SYNOPSIS
The adrenal cortices produce a variety of steroid hormones (corticosteroids) that play vital roles in a number of physiologic processes, including: electrolyte and fluid balance; cardiovascular homeostasis; carbohydrate, protein and lipid metabolism; immune and inflammatory responses; and sexual development and reproductive function. While permanent adrenocortical insufficiency is rare in all species, emerging evidence in both human and equine medicine suggests that transient, reversible adrenocortical dysfunction resulting in cortisol insufficiency frequently develops during critical illness. This syndrome is termed relative adrenal insufficiency (RAI) or critical illness-related corticosteroid insufficiency (CIRCI), and can contribute substantially to morbidity and mortality associated with the primary disease. Thus, this review will primarily cover the mechanisms, diagnosis and clinical consequences of adrenocortical insufficiency, with particular focus on our current understanding of RAI/CIRCI in horses and foals.
Keywords: Adrenal gland, adrenal insufficiency, CIRCI, cortisol, hypoadrenocorticism
The adrenal cortices produce a variety of steroid hormones (corticosteroids), including mineralocorticoids (e.g., aldosterone), glucocorticoids (e.g., cortisol), and adrenal androgens (e.g., dehydroepiandrosterone, DHEA). These corticosteroids play vital roles in a number of physiologic processes, including: electrolyte and fluid balance; cardiovascular homeostasis; carbohydrate, protein and lipid metabolism; immune and inflammatory responses; and sexual development and reproductive function. Like other endocrine organs, adrenocortical dysfunction may manifest as either abnormal increases or decreases in activity. Increased adrenocortical activity (hyperadrenocorticism) may occur in horses with Pituitary Pars Intermedia Dysfunction, but primary hyperadrenocorticism and permanent adrenocortical insufficiency (hypoadrenocorticism, Addison's Disease) are rare in horses. However, emerging evidence in both human and equine medicine suggests that transient, reversible adrenocortical dysfunction resulting in cortisol insufficiency frequently develops during critical illness. This syndrome is termed relative adrenal insufficiency (RAI) or critical illness-related corticosteroid insufficiency (CIRCI), and can contribute substantially to morbidity and mortality associated with the primary disease. Thus, this review will primarily cover the mechanisms, diagnosis and clinical consequences of adrenocortical insufficiency, with particular focus on our current understanding of RAI/CIRCI in horses and foals.
I. ADRENOCORTICAL ANATOMY AND PHYSIOLOGY
The adrenal glands are located at the craniomedial aspect of each kidney, and each gland is divided into an outer cortex that secretes corticosteroids and an inner medulla that secretes catecholamines. The adrenal cortices are divided into 3 cellular zones: 1) the outer zona glomerulosa; 2) the middle zona fasciculata, which is the largest zone and comprises 75% of the weight of the entire adrenal gland; and 3) the narrow inner zona reticularis.1 Cells in the zona glomerulosa are primarily responsible for the secretion of mineralocorticoids (e.g., aldosterone), while zona fasciculata cells synthesize and secrete glucocorticoids (e.g., cortisol). Cells in the zona reticularis also secrete small amounts of glucocorticoids, but predominantly produce adrenal androgens such as DHEA and androstenedione.
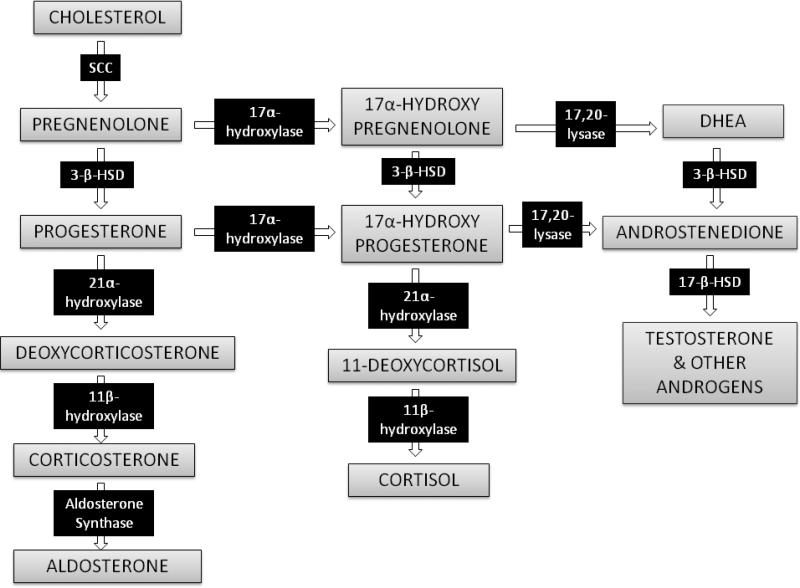
All corticosteroid hormones are structurally similar and share a common synthetic pathway (Figure 1). All steroid hormones are synthesized from cholesterol, and contain a common sterol backbone with three 6-carbon and one 5-carbon rings. Steroid hormone synthesis occurs in the mitochondria and endoplasmic reticulum, and begins with mitochondrial uptake of cytoplasmic cholesterol via the steroidogenic acute regulatory (StAR) transporter protein.3 Binding of a regulatory hormone to adrenocortical cell surface receptors stimulates cyclic AMP-mediated uptake of circulating plasma lipoproteins and liberation of cholesterol via lysosomal lipases to provide the major source of such cholesterol for steroid hormone synthesis.1 De novo cholesterol synthesis also occurs in the adrenal cortex and can provide an alternative source of cytoplasmic cholesterol for corticosteroid synthesis.1
Figure 1.
Biosynthetic pathway for adrenal corticosteroids. The enzymes responsible for catalyzing each biotransformation are shown in the black boxes over the arrows. SCC = side chain cleavage enzyme. 3-β-HSD = 3-β-hydroxysteroid dehydrogenase. DHEA = dehydroepiandrosterone.
