Abstract
Patients with schizophrenia exhibit poor working memory (WM). Although several subcomponents of WM can be measured, evidence suggests the primary subcomponent affected in schizophrenia is span capacity (WMC). Indeed, the NIMH-funded MATRICS initiative recommended assaying the WMC when assessing the efficacy of a putative therapeutic for FDA approval. Although dopamine D1 receptor agonists improve delay-dependent memory in animals, evidence for improvements in WMC due to dopamine D1 receptor activation is limited. In contrast, the dopamine D2-family agonist bromocriptine improves WMC in humans. The radial arm maze (RAM) can be used to assess WMC, although complications due to ceiling effects or strategy confounds have limited its use. We describe a 12-arm RAM protocol designed to assess whether the dopamine D1-family agonist SKF 38393 (0, 1, 3, and 10 mg/kg) or bromocriptine (0, 1, 3, and 10 mg/kg) could improve WMC in C57BL/6N mice (n=12) in cross-over designs. WMC increased and strategy usage decreased with training. The dopamine D1 agonist SKF 38393 had no effect on WMC or long-term memory. Bromocriptine decreased WMC errors, without affecting long-term memory, consistent with human studies. These data confirm that WMC can be measured in mice and reveal drug effects that are consistent with reported effects in humans. Future research is warranted to identify the subtype of the D2-family of receptors responsible for the observed improvement in WMC. Finally, this RAM procedure may prove useful in developing animal models of deficient WMC to further assess putative treatments for the cognitive deficits in schizophrenia.
Keywords: Working memory, span capacity, dopamine D1 receptor, dopamine D2 receptor, bromocriptine, mice
1.1. Introduction
Working memory (WM) is impaired in a number of neuropsychiatric disorders including schizophrenia. Deficient WM was identified by the National Institute of Mental Health (NIMH)-funded Measurement And Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative as a core cognitive deficit that requires treatment [1–4]. Since WM is a multi-component cognitive domain, considerable time has been spent assessing the different components of WM and creating a unified theory on the construct of WM [5–12]. The generally accepted theory of WM consists of a central executive which acts to coordinate a series of subsystems that includes the phonological loop, the visuospatial sketch pad [7], and the episodic buffer [13].
This complexity of the WM domain has complicated the search for procognitive treatments for disorders such as schizophrenia. The two WM tasks chosen by MATRICS to assess whether a putative therapeutic drug is efficacious for FDA approval provided measures of WM span capacity (WMC [14]). This choice is consistent with a recent analysis of patients with schizophrenia suggesting that the primary WM deficit is of impaired span capacity [15]. These MATRICS-chosen tasks assess the capacity of buffer systems for the number of items of information they can hold at one time, while not assessing the ability of the central executive to coordinate those systems. The WMC of humans is classically 7±2 items [16], while a reduced capacity is seen in patients with schizophrenia [15, 17] or subjects having a high risk for developing schizophrenia [18]. Hence, treatments need to be developed that improve WMC.
The dopamine D1 receptor was highlighted as one of the most promising targets for developing procognitive therapeutics to treat schizophrenia [19]. Much of the research that promotes this receptor as a viable target stems from assessments of D1 agonist effects on delay-dependent (DD) WM in rats and monkeys [20–24]. Given that DD WM and WMC are both pharmacologically and anatomically dissociable, there is little if any evidence that a D1 agonist might improve WMC. For example, DD memory tasks require and activate the hippocampus [25–37], a structure that is not required for non-DD memory tasks such as WMC tasks as evidenced by hippocampal lesions [25, 33, 37, 38] and functional magnetic resonance imaging studies in humans [33, 34, 39–42]. Also in humans, the muscarinic antagonist scopolamine impairs DD memory but not WMC [43–46], while the D2-family agonist bromocriptine specifically improves WMC [47–49]. In rodent studies, bromocriptine has failed to improve memory in normal rodents [50, 51] but has ameliorated some memory deficits produced by stress [51] or traumatic brain injury [52]. None of these studies used tasks that assessed the WMC however.
One confusion that has arisen when attempting to develop procognitive therapeutics for WM has been the discordant use of the term WM in man when compared to animals. When the term WM appears in animal studies, the task used often assesses short term memory for a single object, stimulus, or location consistent with DD WM [4]. Thus, there are few tasks with cross-species translational validity. The radial arm maze (RAM) has often been used in the past to assess spatial WMC, but most research has limited applicability to WM due to: 1) the inclusion of delays [4, 53, 54]; 2) ceiling effects (when in an 8-arm maze half of the arms are used to also assess reference memory (RM [55, 56]); or 3) testing occurring during learning, thus WMC is confounded by simple strategy use [54, 57].
We have developed an automated 12-arm RAM procedure for use in mice. This task utilizes only a 1-second delay between trials to counter simple strategy formation without introducing the delay as a challenge. Moreover, only two arms are used to assay RM while ten arms are used to assay WMC. Including ten WM arms avoids the confound of there being a limited number of items to be stored online, but also permits room for observing improvements in performance, as seen in human span tasks [47–49]. Thus, we hypothesized that we could assess WMC in mice in the RAM without being confounded by strategy use by including a 1-second delay. Moreover, consistent with rats and non-human primates, we hypothesized that the dopamine D1 receptor agonist SKF 38393 would not affect WMC or long term memory in mice due to the absence of a DD component of the task. Furthermore, we hypothesized that, consistent with humans, the D2 receptor agonist bromocriptine would improve WMC in mice, while not affecting long term memory [50].
