and Treatment. 2010;3:1–14. doi: 10.4137/RRT.S4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stringer EM, Dieder EK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in four African countries. JAMA. 2010;304(3):293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 19.Jani I, Langa J, Vaz P, et al. Challenges in introducing infant diagnosis of HIV using dried blood spots for DNA PCR in primary health care settings in Mozambique (Abstract TUPEB117. Presented at: International AIDS Society Conference; Sydney. 2007. [Google Scholar]
- 20.De Baets AJ, Bulterys M, Abrams EJ, et al. Care and treatment of HIV-infected children in Africa: issues and challenges at the district hospital level. Pediatr Infect Dis J. 2007;26:163–73. doi: 10.1097/01.inf.0000253040.82669.22. [DOI] [PubMed] [Google Scholar]
- 21.Ahoua L, Ayikoru H, Gnauck K, et al. Evaluation of a five-year programme to prevent mother-to-child transmission of HIV infection in northern Uganda. J Trop Pediatr. 2010;56:43–52. doi: 10.1093/tropej/fmp054. [DOI] [PubMed] [Google Scholar]
- 22.Ioannidis JP, Taha TE, Kumwenda N, et al. Predictors and impact of losses to follow-up in an HIV-1 perinatal transmission cohort in Malawi. Int J Epidemiol. 1999;28(4):769–775. doi: 10.1093/ije/28.4.769. [DOI] [PubMed] [Google Scholar]
- 23.Goverment Report. Maputo: 2003. Moçambique Inquérito Demográfico e de Saúde. [Google Scholar]
- 24.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: Wiley; 1987. [Google Scholar]
- 25.R-software (computer program). Version 2.9.2. [April 1, 2010]; www.r-project.org.
- 26.KIDS-ART-LINC Collaboration Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 27.Sherman GG, Jones SA, Coovadia AH, Urban MF, et al. PMTCT from research to reality--results from a routine service. S Afr Med J. 2004;94:289–92. [PubMed] [Google Scholar]
- 28.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10:1242–50. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 29.Doherty T, Besser M, Donohue S, et al. An evaluation of the prevention of mother-to-child transmission (PMTCT) of HIV initiative in South Africa - lessons and key recommendations. [January 12, 2010];Publications - Health Systems Trust. Available at: http://www.hst.org.za/publications/599.
- 30.Bwirire LD, Fitzgerald M, Zachariah R, et al. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans R Soc Trop Med Hyg. 2008;102:1195–1200. doi: 10.1016/j.trstmh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Nyandiko WM, Otieno-Nyunya B, Musick B, et al. Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr. 2010;54(1):42–50. doi: 10.1097/QAI.0b013e3181d8ad51. [DOI] [PubMed] [Google Scholar]
- 32.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53(3):405–11. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aber JL, Bennet NG, Jiali LI, et al. The effects of poverty on child health and development. Annual Review of Public Health. 1997;18:463–83. doi: 10.1146/annurev.publhealth.18.1.463. [DOI] [PubMed] [Google Scholar]
- 34.Sam I, Ball CS, Blott MJ, et al. Loss to follow-up of HIV-exposed infants in South London. Int J STD AIDS. 2003;14:291–92. doi: 10.1258/095646203321264999. [DOI] [PubMed] [Google Scholar]
- 35.Duflo E. Child health and household resources in South Africa: evidence from the old age pension program. The American Economic Review. 2000;90:393–98. doi: 10.1257/aer.90.2.393. [DOI] [PubMed] [Google Scholar]
- 36.Cauldbeck MB, O'Connor C, O'Connor MB, et al. Adherence to anti-retroviral therapy among HIV patients in Bangalore, India. AIDS Res Ther. 2009;6:7. doi: 10.1186/1742-6405-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlucci JG, Kamanga A, Sheneberger R, et al. Predictors of adherence to antiretroviral therapy in rural Zambia. J Acquir Immune Defic Syndr. 2008;47(5):615–22. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelzer K, Mlambo G. Factors determine HIV viral testing of infants in the context of mother-to-child transmission programs. Acta Paediatrica. 2010;99(4):590–596. doi: 10.1111/j.1651-2227.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 39.Zolfo M, De Weggheleire A, Schouten E, et al. Time for “test and treat” in prevention of mother-to-child transmission programs in low- and middle-income countries. J Acquir Immune Defic Syndr. 2010;55(3):287–9. doi: 10.1097/QAI.0b013e3181eef3da. [DOI] [PubMed] [Google Scholar]
- 40.Stringer JS, Sinkala M, Maclean CC, et al. Effectiveness of a city-wide program to prevent mother-to-child HIV transmission in Lusaka, Zambia. AIDS. 2005;19:1309–15. doi: 10.1097/01.aids.0000180102.88511.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reithinger R, Megazzini K, Durako SJ, et al. Monitoring and evaluation of programmes to prevent mother to child transmission of HIV in Africa. BMJ. 2007;334:1143–6. doi: 10.1136/bmj.39211.527488.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86:57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The United States President's Emergency Fund for AIDS Relief. Partnership to Fight HIV/AIDS in Mozambique. [March 28, 2010]; Available at: http://www.pepfar.gov/countries/mozambique/index.htm.
- 44.Potter D, Goldenberg RL, Chao A, et al. Do targeted HIV programs improve overall care for pregnant women?: Antenatal syphilis management in Zambia before and after implementation of prevention of mother-to-child HIV transmission programs. J Acquir Immune Defic Syndr. 2008;47:79–85. doi: 10.1097/QAI.0b013e31815d2f71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besser M, Sogaula N, Goheen M, et al. Improving uptake of early infant HIV diagnosis through simple interventions: lessons learned at mother2mother's innovation center, South Africa (THPE0286). Presented at: XVIII International AIDS Conference; Vienna. 2010. [Google Scholar]
- 46.Ahmed S, Kanjelo K, Nanthuru D, et al. Using community health workers to improve identification, enrollment into care, and outcomes for HIV-exposed infants at the Kawale Health Centre in Lilongwe, Malawi (WEPE0842). Presented at: XVIII International AIDS Conference; Vienna. 2010. [Google Scholar]
- 47.Antiretroviral drugs for treating pregnant women and prevent HIV infections in infants: recommendations for a public health approach -- 2010 version. [October 5, 2010];World Health Organization. HIV Program. 2010 Available at: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.