Once cholesterol is taken up into the mitochondrion via StAR, it is converted to pregnenolone via side chain cleavage enzyme (p450scc, cholesterol desmolase). Both cholesterol delivery to the mitochondria via StAR and p450scc-catalyzed pregnenolone synthesis have been proposed as the rate limiting step in corticosteroid biosynthesis.4, 5 All other steroid hormones are derivatives of pregnenolone; which specific steroids are produced by a particular adrenocortical cell depends on which biosynthetic enzymes are expressed in that cell (Figure 1).1, 6
Glucocorticoids: Synthesis, Secretion and Systemic Effects
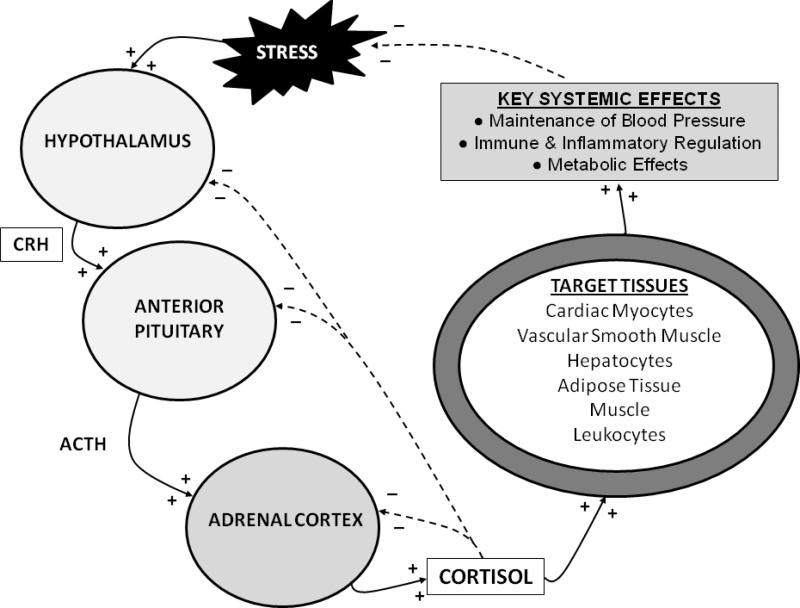
Glucocorticoids are the predominant adrenal corticosteroid and the most prevalent circulating steroid hormone. Their synthesis is regulated by the hypothalamic-pituitary-adrenal (HPA) axis (Figure 2), and they play an integral role in the endocrine response to stress. The HPA axis is activated when physiologic, pathophysiologic, or environmental stressors activate peripheral and central nervous system components, whose signals are then interpreted and integrated in the hypothalamus. Activation of the hypothalamic paraventricular nuclei culminates in the release of the peptide hormone corticotropin-releasing hormone (CRH) into the hypothalamic-hypophyseal portal vessels. CRH then acts locally in the adjacent anterior pituitary gland to activate type 1 CRH-receptors on the cell surface of pituitary corticotroph cells to induce the release of adrenocorticotropic hormone (ACTH, corticotrophin) into the systemic circulation.7
Figure 2.
The hypothalamic-pituitary-adrenal (HPA) axis. Stimulatory interactions are illustrated with solid arrows and + signs, and inhibitory interactions (negative feedback) are illustrated with dashed arrows and – signs. CRH = corticotropic releasing hormone. ACTH = adrenocorticotropic hormone.
ACTH binds cell surface receptors (melanocortin 2 receptor, MC2R) on adrenocortical cells and stimulates the adrenal glands to primarily synthesize and secrete cortisol, but to a less extent also aldosterone. Five ACTH receptor types have been characterized in humans, the majority of which bind both ACTH and α- and γ-melanocyte stimulating hormone (α- and γ-MSH).8 MC2R is the only ACTH receptor type that is expressed in the human adrenal cortex, and the only type that does not bind MSH.8 MC2R is a G protein-coupled transmembrane receptor that acts via adenylate cyclase to increase cyclic AMP levels, which then activate a variety of enzymes critical for cortisol synthesis.8 At present, melanocortin receptor subtype expression in the equine adrenal cortex has not been characterized, but is presumed to be similar to that in humans.
The critical enzymes necessary for cortisol synthesis are expressed in zona fasciculata cells, and include 3-β-hydroxysteroid dehydrogenase (3-β-HSD), 17-α-hydroxylase, 21-α-hydroxylase, and 11-β-hydroxylase (Figure 1).3 This latter enzyme catalyzes the final step in cortisol synthesis from the 11-deoxycortisol precursor molecule, and is present only in glucocorticoid-producing cells.3
Cortisol is not stored in adrenocortical cells, but rather is secreted into the systemic circulation immediately following ACTH-induced synthesis.5 Like all steroid hormones, cortisol is lipophilic and thus is transported in the plasma predominantly bound to plasma proteins including cortisol-binding globulin (CBG) and albumin.9 In most adult mammals, including horses, approximately 90% of circulating cortisol is bound.6, 9-12 As cortisol receptors are located in the cytoplasm of steroid-responsive cells, though, it is only the free, unbound portion of circulating cortisol that is available to enter cells via diffusion across the plasma membrane and to bind these intracellular glucocorticoid receptors (GRs).13
Binding of cortisol to the cytoplasmic GR causes conformational changes that allow dissociation of regulatory heat shock proteins (HSPs), permitting the cortisol-GR complex to dimerize, localize to the nucleus, bind DNA at glucocorticoid-response-elements (GREs), and regulate transcription of glucocorticoid-responsive genes.1 In humans, there are two GR isoforms, GRα and GRβ, but GRβ appears to be transcriptionally inactive and may function as an endogenous inhibitor of GRα activity.13 To the authors’ knowledge, equine GR isoforms and their respective activities are not well characterized.
Many cell types are sensitive to glucocorticoids, permitting cortisol to exert diverse effects necessary for stress responses to both health and disease. Essential glucocorticoid-mediated physiologic responses include maintenance of blood pressure, provision of energy to tissues, and control of an appropriate inflammatory response.1, 5, 14 The sum of cortisol's systemic effects serves to reduce the physiologic stressors that initially activated the HPA axis to ultimately reduce HPA axis tone. In addition, cortisol itself acts via negative feedback mechanisms at hypothalamic, pituitary and adrenal levels to further down-regulate HPA axis activity. Thus, with an intact HPA axis, plasma cortisol concentrations are maintained at a level appropriate for the existing degree of physiologic stress.
Mineralocorticoids: Synthesis, Secretion and Systemic Effects
Mineralocorticoids (e.g. aldosterone) are produced in small quantities relative to glucocorticoids, but have equally important physiologic roles. Angiotensin II and plasma potassium are the primary stimulants of aldosterone synthesis and secretion, but acute release of ACTH also induce aldosterone synthesis to a lesser degree. The renin-angiotensin-aldosterone system is activated by hypovolemia/hypotension or increases in plasma osmolality, and culminates in adrenocortical aldosterone secretion. Alternatively, or in conjunction with the above mechanisms, increased serum potassium concentration also stimulates aldosterone secretion. Both angiotensin II and potassium increase transcription of the aldosterone synthase gene in zona glomerulosa cells via calcium-mediated activation of cAMP response elements – angiotensin II by receptor-mediated activation of phospholipase C and potassium via opening of voltage-gated calcium channels.5
Like glucocorticoids, aldosterone circulates in the plasma bound to albumin or CBG, and primarily acts by binding cytosolic mineralocorticoid receptors (MRs) and altering transcription of genes necessary for sodium and potassium transport.15 MRs are predominantly expressed in sodium-transporting epithelia in the distal renal tubules and colon, with lesser expression in the rest of the intestinal tract and the heart.5 Aldosterone induces transcription of an aldosterone-regulated kinase that increases activity of apical membrane sodium channels.15 The net effect of this is increased sodium flux across epithelial cells, resulting in increased renal and intestinal sodium and water resorption and concurrent stimulation of potassium excretion via the basolateral Na+/K+ ATP-ase.15 Mineralocorticoids are vital for appropriate fluid and electrolyte balance, and mineralocorticoid deficiency rapidly results in hyponatremia, hyperkalemia, and hypovolemia.