2.1. Materials and Methods
2.1.1. Apparatus
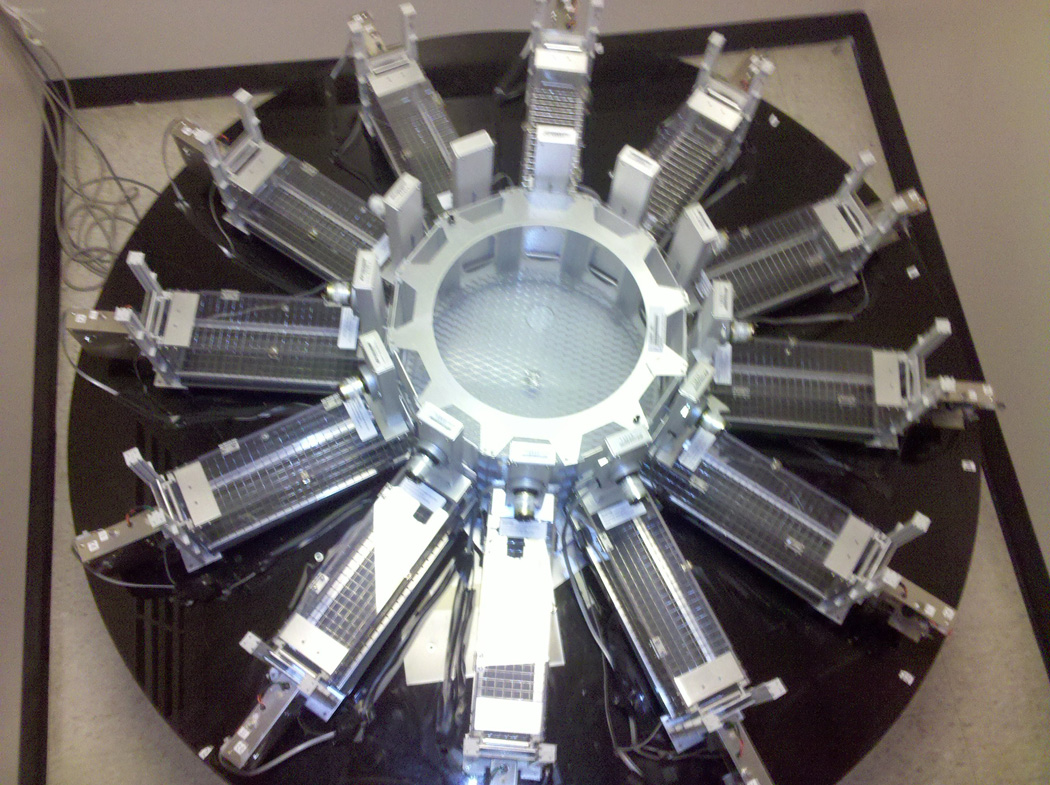
A fully automated 12-arm radial arm maze (RAM) was used in these experiments (Coulbourn Instruments, Whitehall, PA). This equipment was interfaced with Med Associates controlling software (St. Albans, VT). The RAM consisted of twelve arms radiating from a dodecahedral central hub (43 cm in diameter), with each arm (36 × 10 × 12 cm) separated by thirty degrees. Automated guillotine doors separated access from the central hub to each of the twelve arms. The walls and ceiling of each arm were made of acrylic glass while the floor consisted of a metal grid. Activity in each arm was monitored using an infrared beam 5.5 cm from the entrance. A dipper mechanism was located at the distal end of each arm. Each dipper provided liquid reinforcement via computer-controlled dipping into a liquid-filled bowl below the dipper. Head entry to the dipper mechanism was monitored using an infrared beam. The RAM was fixed to a circular acrylic table (124 cm in diameter) raised 53 cm from the floor. The table was painted black to avoid a putative confound of depth perception-induced anxiety [58]. Seven extra-maze spatial cues were created and fixed at conspicuous locations around the four walls. The room was lit by dim fluorescent lighting to create a low illumination at the RAM height (110 lux). A tracking camera was positioned above the center of the maze to track animal behavior. A constant background white noise (60 db A scale) was provided throughout training and testing to minimize distraction from external sounds.
2.1.2. Animals
Twelve C57Bl/6N male mice were obtained from Charles River Laboratories (Wilmington, MA). Training began at approximately 3 months of age, with mice weighing between 20–30 g. Animals were housed in groups of four per ventilated cage. Mice were maintained at 85% of free-feeding weight with water available ad libitum and housed in a vivarium on a reversed day-night cycle (lights on at 8.00 PM, off at 8.00 AM). Mice were brought to the laboratory 60 min before testing between 9.00 AM and 6.00 PM. All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care
2.2.1. Habituation to the Testing Environment
Liquid reinforcement in the form of strawberry milkshake was used to avoid satiety issues as well as drug-induced confounds in eating solid food reinforcement [59]. Acclimation to food reinforcement occurred prior to training in the home cage to avoid a neophobic response to the reinforcer during training. To acclimate the mice to the testing environment, they were placed in the RAM with all 12 doors opened and food dippers raised to provide reinforcement at the end of each arm. To encourage exploration of the RAM, strawberry milkshake was randomly placed throughout the apparatus. Habituation occurred for seven consecutive days with exposure time initially at fifteen minutes (days 1–3), then reduced to ten minutes (days 4 & 5), and finally to seven minutes (days 6 & 7).
2.2.2. RAM protocol
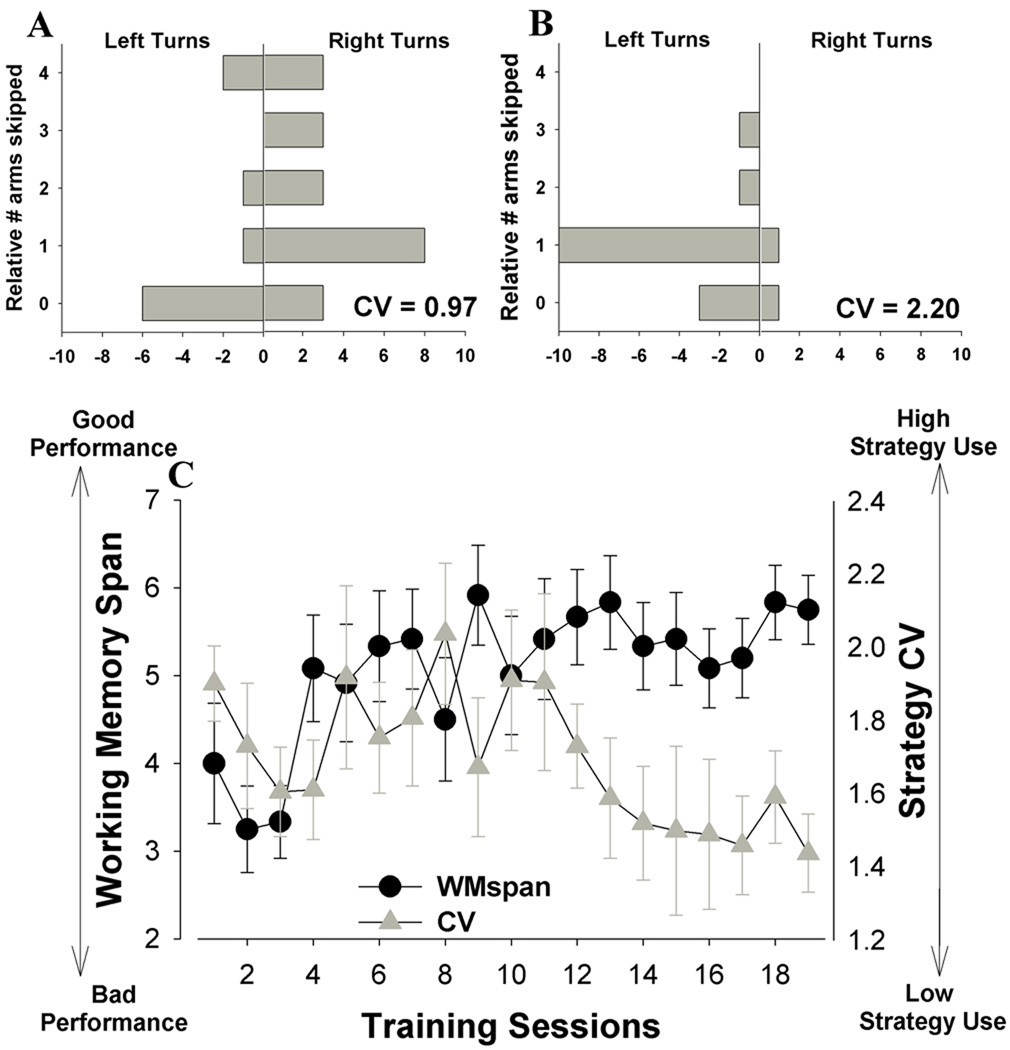
The protocol (Fig. 1) was designed to assay both working and reference memory. To assess WMC within a session, ten arms were allocated as WM arms which were only baited at the start of each session. The two remaining arms were allocated as RM arms which were never baited throughout training or testing. For each session, mice were placed in the hub with all doors closed. At the start of each session, all twelve doors were raised allowing entry into all 12 arms. Upon entering an arm, entry into the other 11 arms was barred by closing the doors. Upon retrieval of the food reward from the magazine, returning to the hub resulted in all 11 doors being raised after a 1 s delay. The 1 s delay was instituted to interfere with the development of simple turn left/right strategies [59]. The mouse was again able to enter any of the 12 arms. The WMC was measured as the number of WM arms entered prior to a repeat entry. For repeat entries into a WM arm (recorded as a WM error), a magazine head entry was not required to initiate the next arm to reopen. Likewise, for entries into RM arms (recorded as a RM error), magazine head entries were not required to reopen the remaining doors. Percentages were calculated to examine WM and RM errors as a function of total arm entries. The total number of WM errors were totaled and divided by the total number of WM arms entered (%WM errors). The total number of RM errors were totaled and divided by the total number of RM arms entered (%RM errors). The total number of WM and RM arms entered including repeat entries were also measured (Arms). To assess the development and use of a simple turn left/right strategy, we totaled each of all the possible relative transitions from one arm to another. The degree of strategy use was quantified by calculating the coefficient of variation (StrategyCV) of the totals for each subject. If the subject utilized a varied relative arm choice strategy (e.g. the mouse turned left and right evenly skipping 1, 2, 3, and 4 arms with little consistency in arm selection), the relative number of arms skipped would be evenly distributed resulting in a low StrategyCV score (Fig. 2A). If however, a predictable strategy was utilized, the distribution of the total number of arms skipped would be uneven (e.g. the mouse always turned left and skipped one arm), resulting in a high StrategyCV (Fig.2B).Sessions continued until each WM arm was entered or 7 minutes had elapsed, irrespective of the total WM or RM arms entered. Drug testing began once a stable level of performance had been reached by the mice as measured by WMC, WM, and RM errors. Stability of performance was assessed by comparing the performance at each measure over 4 consecutive days (Tuesday – Friday; one-way repeated measures analysis of variance) where a main effect of day was not observed for any measure.
Figure 1. 12-arm Radial Arm Maze.
The fully automated radial arm maze has 12 arms radiating from a central hub. Guillotine doors separate the arms and the hub. Arm entries and food reward area entries (located at the distal end of the arms) are monitored by infrared beams. Ten arms are designated working memory arms (baited once per session) and two arms are reference memory arms (never baited).
Figure 2. Spatial coefficient of variation (CV) and frequency distribution for low and high strategy use performers.
The spatial CV performances of C57BL/6N mice over time in the RAM were compared. Performance was measured by the relative transitions from one arm to another. If the subject employed a varied relative arm choice strategy (A), the total number of arms skipped was more evenly distributed resulting in a low StrategyCV score. If however, a predictable strategy is employed, the distribution of the total number of arms skipped was uneven (B) resulting in a high StrategyCV. Thus, the degree of strategy use was quantified by calculating the CV of the totals for each subject over a period of training sessions. During training, mice initially adopted a strategy and had a high StrategyCV score, their ordered strategy significantly declined with training however (C). As training progressed and the use of simple strategies declined, working memory span improved toward a stable performance (C). Data presented as mean ± s.e.m.
2.3.1. Experiment 1: Examination of the effects of bromocriptine (1, 3, and 10 mg/kg) on RAM performance in mice
The dopamine D2 receptor family agonist bromocriptine improves working memory WMC in humans and does not affect long term memory. To assess whether the current RAM paradigm would reproduce these effects in mice, we examined the effects of bromocriptine doses (0, 1, 3, 10 mg/kg) on performance in a within-subjects design.