Adrenal androgens: Synthesis, Secretion and Systemic Effects
In adult males, circulating androgens are primarily of testicular origin, but in females more than half of plasma androgens may originate from the adrenal cortices. ACTH is necessary for the synthesis and secretion of adrenal androgens, but another factor (or factors) that is as-yet-unidentified is also required for adrenal androgen synthesis.5 Like other adrenal steroids, adrenal androgens are transported in the plasma bound to albumin and CBG, and act via cytosolic steroid hormone receptors to modulate gene transcription. Adrenal androgens – such as DHEA and androstendione – can be extraglandularly metabolized to active testosterone and estrogens, and play a particular important role in early pubertal sexual development in most species. Increased adrenal androgen production is postulated to play a role in mares with estrus-related behavior problems, though this has not been definitively proven.16
II. METHODS FOR DIAGNOSIS OF ADRENOCORTICAL INSUFFICIENCY
Testing methods used to diagnose adrenocortical insufficiency in both human and veterinary medicine fall into two general categories: 1) assessment of endogenous basal adrenocortical activity by measurement of circulating or excreted hormone concentrations; and 2) dynamic assessment of adrenocortical responsiveness by assessment of hormone production following administration of exogenous regulatory hormones.17
In general, measurement of basal plasma hormone concentrations offers a rapid and safe means of assessing adrenocortical function. Well-established commercial radio- or chemiluminescent immunoassays are readily available for measurement of equine cortisol and can provide results rapidly.6, 17 Commercial immunoassays are also available for most other adrenal corticosteroids, and measurement of aldosterone and adrenal androgens has been described in horses.18-20 However, cortisol and other adrenal steroids are typically secreted in a pulsatile fashion and plasma concentrations can vary widely during a 24-hour period in both healthy and sick individuals with time of day, season, emotional state, and moment-to-moment changes in physiologic stressors.5, 17 Thus, documentation of a single random low basal concentration of any steroid hormone should be interpreted with caution as true steroid deficiency is not always indicated.
Assessment of cortisol concentrations in conjunction with regulatory hormone (ACTH, CRH) concentrations can provide a more comprehensive picture of HPA axis function. Plasma CRH and ACTH concentrations are easily assessed in most species via commercially available immunoassays, but as the majority of CRH is secreted into the hypothalamic-hypophyseal portal vessels rather than into the systemic circulation, accurate measurement of CRH requires sophisticated sampling methodology. In addition, while in healthy individuals ACTH and cortisol concentrations are fairly closely correlated, ACTH-cortisol dissociation is not uncommon during illness.21
Dynamic tests are designed to circumvent some of these limitations. The most commonly used dynamic test to diagnose cortisol insufficiency is the ACTH stimulation test, during which the cortisol response to exogenous synthetic ACTH (usually ACTH1-24, cosyntropin) is measured. Typically, in the classic “high-dose” ACTH stimulation test, serum cortisol concentration is measured just prior to and 30-90 minutes after intravenous or intramuscular administration of a supraphysiologic quantity of ACTH (1.0-2.0 μg/kg, approximately 100 – 250 μg in people and foals). This high-dose ACTH stimulation test produces a maximal adrenal response, so an inadequate increase in cortisol concentration in this test is used to diagnose both absolute, irreversible adrenal insufficiency (e.g., Addison's disease) and transient RAI/CIRCI.5, 22-24
However, the high-dose ACTH stimulation test may be less sensitive for diagnosis of the transient, reversible HPA axis suppression that occurs in RAI/CIRCI. In some patients with RAI/CIRCI, the adrenal gland fails to produce an appropriate cortisol response to physiologic concentrations of ACTH but may still respond adequately to supraphysiologic ACTH concentrations.25, 26 A measurable cortisol response can be produced by administration of lower ACTH doses (0.01-0.2 μg/kg, 1-10 μg in people and foals ),25, 26 and such “low-dose” stimulation tests may more accurately diagnose RAI/CIRCI in critically ill patients.25-27
It is important to note, however, that both the high-dose and low-dose ACTH stimulation tests only evaluate the adrenal component of the HPA axis. Therefore, patients with cortisol insufficiency due to impaired HPA axis function at the hypothalamic and/or pituitary levels may produce an appropriate cortisol response to exogenous ACTH, and may be falsely interpreted as having intact HPA axis function. The CRH stimulation test is occasionally used to assess function of the higher levels of the HPA axis function, and is most helpful for distinguishing among adrenocortical, hypothalamic and pituitary failure if both ACTH and cortisol concentrations are measured before and after CRH stimulation.5 Effects of various doses of ovine CRH on cortisol concentrations in adult horses have been determined, and a CRH stimulation test protocol for use in horses has been described.28 However, this test is expensive and in people is less sensitive for the diagnosis of HPA axis hypofunction than other means,29 so the CRH stimulation test is not currently recommended as a first-line means of HPA axis assessment.
The metyrapone test may be more useful for assessing the integrity of the entire HPA axis. In people, metyrapone (30 mg/kg), a specific inhibitor of the adrenocortical steroidogenic enzyme 11-β-hydroxylase, is administered orally at midnight.5, 30 Because 11-β-hydroxylase catalyzes the conversion of 11-deoxycortisol to cortisol in the last step of adrenocortical cortisol synthesis, metyrapone administration results in a fall in circulating cortisol concentrations. With an intact HPA axis, this drop in plasma cortisol stimulates increased HPA axis activity, resulting in increased CRH and ACTH secretion and subsequent stimulation of adrenocortical cortisol synthesis. However, since the last step in cortisol synthesis is inhibited by metyrapone, cortisol concentrations remain low while concentrations of the precursor 11-deoxycortisol rise. A decrease in cortisol concentrations to below 7.0 μg/dl 8-10 hours after metyrapone administration confirms that 11-β-hydroxylase suppression was adequate. Increases in ACTH and 11-deoxycortisol concentrations at this time suggest an intact HPA axis, while low ACTH and 11-deoxycortisol suggest HPA axis dysfunction at the hypothalamic and/or pituitary levels.5, 30 However, if ACTH concentrations increase but 11-deoxycortisol remains low, primary adrenocortical dysfunction is implied. Measurement of 11-deoxycortisol is described in the horse,31 but commercial assays are not readily available in all clinical settings, and many assays require a cumbersome extraction step. Furthermore, while intravenous infusions of metyrapone have been described in horses,32 oral metyrapone has not been used for HPA axis function assessment in ill horses to date.