2.3.2. Experiment 2: Examination of the effects of SKF 38395 (1, 3, and 10 mg/kg) on RAM performance in mice
The partial dopamine D1 receptor family agonist SKF 38393 improves DD WM in rodents and primates. To assess whether SKF 38393 could improve WMC, we examined the effects of SKF 38393 doses (0, 1, 3, and 10 mg/kg) on performance of mice in a within-subjects design.
2.4.1. Drugs
Prior to testing, mice were habituated to saline injections for 3 days. Bromocriptine (Sandoz Research Institute, East Hanover, NJ), an ergot-derived dopamine D2 receptor family agonist was diluted in a 2% Tween/98% water solution. Bromocriptine was administered intraperitoneally 20 minutes prior to the start of testing in a 10 ml/kg injection volume, with doses chosen based on earlier studies [60, 61]. SKF 38393 (A.G. Scientific, Institute, San Diego, CA), a partial dopamine D1 receptor family agonist was diluted in saline (0.9%) and administered intraperitoneally in a 10 ml/kg injection volume 10 min prior to testing, with doses based on previous studies [20, 62]. Drugs were administered in a cross-over design, with drug treatment on Tuesdays and Fridays, saline on Monday, Wednesday, and Thursday, with a 1-week washout period between drug challenges.
2.5.1. Statistical Analysis
Acquisition of the task was analyzed using a one-way repeated measures analysis of variance (ANOVA) with day as the within subject factor. During drug testing, the dependent measures were analyzed using a one-way repeated measures ANOVA with drug dose as the within subject factor. Specific measures were considered significant if a P-value of 0.05 and lower was obtained. Tukey post hoc analyses were performed on significant main effects.
3.1. Results
3.1.1. RAM acquisition
The mice readily improved performance in the RAM acquiring maximal levels of WMC within 9 days. This acquisition was evidenced as a main effect of day on WMC (F(18,198)=2.3, p<0.005; Fig. 2C). Furthermore, strategy CV decreased over time (F(18,198)=2.2, p<0.005; Fig. 2C), suggesting that the mice rejected the use of a simple strategy to perform the task. Thus, during training we observed a dissociation between WMC and strategy CV.
3.1.2. Experiment 1: The effects of bromocriptine on RAM performance
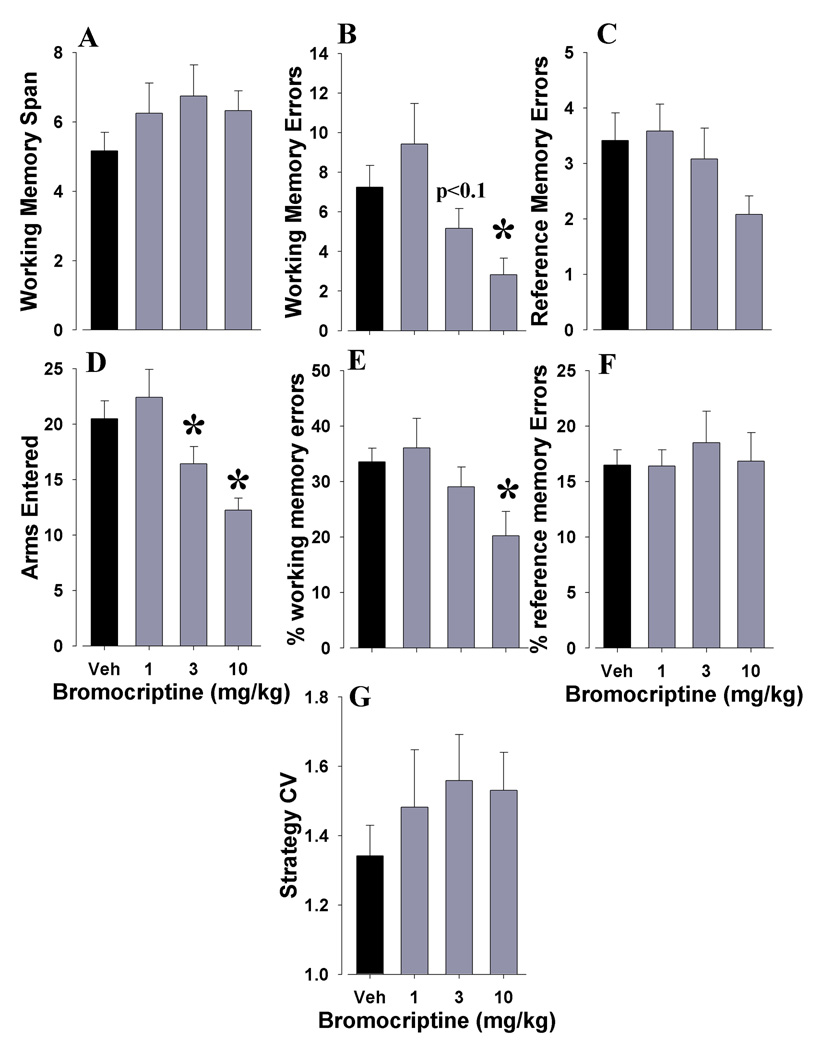
The mice were administered a dopamine D2-family agonist, bromocriptine. The protocol during testing remained the same as training to avoid possible learning confounds. Although bromocriptine administration appeared to increase WMC, this effect was not significant (F<1, ns; Fig. 3A). A significant reduction in WM span errors (F(3,33)=5.2, p<0.005; Fig. 3B) was observed however. Bromocriptine had no effect on RM errors (F(3,33)=2.2, ns; Fig. 3C). Bromocriptine administration reduced the total arms entered (F(3,33)= 7.6, p<0.001; Fig. 3D). A reduction in percentage of WM errors (F(3,33)=3.3, p<0.05; Fig. 3E) was observed, while the percentage of RM errors was unaffected (F<1, ns; Fig. 3F). Bromocriptine did not alter strategyCV (F(3,33)=1.2, ns; Fig. 3G).