The insulin tolerance test (ITT) is currently considered the gold-standard test for diagnosis of HPA axis hypofunction,33 and has been described in both foals34 and adult horses.35 In the ITT, hypoglycemia is induced by administration of exogenous insulin, and the cortisol response to the physiologic stress of this hypoglycemia is measured. A blunted or absent increase in cortisol following hypoglycemia implies HPA axis dysfunction. ACTH can also be measured in conjunction with cortisol to differentiate between adrenal and central HPA axis dysfunction. However, given the risks associated with severe hypoglycemia such as is induced in the ITT,33 as well as the potential for pre-existing glucose derangements or peripheral insulin resistance during severe illness,36 the clinical utility of the ITT in critically ill patient is limited.
Some of the physiologic consequences of CIRCI may be associated with cortisol resistance in peripheral tissues rather than circulating cortisol insufficiency.37-40 In such cases, the above tests may suggest intact HPA axis function, with appropriate endogenous cortisol concentrations and normal responses to dynamic testing, despite clinical evidence of cortisol insufficiency such as persistent hypotension and inflammatory dysregulation. Unfortunately, methodology for assessing cortisol activity at the tissue level is currently limited,37-40and is not readily available for use in clinical patients with suspected RAI/CIRCI.
III. CURRENT UNDERSTANDING OF ADRENOCORTICAL FUNCTION IN HEALTHY FOALS AND ADULT HORSES
Horses
Basal cortisol concentrations in healthy adult horses at rest are reported in the approximate range of 1.1 to 14.3 μg/dl (30 to 395 nmol/L), consistent with resting cortisol concentrations in other domestic animal species.19, 28, 41-45 In addition to the pulsatile (ultradian) rhythm to corticosteroid secretion described above, adult horses exhibit a circadian rhythm to cortisol secretion similar to other species, with peak secretion in the morning and the nadir in the evening.5, 32, 44 This circadian, rhythm, though, is easily disrupted in horses by simple routine changes.32 Cortisol responses to ACTH stimulation testing in adult horses are described, and suggest adults horses show a comparable dose-dependent effect of exogenous ACTH on cortisol concentrations as is described in other species.5, 45, 46 In general, an approximately 2.5-to-5-fold increase in cortisol concentration is observed within 30 minutes after administration of a 0.1 – 10 μg/kg dose of exogenous ACTH (cosyntropin).46 Administration of higher doses (2-10 μg/kg) result in a sustained and greater, (5-10 fold) increase in cortisol concentration by 90 to 180 minutes after ACTH administration.46
Reported resting plasma concentrations of aldosterone and androstenedione in healthy adult horses have also been described. Basal aldosterone concentrations have been described within a range of approximately 14 to 50 pg/ml.18, 19, 47 Androstenedione concentrations in adult horses reportedly fall within a range of <0.050 to 0.986 ng/ml.16, 19 Sex and age variations in adrenal androgen concentrations may be relevant19 but at present are poorly understood in horses.
Foals
HPA axis function in the fetal and neonatal foal has been investigated in several studies with a variety of methodologies, and does differ from both adult horses and other mammalian species in key ways that may impact the neonatal foal's ability to respond to stress. Compelling evidence shows that maturation of the HPA axis occurs in the days just prior to parturition and continues during the first several weeks of life, much later than is described in other species.48-51 For example, premature foals have lower serum cortisol concentrations (< 3 μg/dl) in the two hours after birth than full-term foals (12-14 μg/dl).51 Concurrently, premature foals exhibit significantly higher endogenous ACTH concentrations than term foals (650 pg/ml vs 300 pg/ml at 30 minutes postpartum).51
These low baseline cortisol and concurrent high ACTH concentrations in premature foals imply that foals may have impaired adrenocortical sensitivity to ACTH, limited cortisol synthetic capacity, or both, as compared to adult horses. ACTH stimulation tests support this theory, as premature foals show a blunted cortisol response to exogenous ACTH, with only a 28% increase in plasma cortisol 30 to 60 minutes following stimulation, as compared with a 208% increase in normal term foals.48 MC2R density or activity has not been described in foals or horses of any age, but there is some evidence to suggest that the fetal foal's adrenal gland may be incapable of synthesizing cortisol until very late in gestation. Immunohistochemical localization showed that key steroidogenic enzymes necessary for cortisol synthesis (p450scc and 3-β-HSD) are absent or present in very low amounts until just prior to parturition in the foal,52, 53 much later than is described in other species.54
Furthermore, adrenocortical function may not be fully mature at birth even in full-term foals. By 12-24 hours of age, mean basal cortisol concentrations are lower in healthy neonatal foals (approximately 2.0 to 3.6 μg/dl) than reported mean concentrations in healthy adult horses (approximately 3-7 μg/dl), despite comparable or higher concurrent ACTH concentrations in foals.16, 42, 55-57 This apparent decreased cortisol response to endogenous ACTH in the neonatal foal is supported by further evidence of limited cortisol responses to exogenous ACTH in the same study: in neonatal foals during the first week of life, the increase in cortisol concentration following administration of both a low (10 μg) and a high (100 μg) dose of ACTH was approximately half that seen in adult horses in response to a comparable dose of ACTH.46, 56 Insulin-induced cortisol responses in foals within 12 hours of birth are also less than half of the responses achieved in 7 to 14 day old foals.34 Furthermore, this decreased adrenocortical responsiveness appears to persist during the first few months of life, as 12-week-old foals showed significantly greater cortisol responses to a low-dose (0.1 μg/kg) ACTH stimulation test than younger foals.58 The specific cause of these impaired cortisol responses in neonatal foals is not known, but regardless of the causative mechanism or mechanisms, this evidence of fetal and neonatal HPA axis immaturity is certain to impact, and may possibly impair, the foal's ability to respond to physiologic stress and disease during the neonatal period.
In contrast to cortisol, newborn foals appear to be able to mount a substantial aldosterone response to hypovolemia/hyponatremia;20,61 in fact, one study suggested that the aldosterone response in foals appears to be exaggerated in comparison to that in adult horses and may reflect differences in tubular sensitivity to aldosterone between neonates and adults.20 However, well-established reference ranges for plasma aldosterone or adrenal androgens concentrations in foals are not currently available.