Figure 3. Bromocriptine decreases working memory span errors without affecting long term memory.
The effects of bromocriptine administration on C57BL/6N mice performing the RAM were assessed. Bromocriptine appeared to increase working memory span capacity in mice, although this effect was not significant (A). Bromocriptine significantly reduced working memory span errors (B) however. This effect on working memory span errors was not evident across different forms of memory since reference memory errors were unaffected (C). Bromocriptine reduced the total number of arms entered (D). When measured as a percentage of total arms entered, bromocriptine still significantly reduced working memory span errors (E) with no concomitant effect on reference memory errors (F). Finally, bromocriptine did not alter the predictability of strategy use (I) suggesting that its effects on working memory span errors were not due to altered learning. Data presented as mean+s.e.m., and * denotes p<0.05 when compared to vehicle control.
3.1.3. Experiment 2: The effect of SKF 38393 on RAM performance
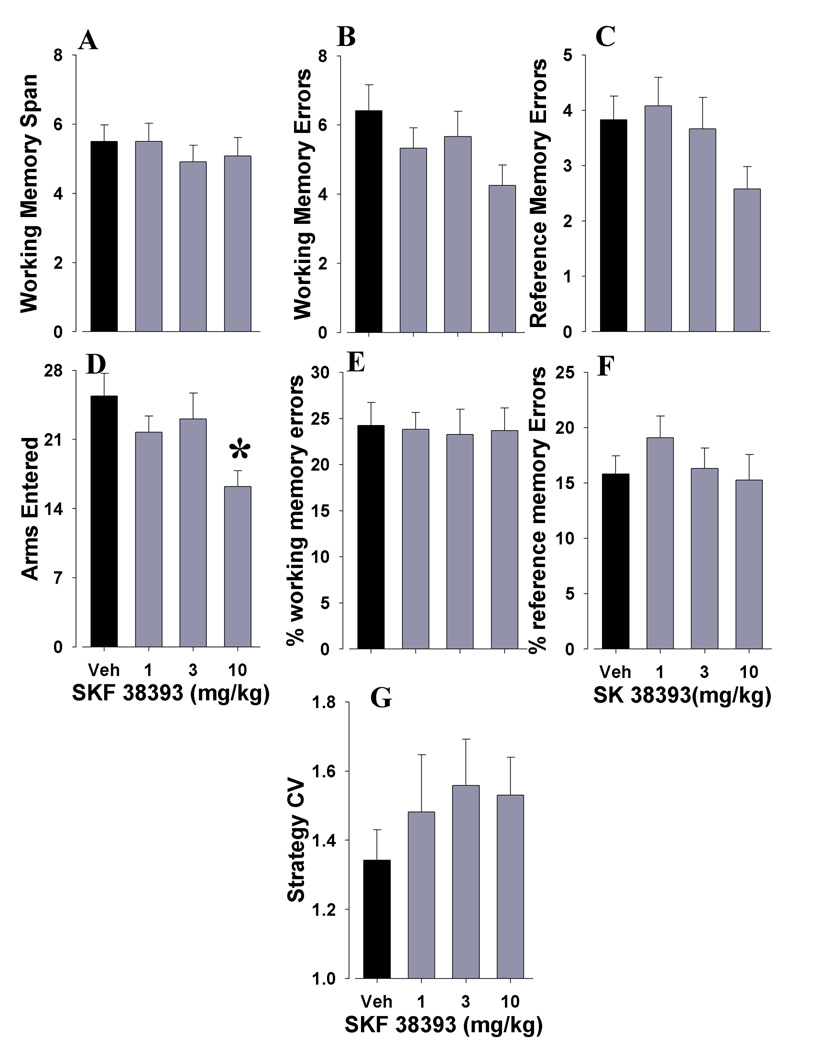
We examined the effects of the dopamine D1-family agonist SKF 38393 on WMC and RM in mice using the RAM. In mice performing the RAM, SKF 38393 did not affect WMC (F(3,33)<1, ns; Fig. 4A), WM span errors (F(3,33)=2.3, ns; Fig. 4B), or RM errors (F(3,33)=1.8, ns; Fig. 4C). A reduction in total arms entered (F(3,33)=5.7, p<0.003; Fig. 4D) was observed. SKF 38393 had no effect on percentage of WM errors (F<1, ns; Fig. 4E), or percentage of RM errors (F<1, ns; Fig. 4F). Finally, SKF 38393 had no effect on strategyCV (F<1, ns; Fig. 4G).
Figure 4. SKF 38393 did not affect working memory span capacity or long term memory.
The effects of SKF 38393 administration on C57BL/6N mice performing the RAM were assessed. SKF 38393 did not affect any aspect of working memory including working memory span capacity (A) and working memory errors (B) in mice. SKF 38393 did not affect reference memory errors (C) but did reduce the total number of arms entered (D) for the highest dose only. When measured as a percentage of total arms entered, SKF 38393 had no effect on working memory span errors (E) or on reference memory errors (F). Finally, SKF 38393 did not alter the predictability of strategy use (G) suggesting that its effect on total arms entered was not due to altered learning. Data presented as mean+s.e.m., and * denotes p<0.05 when compared to vehicle control.
4.1. Discussion
In the present studies, we assessed whether a dopamine D1 or D2 receptor agonist can improve WMC in mice as assessed in the RAM. The dopamine D2 receptor family agonist bromocriptine improved spatial WMC as measured by a reduction in errors while not affecting RM, an effect that is consistent with published reports in healthy humans. In contrast, the dopamine D1 receptor family agonist SKF 38393 affected neither spatial WMC nor RM. Thus, dopamine D1 receptor agonist SKF 38393 effects on WM may be limited to DD memory tasks.