IV. ADRENOCORTICAL INSUFFICIENCY
General Mechanisms of Adrenocortical Dysfunction Resulting in Cortisol Insufficiency
Cortisol insufficiency can result from HPA axis impairment at one or several levels, and may be transient or permanent.5 Permanent HPA axis dysfunction results from destruction of one or more glandular components of the axis, and is infrequent in both human and veterinary medicine. Immune-mediated adrenocortical destruction (e.g., Addison's Disease) is the most common manifestation of permanent HPA axis hypofunction in both human and veterinary patients,5, 62 but has not been described in horses to date. Patients with Addison's Disease cannot mount an appropriate cortisol response to stress and thus frequently present with hemodynamic instability and collapse.5, 62 Aldosterone deficiency, in addition to cortisol deficiency, is a typical feature of Addison's Disease, and results in fluid and electrolyte derangements that contribute to the development of hypovolemia, hypotension and cardiovascular collapse in affected individuals.5, 62
Irreversible adrenocortical, hypothalamic or pituitary destruction may also result from neoplastic infiltration, or from invasion of the glands by infectious organisms, but is rare in all species.62, 63 Severe adrenocortical hemorrhage and necrosis resulting in adrenal insufficiency – Waterhouse-Friderichsen syndrome – is also described in both people and horses with septic and endotoxic shock, and is believed to result from vascular derangements and ischemia associated with the primary disease. 64 Finally, congenital abnormalities in CRH-R1, MC2R, or GR receptor structure or function and congenital absence of synthetic enzymes necessary for pituitary or adrenocortical hormone synthesis are described in people, and can also result in HPA axis hypofunction.5
While irreversible HPA axis hypofunction due to destruction of HPA axis components is uncommon,5, 25, 65, 66 recent evidence suggests that transient HPA axis dysfunction (RAI/CIRCI) may occur in a substantial number of critically ill patients with a variety of conditions, including acute respiratory distress syndrome,23, 24 major trauma,67 and cardiothoracic surgery.68 The predominant diseases, though, associated with the development of RAI/CIRCI in people and infants are sepsis and septic shock.22, 25, 27, 39, 69-72 RAI/CIRCI can result from temporary suppression of HPA axis activity at one or more levels, resulting in: 1) inhibition of CRH and/or ACTH secretion, 2) decreased sensitivity to CRH or ACTH at their respective target tissues, 3) exhaustion of adrenocortical cortisol synthetic capacity (“loss of adrenal reserve”), or 4) impaired response to cortisol in the peripheral tissue resistance.14, 23, 39
The specific mechanisms leading to the development of RAI/CIRCI are poorly understood, but may involve a combination of factors, including: 1) direct damage to HPA axis components from the primary disease, 2) inhibition of cortisol production by medications used to treat the primary disease, and/or 3) suppression of activity of one or more components of the HPA axis by infectious organisms or the host's own immune and inflammatory response.23, 25, 26, 39 Periods of hypotension associated with hypovolemic or septic shock can result in decreased adrenal perfusion and ischemic injury to the metabolically active adrenocortical cells.64, 73 If this occurs for prolonged periods or to an extreme degree the damage may be irreversible, as seen in patients with Waterhouse-Friderichsen syndrome described above, but milder circulatory insults may result in transient injury and impaired function. In addition, the anesthetic agent etomidate, which is used frequently in critically ill people and animals, is known to directly inhibit adrenocortical cortisol synthesis by inhibiting 11-β-hydroxylase,74 the enzyme that catalyzes the key last step in the synthesis of active cortisol. Several antimicrobial agents, (e.g., ketoconazole and rifampin), which are often used in septic people and animals, also can inhibit adrenal steroid synthesis.23
It is the patient's own immune and inflammatory response, though, that appears to play the most vital role in the development of RAI/CIRCI during critical illnesses such as sepsis. Bacterial components such as endotoxin (the lipopolysaccharide component of gram negative bacterial cell walls) and host pro-inflammatory cytokines participate in initiating and maintaining the HPA axis response to sepsis. These factors can directly stimulate HPA axis activity at the multiple levels, ultimately resulting in stimulation of cortisol synthesis and secretion.39, 64, 75-77
However, bacterial ligands and inflammatory cytokines may also be capable of suppressing HPA axis function at one or more levels, in the face of an overwhelming bacterial infection or excessive host inflammatory response. For example, inducible nitric oxide synthase-mediated death of hypothalamic neurons in cardioregulatory centers has been described in patients that died of septic shock, and may be involved in HPA axis dysfunction in such patients.78 Bacterial endotoxin has been shown to directly decrease pituitary CRH receptor gene expression in both rats and cattle.79, 80 In addition, TNF-α can directly impair both pituitary ACTH release and adrenocortical cortisol synthesis.23 Finally, cholesterol availability for corticosteroid synthesis may be limited during sepsis, as several studies have shown decreased levels of plasma high-density lipoprotein (HDL) in critically ill individuals and correlated these decreased HDL levels with blunted cortisol responses to ACTH stimulation testing.23
Furthermore, several studies suggest that peripheral tissue cortisol resistance may develop in some critically ill patients. Specifically, impaired GR binding affinity has been documented in an ovine model of acute lung injury81 and in a rodent burn model.38 This effect was partially ameliorated in the rodent model when TNF-α and IL-1β were reduced by neutralizing antibodies.38 Furthermore, nuclear localization of cortisol-GR complexes was impaired in human leukocytes cells exposed to plasma from patients that died of acute respiratory distress syndrome.40 Thus, the complex relationship between endocrine and immune regulation of HPA axis function determines overall cortisol production and activity during sepsis. Any imbalance in these interactions can result in absolute or functional cortisol insufficiency and may play a critical role in the pathogenesis of RAI/CIRCI.
Critical Illness Related Corticosteroid Insufficiency (CIRCI) in Foals
Our current understanding of HPA axis function in critically ill horses and foals is limited, but several studies suggest that RAI/CIRCI may indeed occur in septic neonatal foals. One case report of transient HPA axis dysfunction, as evidenced by both a low basal cortisol concentration and an impaired cortisol response to a high-dose ACTH stimulation test, has been described in a septic neonatal foal.82 In addition, two independent studies that measured basal ACTH and cortisol concentrations in healthy and septic neonatal foals found significantly increased ACTH:cortisol ratios in non-surviving septic foals.83, 84 Such high ACTH:cortisol ratios, with high ACTH concentrations and low corresponding cortisol concentrations, suggest HPA axis dysfunction at the level of the adrenal gland may occur in the septic full-term foal.
HPA axis function has been further characterized in hospitalized foals using ACTH stimulation tests in two studies.85, 86 Neither study identified a significant difference in peak or delta cortisol responses between groups of age-matched healthy and ill foals in response to a low- dose ACTH stimulation test (0.1 μg/kg)86 or in response to a paired low-dose (10 μg) / high-dose (100 μg) ACTH stimulation test.85 However, when human diagnostic criteria for RAI/CIRCI24 were adapted and applied to a group of 72 hospitalized foals, approximately 50% of all hospitalized foals and approximately 40% of septic foals met these criteria.85 Furthermore, increased disease severity and poorer prognoses were correlated with decreased cortisol responses to ACTH stimulation in foals in both studies. Specifically, non-surviving foals had significantly lower cortisol responses to low-dose ACTH stimulation as compared to survivors,86 and foals that met criteria for RAI/CIRCI (as characterized by an inadequate cortisol response to a high-dose ACTH stimulation test) had a significantly greater incidence of shock, multiple organ dysfunction syndrome, and non-survival than foals with an adequate cortisol response to ACTH.85
In concert, these studies provide evidence that RAI/CIRCI occurs in critically ill and septic neonatal foals with comparable frequency and impact as in septic people, and suggest that the mechanisms and management of RAI/CIRCI in critically ill foals warrant further investigation. At present, cortisol replacement therapy in the form of hydrocortisone at approximately 1-4 mg/kg/day is recommended for the management of RAI/CIRCI in septic people and infants.24, 87 Such therapy has not been critically evaluated in neonatal foals to date, but recent evidence suggests that in healthy 2-to-6-day-old foals, a short tapering course of hydrocortisone (1.3 mg/kg/day divided q 4 hours IV) has potentially beneficial anti-inflammatory effects without significantly impairing innate immune function.88
Adrenocortical Insufficiency in Adult Horses
Adrenocortical insufficiency is not well described in adult horses. Transient adrenal insufficiency characterized by low basal ACTH and cortisol concentrations and impaired cortisol responses to ACTH stimulation testing has been described in one horse following abrupt cessation of long term anabolic steroid supplementation.89 A syndrome of adrenal exhaustion resulting in lethargy, anorexia, and poor performance is also anecdotally described in race horses, and has been attributed to adrenal insufficiency associated with long-term steroid administration or chronic stress.66 However, measurement of basal cortisol concentrations and ACTH stimulation testing in race and endurance horses has not provided convincing evidence for adrenal insufficiency in these equine athletes.66, 90 Decreased cortisol responses to ACTH are also described in mares with abnormal estrus related behavior,16 but the clinical significance of this behavior is not known. The potential for iatrogenic adrenal insufficiency associated with HPA axis suppression by exogenous steroids, though, should be considered in horses on chronic glucocorticoid or anabolic steroid supplementation, and care should to avoid abrupt cessation of such therapy.
In addition, the adrenal gland is extremely vulnerable to ischemic injury associated with endotoxic or hypovolemic shock in horses as in many other species. Adrenocortical hemorrhage, and necrosis similar to the human Waterhouse-Friedrichsen syndrome, is a common finding at necropsy examination of adult horses that succumbed to acute severe gastrointestinal disease and other diseases associated with endotoxic shock.73 In theory, such adrenal injury could contribute to long-term adrenocortical insufficiency in surviving horses, though this has not been documented in horses to date. Further, to the authors’ knowledge, classic hypoadrenocorticism (Addison's Disease) resulting from immune-mediated adrenocortical destruction and manifesting with glucocorticoid and mineralocorticoid deficiency has not been described in horses.
Hypoaldosteronism
In people, primary hypoaldosteronism is uncommon, and most frequently occurs as a result of congenital defects in some component of the aldosterone biosynthetic pathway.5 Hypoaldosteronism is also reported in renal diseases that damage the juxtaglomerular apparatus integral to function of the renin-angiotenin-aldosterone system and peripheral resistance to aldosterone are also reported in people.5 To the authors’ knowledge, such syndromes have not been described in horses. However, hypoaldosteronism is a documented cause of renal tubular acidosis in people,91 and should not be overlooked as possible cause of RTA in horses.
Sex Steroid Insufficiency
Adrenal androgen deficiency can result in abnormal or delayed sexual development, but due to the relatively greater contribution of gonadal androgens to sexual development, clinical disease associated with sole defects in adrenal androgen production are uncommon in all species,93 and have not been described in horses to the authors’ knowledge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gravanis A, Margioris A. Pharmacology of glucocorticoids: An overview. In: Margioris A, Chrousos G, editors. Contemporary Endocrinology: Adrenal Disorders. Humana Press; Totowa, NJ: 2001. [Google Scholar]
- 2.Alesci S, Koch C, Bornstein S, Pacak K. Adrenal androgens regulation and adrenopause. Endocrine Regulations. 2001;35:95–100. [PubMed] [Google Scholar]
- 3.Wiebke A, Stewart P. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocr Metab Clin N Amer. 2005;34(2):293–313. doi: 10.1016/j.ecl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Kallen C, Arakane F, Sugawara T, et al. Structure, function and regulated expression of the steroidogenic acute regulatory (StAR) protein. In: Margioris A, Chrousos G, editors. Contemporary Endocrinology: Adrenal Disorders. Humana Press; Totowa, NJ: 2001. pp. 107–117. [Google Scholar]
- 5.Stewart P. The Adrenal Cortex. In: Kronenberg H, Melmed S, Polonsky K, Larsen P, editors. Williams Textbook of Endocrinology. 11 ed. Saunders Elsevier; Philadelphia: 2008. pp. 445–503. [Google Scholar]
- 6.Rijnberk A, Mol J. Adrenocortical Function. In: Kaneko J, Harvey J, Bruss M, editors. Clinical Biochemistry of Domestic Animals. 5th ed. Academic Press; San Diego: 1997. pp. 553–570. [Google Scholar]
- 7.Maier C, Kotzmann H, Luger A. CRH-receptors and their ligands. In: Gaillard R, editor. The ACTH Axis: Pathogenesis, Diagnosis, and Treatment. Kluwer Academic Publishers; Boston: 2003. pp. 65–83. [Google Scholar]