Bromocriptine improved WMC in mice in the present studies, paralleling studies in humans [47, 48]. Previous assessment of bromocriptine effects in the RAM have been hampered by ceiling effects due to the use of only four WM arms [51] and by learning effects when drug administration occurred during training [63]. The present studies suggest however that when mice are trained to a stable level of performance in the task and performance is not at ceiling, a significant reduction in WMC errors can be observed after bromocriptine administration. This reduction in WMC errors was still apparent when the reductions in arm entries were taken into account. These data suggest that with bromocriptine, mice can maintain locations significantly better than with saline. Since bromocriptine did not affect long-term memory or strategy use, it appears that the 12-arm RAM protocol employed here can identify drugs that selectively affect WMC consistent with observations in humans. The dopamine D1 receptor family agonist SKF 38393 did not improve WMC however, despite improving DD WM performance in rats and non-human primates [20, 21, 23, 24]. This study provides further support for the pharmacological dissociation of DD and WMC performance [6, 13, 15, 64–66]. As described above, utilizing delays in a memory task leads to hippocampal mediation of performance as opposed to the frontal mediation of WMC tasks used in humans (reviewed in [25]). Thus, it is recognized that DD tasks do not assess WMC in a manner that is consistent with human tasks [4, 53, 54]. The NIMH-funded MATRICS initiative chose to assess WMC for assessing putative procognitive therapeutics to treat schizophrenia [14] because WMC deficits may be the primary WM deficit in patients with schizophrenia [15], these data highlight the need to examine D2-family agonists as a potential therapeutic target for schizophrenia. The practical utility of a non-selective D2 agonist for schizophrenia patients treated with antipsychotics (D2 receptor antagonists) would be limited, thus focus should be placed on identifying the dopamine receptor subtype that mediates these effects. The fact that these data were generated using C57BL/6 mice would also suggest that while examining the receptor(s) that mediate(s) the effects of bromocriptine in normal animals, D2-family agonists should also be tested in animal models of schizophrenia using this RAM protocol.
The dopamine D2 family agonist bromocriptine acts primarily by stimulating the postsynaptic dopamine D2 and D3 receptors with lower affinity for dopamine D4 receptors [67–69]. Some reports suggest bromocriptine may exhibit greater selectivity for the dopamine D3 receptors [70]. Pramipexole is more selective for dopamine D3 receptors and, consistent with bromocriptine, the selective D3 agonist may improve the WM of poor performers while not affecting good performers [71]. The differential selectivities of these drugs for D2 and D3 receptors are limited however. The use of dopamine D3 receptor knockout mice in this task ± bromocriptine may elucidate the role of the D3 receptor in the improvement of WM more clearly. The cognitive performance of dopamine D3 receptor KO mice has not been studied extensively but reports suggest that these mice perform better than wildtype controls in some memory and learning tasks [72, 73], although delay-dependent memory may be adversely affected [74]. The effects of drugs on behavior in these mice have been limited to locomotor activities [75, 76] and prepulse inhibition [77, 78] however and should be examined in terms of WMC.
Here we introduce a novel measurement of strategy use in the RAM – StrategyCV. StrategyCV provides a valuable insight into the strategy use by mice in the 12-arm RAM. This measure assesses the use of a simple turn left/right strategy in the RAM by totaling each of all the possible transitions from one arm to another. If the subject employed a predictable strategy, the distribution of the transitions would be uneven. If however, a varied arm choice strategy was employed, the distributions would be more evenly distributed. Mice readily achieved maximal performance in the 12-arm RAM with training, while their use of strategies diminished. Once fully trained, strategy scores and WMC performance remained consistent per individual. This measure was based on the spatial CV measure used to assess the predictability of patterns of movements of animals in the Behavioral Pattern Monitor (BPM) [79–81]. Spatial CV in the BPM was found to be modulated by differing neurotransmitter systems [79]. The data presented here suggest that as mice learn to perform in the RAM, their use of a predictable strategy diminishes over time as WMC improves. This pattern is in contrast with the BPM where a rat’s movement becomes more predictable over time when the animal is not confined to small areas [80]. Consequently, by rejecting such a strategy in the RAM, the overall performance of mice improved and became more stable. Importantly, the StrategyCV enabled us to examine the possible contribution of altered strategies as a potential confound on the effects of drug manipulations. In the present studies, neither bromocriptine nor SKF 38393 administration altered the predictability of strategy use on mice in the RAM.
4.2. Conclusion
The data presented here indicate that WMC can be measured by a procedure that can dissociate between dopamine D1 and D2 receptor family agonists in the RAM. Importantly, the D2 family agonist bromocriptine improved WMC in mice in a manner consistent with human WMC tasks. Thus, the current paradigm could provide a platform by which the neurobiological mechanisms of WMC and experimental procognitive therapeutics can be assessed. Moreover, given that bromocriptine improved WMC, future research should be designed to identify the subtype of the D2-family of receptors responsible for the observed improvement because the practical utility of a non-selective D2 agonist for schizophrenia patients treated with antipsychotics (D2 receptor antagonists) would be limited. To this end assessing the effects of co-administration of bromocriptine with antipsychotics in animal model of schizophrenia would prove useful. This research is possible given the availability of dopamine D3 receptor mutant mice and the ability to assess WMC in mice using the current procedure. Finally, the addition of the StrategyCV measure may provide a useful means to evaluate whether future drug-induced alterations of behavior affect WMC specifically or also alters strategy use.