- 8.Clark A, King P. The ACTH receptor and its mutations. In: Gaillard R, editor. The ACTH Axis: Pathogenesis, Diagnosis, and Treatment. Kluwer Academic Publishers; Boston: 2003. pp. 171–190. [Google Scholar]
- 9.Lewis J, Bagley C, Elder P, Bachmann A, Torpy D. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359:189–194. doi: 10.1016/j.cccn.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Adcock R, Kattesh H, Robert M, Saxton A, Carroll J. Relationships between plasma cortisol, cortisosteroid-binding globulin (CBG) and the free cortisol index (FCI) in pigs over a 24 hours period. J An Vet Adv. 2006;5:85–91. doi: 10.1080/10253890701248020. [DOI] [PubMed] [Google Scholar]
- 11.Irvine C, Alexander S. Measurement of free cortisol and the capacity and association constant of cortisol-binding proteins in plasma of foals and adult horses. J Reprod Fert Suppl. 1987;35:19–24. [PubMed] [Google Scholar]
- 12.Meyer H, Rothuizen J. Determination of the percentage of free cortisol in plasma in the dog by ultrafiltration/dialysis. Domest Anim Endocrin. 1993;10:45–53. doi: 10.1016/0739-7240(93)90007-x. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaides N, Galata Z, Kino T, Chrousos G, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marik P, Zaloga G. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002;122:1784–1796. doi: 10.1378/chest.122.5.1784. [DOI] [PubMed] [Google Scholar]
- 15.Young W. Endocrine Hypertension. In: Kronenberg H, Melmed S, Polonsky K, Larsen P, editors. Williams Textbook of Endocrinology. 11th ed. Saunders; Elsevier, Inc.; Philadelphia: 2008. pp. 505–537. [Google Scholar]
- 16.Hedberg Y, Dalin A, Forsberg M, et al. Effect of ACTH (tetracosactide) on steroid hormone levels in the mare. Part A: effect in intact normal mares and mares with possible estrous related behavioral abnormalities. Anim Reprod Sci. 2007;100:73–91. doi: 10.1016/j.anireprosci.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Federman D. The Endocrine Patient. In: Kronenberg H, Melmed S, Polonsky K, Larsen P, editors. Williams Textbook of Endocrinology. 11th ed. Saunders Elsevier; Philadelphia: 2008. pp. 12–17. [Google Scholar]
- 18.Gehlen H, Sundermann T, Rohn K, Stadler P. Aldosterone plasma concentration in horses with heart valve insufficiencies. Res Vet Sci. 2008;85:340–344. doi: 10.1016/j.rvsc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Haffner J, Fecteau K, Eiler H, Tserendorj T, Hoffman R, Oliver J. Blood steroid concentrations in domestic Mongolian horses. J Vet Diagn Invest. 2010;22:537–543. doi: 10.1177/104063871002200407. [DOI] [PubMed] [Google Scholar]
- 20.Hollis A, Boston R, Corley K. Plasma aldosterone, vasopressin, and atrial natriuretic peptide in hypovolaemia: a preliminary comparative study of neonatal and mature horses. Equine Vet J. 2008;40:64–69. doi: 10.2746/042516407X235795. [DOI] [PubMed] [Google Scholar]
- 21.Bornstein S, Engeland W, Erhart-Bornstein M, Herman J. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metabol. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Annane D, Maxime V, Ibrahim F, Alvarez J, Abe E, Boudou P. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174:1319–1326. doi: 10.1164/rccm.200509-1369OC. [DOI] [PubMed] [Google Scholar]
- 23.Marik P. Critical Illness Related Corticosteroid Insufficiency. Chest. 2009;135(1):181–193. doi: 10.1378/chest.08-1149. [DOI] [PubMed] [Google Scholar]
- 24.Marik P, Pastores S, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 25.Marik P, Zaloga G. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31:141–145. doi: 10.1097/00003246-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Kozyra E, Wax R, Burry L. Can 1 ug of cosyntropin be used to evaluate adrenal insufficiency in critically ill patients? Ann Pharmacother. 2005;39:691–698. doi: 10.1345/aph.1E139. [DOI] [PubMed] [Google Scholar]
- 27.Siraux V, De Backer DY, G, Melot C, Gervy C, Mockel J, Vincent J. Relative adrenal insufficiency in patients with septic shock: comparison of low-dose and conventional corticotropin tests. Crit Care Med. 2005;33:2479–2486. doi: 10.1097/01.ccm.0000185641.87051.7c. [DOI] [PubMed] [Google Scholar]
- 28.Reijerkerk E, Visser E, van Reenen C, van der Kolk J. Effects of various doses of ovine corticotrophin-releasing hormone on plasma and saliva cortisol concentrations in the horse. Am J Vet Res. 2009;70:361–364. doi: 10.2460/ajvr.70.3.361. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt I, Lahner H, Mann K, Petersenn S. Diagnosis of adrenal insufficiency: Evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. J Clin Endocrinol Metab. 2003;88:4193–4198. doi: 10.1210/jc.2002-021897. [DOI] [PubMed] [Google Scholar]
- 30.Fiad T, Kirby J, Cunningham S, McKenna T. The overnight single-dose metyrapone test is a simple and reliable index of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol. 1994;40:603–609. doi: 10.1111/j.1365-2265.1994.tb03011.x. [DOI] [PubMed] [Google Scholar]
- 31.Luna S, YTaylor P, Wheeler M. Cardiorespiratory, endocrine and metabolic changes in ponies undergoing intravenous or inhalation anaesthesia. J Vet Pharmacol Ther. 1996;19:251–258. doi: 10.1111/j.1365-2885.1996.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 32.Alexander S, Irvine C, Donald R. Dynamics of the Regulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis Determined Using a Nonsurgical Method for Collecting Pituitary Venous Blood from Horses. Front Neuroendocrinol. 1996;17:1–50. doi: 10.1006/frne.1996.0001. [DOI] [PubMed] [Google Scholar]
- 33.Giordano R, Picu A, Bonelli L, et al. Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparisons of different provocative tests. Clin Endocrinol. 2008;68(6):935–941. doi: 10.1111/j.1365-2265.2007.03141.x. [DOI] [PubMed] [Google Scholar]
- 34.Silver M, Fowden A, Know J, Ousey J, Franco R, Rossdale P. Sympathoadrenal and other responses to hypoglycaemia in the young foal. J Reprod Fertil Suppl. 1987;35(607-614) [PubMed] [Google Scholar]
- 35.Alexander S, Roud H, Irvine C. Effect of insulin-induced hypoglycemia on secretion patterns and rates of corticotropin-releasing hormone, arginine vasopressin and adrenocorticotrophin in horses. J Endocrinol. 1997;153(3):401–409. doi: 10.1677/joe.0.1530401. [DOI] [PubMed] [Google Scholar]
- 36.Van den Berghe G, editor. Endocrinology of Critical Illness. Saunders; Philadelphia: 2006. Critical Care Clinics; No. 22, no. 1. [Google Scholar]
- 37.Cohen J, Venkatesh B. Assessment of tissue cortisol activity. Crit Care Resusc. 2009;11(4):287–289. [PubMed] [Google Scholar]