Research Highlights from assessing working memory span capacity in mice
Important to identify the subcomponent of working memory that is assessed
Working memory span capacity (WMC) is specifically impaired in patients with schizophrenia
Consistent with humans, the dopamine D2-family agonist bromocriptine improved WMC in mice
The dopamine D1-family agonist SKF 38393 did not improve WMC in mice
The drug effects were not confounded by changes in strategy as measured by StrategyCV
Acknowledgements
We thank Drs. Victoria Risbrough and Susan Powell, as well as Mahálah Buell for their support. This study was supported by NIH grants R21-MH085221, R21-MH091571, and R01-MH071916, and the Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kern RS, Green MF, Nuechterlein KH, Deng BH. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophrenia research. 2004;72:11–19. doi: 10.1016/j.schres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. The American journal of psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 3.Webber MA, Marder SR. Better pharmacotherapy for schizophrenia: what does the future hold? Current psychiatry reports. 2008;10:352–358. doi: 10.1007/s11920-008-0056-8. [DOI] [PubMed] [Google Scholar]
- 4.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neuroscience and biobehavioral reviews. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JR, Reder LM, Lebiere C. Working memory: activation limitations on retrieval. Cogn Psychol. 1996;30:221–256. doi: 10.1006/cogp.1996.0007. [DOI] [PubMed] [Google Scholar]
- 6.Baddeley A. The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baddeley AD. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- 8.Conway AR, Cowan N, Bunting MF. The cocktail party phenomenon revisited: the importance of working memory capacity. Psychon Bull Rev. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson KA, Kintsch W. Long-term working memory. Psychological review. 1995;102:211–245. doi: 10.1037/0033-295x.102.2.211. [DOI] [PubMed] [Google Scholar]
- 10.Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychological review. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- 11.Norman DA, Wickelgren WA. Short-term recognition memory for single digits and pairs of digits. J Exp Psychol. 1965;70:479–489. doi: 10.1037/h0022544. [DOI] [PubMed] [Google Scholar]
- 12.Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion -85. [DOI] [PubMed] [Google Scholar]
- 13.Baddeley A. The concept of episodic memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1345–1350. doi: 10.1098/rstb.2001.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophrenia bulletin. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- 15.Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, et al. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Archives of general psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Reviews. 1956;63:81–97. [PubMed] [Google Scholar]
- 17.Stone M, Gabrieli JD, Stebbins GT, Sullivan EV. Working and strategic memory deficits in schizophrenia. Neuropsychology. 1998;12:278–288. doi: 10.1037//0894-4105.12.2.278. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor M, Harris JM, McIntosh AM, Owens DG, Lawrie SM, Johnstone EC. Specific cognitive deficits in a group at genetic high risk of schizophrenia. Psychological medicine. 2009;39:1649–1655. doi: 10.1017/S0033291709005303. [DOI] [PubMed] [Google Scholar]
- 19.Tamminga CA. Accelerating new knowledge in schizophrenia. The American journal of psychiatry. 2008;165:949–951. doi: 10.1176/appi.ajp.2008.08060815. [DOI] [PubMed] [Google Scholar]
- 20.Rios Valentim SJ, Jr, Gontijo AV, Peres MD, Rodrigues LC, Nakamura-Palacios EM. D1 dopamine and NMDA receptors interactions in the medial prefrontal cortex: modulation of spatial working memory in rats. Behav Brain Res. 2009;204:124–128. doi: 10.1016/j.bbr.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 22.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 23.Amico F, Spowart-Manning L, Anwyl R, Rowan MJ. Performance- and task-dependent effects of the dopamine D1/D5 receptor agonist SKF 38393 on learning and memory in the rat. Eur J Pharmacol. 2007;577:71–77. doi: 10.1016/j.ejphar.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- 26.Carrozzo M, Koch G, Turriziani P, Caltagirone C, Carlesimo GA, Lacquaniti F. Integration of cognitive allocentric information in visuospatial short-term memory through the hippocampus. Hippocampus. 2005;15:1072–1084. doi: 10.1002/hipo.20126. [DOI] [PubMed] [Google Scholar]
- 27.Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behavioral neuroscience. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behavioral neuroscience. 1986;100:155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
- 29.Zola SM, Squire LR. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus. 2001;11:92–98. doi: 10.1002/hipo.1027. [DOI] [PubMed] [Google Scholar]
- 30.Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Clark CR, Moores KA, Lewis A, Weber DL, Fitzgibbon S, Greenblatt R, et al. Cortical network dynamics during verbal working memory function. Int J Psychophysiol. 2001;42:161–176. doi: 10.1016/s0167-8760(01)00164-7. [DOI] [PubMed] [Google Scholar]
- 32.Friedman HR, Goldman-Rakic PS. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8:4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy DA, Hopkins RO, Squire LR. Impaired odor recognition memory in patients with hippocampal lesions. Learning & memory (Cold Spring Harbor, NY. 2004;11:794–796. doi: 10.1101/lm.82504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cereb Cortex. 2005;15:303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
- 35.Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14:808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- 36.Schon K, Quiroz YT, Hasselmo ME, Stern CE. Greater working memory load results in greater medial temporal activity at retrieval. Cereb Cortex. 2009;19:2561–2571. doi: 10.1093/cercor/bhp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- 38.Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minamoto T, Osaka M, Osaka N. Individual differences in working memory capacity and distractor processing: possible contribution of top-down inhibitory control. Brain Res. 2010;1335:63–73. doi: 10.1016/j.brainres.2010.03.088. [DOI] [PubMed] [Google Scholar]
- 40.Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, et al. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbs SE, D'Esposito M. A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience. 2006;139:359–371. doi: 10.1016/j.neuroscience.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 42.Osaka M, Osaka N, Kondo H, Morishita M, Fukuyama H, Aso T, et al. The neural basis of individual differences in working memory capacity: an fMRI study. NeuroImage. 2003;18:789–797. doi: 10.1016/s1053-8119(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 43.Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, et al. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology. 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- 44.Mintzer MZ, Griffiths RR. Differential effects of scopolamine and lorazepam on working memory maintenance versus manipulation processes. Cogn Affect Behav Neurosci. 2007;7:120–129. doi: 10.3758/cabn.7.2.120. [DOI] [PubMed] [Google Scholar]
- 45.Rusted JM, Warburton DM. The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology. 1988;96:145–152. doi: 10.1007/BF00177553. [DOI] [PubMed] [Google Scholar]
- 46.Thienel R, Kellermann T, Schall U, Voss B, Reske M, Halfter S, et al. Muscarinic antagonist effects on executive control of attention. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2009;12:1307–1317. doi: 10.1017/S146114570999068X. [DOI] [PubMed] [Google Scholar]
- 47.Kimberg DY, D'Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 48.Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology. 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- 49.Imamura T, Takanashi M, Hattori N, Fujimori M, Yamashita H, Ishii K, et al. Bromocriptine treatment for perseveration in demented patients. Alzheimer Dis Assoc Disord. 1998;12:109–113. doi: 10.1097/00002093-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Marighetto A, Valerio S, Philippin JN, Bertaina-Anglade V, Drieu la Rochelle C, Jaffard R, et al. Comparative effects of the dopaminergic agonists piribedil and bromocriptine in three different memory paradigms in rodents. Journal of psychopharmacology (Oxford, England) 2008;22:511–521. doi: 10.1177/0269881107083836. [DOI] [PubMed] [Google Scholar]
- 51.Srikumar BN, Raju TR, Shankaranarayana Rao BS. Contrasting effects of bromocriptine on learning of a partially baited radial arm maze task in the presence and absence of restraint stress. Psychopharmacology. 2007;193:363–374. doi: 10.1007/s00213-007-0801-4. [DOI] [PubMed] [Google Scholar]
- 52.Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 53.D'Mello GD, Steckler T. Animal models in cognitive behavioural pharmacology: an overview. Brain research. 1996;3:345–352. doi: 10.1016/0926-6410(96)00027-4. [DOI] [PubMed] [Google Scholar]
- 54.Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacology & therapeutics. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joel D, Tarrasch R, Feldon J, Weiner I. Effects of electrolytic lesions of the medial prefrontal cortex or its subfields on 4-arm baited, 8-arm radial maze, two-way active avoidance and conditioned fear tasks in the rat. Brain Res. 1997;765:37–50. doi: 10.1016/s0006-8993(97)00334-x. [DOI] [PubMed] [Google Scholar]
- 56.Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- 57.Ammassari-Teule M, Save E, de Marsanich B, Thinus-Blanc C. Posterior parietal cortex lesions severely disrupt spatial learning in DBA mice characterized by a genetic hippocampal dysfunction. Behav Brain Res. 1998;95:85–90. doi: 10.1016/s0166-4328(97)00213-1. [DOI] [PubMed] [Google Scholar]
- 58.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenk GL. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8):5A. doi: 10.1002/0471142301.ns0805as26. [DOI] [PubMed] [Google Scholar]
- 60.Boyd A. Bromocriptine and psychosis: a literature review. The Psychiatric quarterly. 1995;66:87–95. doi: 10.1007/BF02238717. [DOI] [PubMed] [Google Scholar]
- 61.Platel A, Porsolt RD. Habituation of exploratory activity in mice: a screening test for memory enhancing drugs. Psychopharmacology. 1982;78:346–352. doi: 10.1007/BF00433739. [DOI] [PubMed] [Google Scholar]
- 62.Levin ED, Rose JE. Acute and chronic nicotinic interactions with dopamine systems and working memory performance. Annals of the New York Academy of Sciences. 1995;757:245–252. doi: 10.1111/j.1749-6632.1995.tb17481.x. [DOI] [PubMed] [Google Scholar]
- 63.Reinholz J, Skopp O, Breitenstein C, Winterhoff H, Knecht S. Better than normal: improved formation of long-term spatial memory in healthy rats treated with levodopa. Experimental brain research Experimentelle Hirnforschung. 2009;192:745–749. doi: 10.1007/s00221-008-1654-8. [DOI] [PubMed] [Google Scholar]
- 64.Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychon Bull Rev. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- 65.Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. J Exp Psychol Gen. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- 66.Perry W, Heaton RK, Potterat E, Roebuck T, Minassian A, Braff DL. Working memory in schizophrenia: transient "online" storage versus executive functioning. Schizophrenia bulletin. 2001;27:157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- 67.Kvernmo T, Houben J, Sylte I. Receptor-binding and pharmacokinetic properties of dopaminergic agonists. Current topics in medicinal chemistry. 2008;8:1049–1067. doi: 10.2174/156802608785161457. [DOI] [PubMed] [Google Scholar]
- 68.Newman-Tancredi A, Audinot V, Chaput C, Verriele L, Millan MJ. [35S]Guanosine-5'-O-(3-thio)triphosphate binding as a measure of efficacy at human recombinant dopamine D4.4 receptors: actions of antiparkinsonian and antipsychotic agents. The Journal of pharmacology and experimental therapeutics. 1997;282:181–191. [PubMed] [Google Scholar]
- 69.Seeman P, Van Tol HH. Dopamine receptor pharmacology. Curr Opin Neurol Neurosurg. 1993;6:602–608. [PubMed] [Google Scholar]
- 70.Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, et al. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. The Journal of pharmacology and experimental therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costa A, Peppe A, Dell'Agnello G, Caltagirone C, Carlesimo GA. Dopamine and cognitive functioning in de novo subjects with Parkinson's disease: effects of pramipexole and pergolide on working memory. Neuropsychologia. 2009;47:1374–1381. doi: 10.1016/j.neuropsychologia.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 72.Xing B, Meng X, Wei S, Li S. Influence of dopamine D3 receptor knockout on age-related decline of spatial memory. Neuroscience letters. 2010;481:149–153. doi: 10.1016/j.neulet.2010.06.071. [DOI] [PubMed] [Google Scholar]
- 73.Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Di Marzo V, et al. Enhanced cognitive performance of dopamine D3 receptor "knock-out" mice in the step-through passive-avoidance test: assessing the role of the endocannabinoid/endovanilloid systems. Pharmacol Res. 2010;61:531–536. doi: 10.1016/j.phrs.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D(1), D(2), and D(3) receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- 76.McNamara RK, Logue A, Stanford K, Xu M, Zhang J, Richtand NM. Dose-response analysis of locomotor activity and stereotypy in dopamine D3 receptor mutant mice following acute amphetamine. Synapse (New York, NY. 2006;60:399–405. doi: 10.1002/syn.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, et al. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–2656. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- 78.Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geyer MA, Paulus MP. Multivariate and nonlinear approaches to characterizing drug effects on the locomotor and investigatory behavior of rats. NIDA research monograph. 1992;124:203–235. [PubMed] [Google Scholar]
- 80.Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacology, biochemistry, and behavior. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- 81.Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology. 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]