- 38.Liu D, Su Y, Zhang W, et al. Changes in glucocorticoid and mineralocorticoid receptors of liver and kidney cytosols after pathologic stress and its regulation in rats. Crit Care Med. 2002;30(3):623–627. doi: 10.1097/00003246-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Marik P. Mechanisms and clinical consequences of critical illness associated adrenal insufficiency. Curr Opin Crit Care. 2007;13:363–369. doi: 10.1097/MCC.0b013e32818a6d74. [DOI] [PubMed] [Google Scholar]
- 40.Meduri G, Muthiah M, Carratu P, Eltorky M, Chrousos G. Nuclear factor-κb- and glucocorticoid receptor-α mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Neuroimmunodulation. 2005;12(321-338) doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- 41.Fazio E, Medica P, Aronica V, Grasso L, Ferlazzo A. Circulating beta-endorphin, adrenocorticotrophic hormone and cortisol levels of stallions before and after short road transport: stress effect of different distances. Acta Vet Scand. 2008;50:6. doi: 10.1186/1751-0147-50-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Place N, McGowan C, Lamb S, Schanbacher B, McGowan T, Walsh D. Seasonal variation in serum concentrations of selected metabolic hormones in horses. J Vet Intern Med. 2010;24(3):650–654. doi: 10.1111/j.1939-1676.2010.0500.x. [DOI] [PubMed] [Google Scholar]
- 43.Kudielka B, Buske-Kirschbaum A, Helhammer D, Kirschbaum C. HPA axis responses to laboratory pyschosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 44.Lefcourt A, Bitman J, Kahl S, Wood D. Circadian and ultradian rhythms of peripheral cortisol concentrations in lactating dairy cows. J Dairy Sci. 1993;76:2607–2612. doi: 10.3168/jds.S0022-0302(93)77595-5. [DOI] [PubMed] [Google Scholar]
- 45.Martin L, Behrend E, Mealey K, Carpenter D, Hickey K. Effect of low doses of cosyntropin in serum cortisol concentrations in clinically normal dogs. Am J Vet Res. 2007;68(5):555–560. doi: 10.2460/ajvr.68.5.555. [DOI] [PubMed] [Google Scholar]
- 46.Bousquet-Melou A, Formentini E, Picard-Hagen N, Delage L, Laroute V, Toutain P. The adrenocorticotropin stimulation test: Contribution of a physiologically based model developed in horse for its interpretation in different pathophysiologic situations encountered in man. Endocrinology. 2006;147(9):4281–4291. doi: 10.1210/en.2005-1161. [DOI] [PubMed] [Google Scholar]
- 47.McKeever K, Malinowski K. Endocrine response to exercise in young and old horses. Equine Vet J Suppl. 1999;30:561–566. doi: 10.1111/j.2042-3306.1999.tb05284.x. [DOI] [PubMed] [Google Scholar]
- 48.Silver M, Ousey J, Dudan F, et al. Studies on equine prematurity 2: Post natal adrenocortical activity in relation to plasma adrenocorticotrophic hormone and catecholamine levels in term and premature foals. Equine Vet J. 1984;16(4):278–286. doi: 10.1111/j.2042-3306.1984.tb01927.x. [DOI] [PubMed] [Google Scholar]
- 49.Silver M, Fowden A. Prepartum adrenocortical maturation in the fetal foal: responses to ACTH. J Endocrinol. 1994;142:417–425. doi: 10.1677/joe.0.1420417. [DOI] [PubMed] [Google Scholar]
- 50.Ousey J, Rossdale P, Fowden A, Palmer L, Turnbull C, Allen W. Effects of manipulating intrauterine growth on post natal adrenocortical development and other parameters of maturity in neonatal foals. Equine Vet J. 2004;36(7):616–621. doi: 10.2746/0425164044864598. [DOI] [PubMed] [Google Scholar]
- 51.Rossdale P, Ousey J. Studies on equine prematurity 6: guidelines for assessment of foal maturity. Equine Vet J. 1984;16(4):300–302. doi: 10.1111/j.2042-3306.1984.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 52.Han X, Fowden A, Silver M, et al. Immunohistochemical localisation of steroidogenic enzymes and phenylethanolamine-N-methyl-transferase (PNMT) in the adrenal gland of the fetal and newborn foal. Equine Vet J. 1995;27(2):140–146. doi: 10.1111/j.2042-3306.1995.tb03051.x. [DOI] [PubMed] [Google Scholar]
- 53.Weng Q, Tanaka Y, Taniyama H, et al. Immunolocalization of steroidogenic enzymes in equine fetal adrenal glands during mid-late gestation. J Reprod Devel. 2007;53(5):1093–1098. doi: 10.1262/jrd.18159. [DOI] [PubMed] [Google Scholar]
- 54.Riley S, Boshier D, Luu-The V, Labrie F, Challis J. Immunohistochemical localization of 3 beta-hydroxysteroid/delta 5-delta 4-isomerase, tyrosine hydroxylase, and phenylethanolamine N-methyl transferase in adrenal glands of sheep fetuses throughout gestation and in neonates. J Reprod Fertil. 1992;96(1):127–134. doi: 10.1530/jrf.0.0960127. [DOI] [PubMed] [Google Scholar]
- 55.Donaldson M, McDonnell S, Schanbacher B, Lamb S, McFarlane D, Beech J. Variation in plasma adrenocorticotropic hormone concentration and dexamethasone suppression test results with season, age, and sex in healthy ponies and horses. J Vet Int Med. 2005;19(2):217–222. doi: 10.1892/0891-6640(2005)19<217:vipahc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 56.Hart K, Heusner G, Norton N, Barton M. Hypothalamic-pituitary-adrenal axis assessment in healthy term neonatal foals utilizing a paired low dose/high dose ACTH stimulation test. J Vet Intern Med. 2009;23:344–351. doi: 10.1111/j.1939-1676.2008.00271.x. [DOI] [PubMed] [Google Scholar]
- 57.Irvine C, Alexander S. Factors affecting the circadian rhythm in plasma cortisol concentrations in the horse. Domestic Animal Endocrinology. 1994;11(2):227–238. doi: 10.1016/0739-7240(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 58.Wong D, Vo D, Alcott C, et al. Adrenocorticotropic hormone stimulation tests in healthy foals from birth to 12 weeks of age. Can J Vet Res. 2009;73(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- 59.Hart K, Barton M, Ferguson D, Slovis N, Heusner G. Serum Free Cortisol Fraction in Healthy and Septic Neonatal Foals. 2010. Submitted - In Review. [DOI] [PubMed]
- 60.Hart K, Dirikolu L, Ferguson D, et al. Daily endogenous cortisol production and hydrocortisone pharmacokinetics in adult horses and neonatal foals. 2010. Submitted - In Review. [DOI] [PubMed]
- 61.Broughton Pipkin F, Ousey J, Wallace C, Rossdale P. Studies on equine prematurity 4: Effect of salt and water loss on the renin-angiotensin-aldosterone system in the newborn foal. Equine Vet J. 1984;16(4):292–297. doi: 10.1111/j.2042-3306.1984.tb01929.x. [DOI] [PubMed] [Google Scholar]
- 62.Greco D. Hypoadrenocorticism in small animals. CLin Tech Small Anim Pract. 2007;22(1):32–35. doi: 10.1053/j.ctsap.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 63.de Herder WLS. Overview of Hyper- and Hypoadrencorticism. In: Margioris A, Chrousos G, editors. Contemporary Endocrinology: Adrenal Disorders. Humana Press; Totowa, NJ: 2001. pp. 143–153. [Google Scholar]
- 64.Levin J, Cluff L. Endotoxemia and adrenal hemorrhage. A mechanism for the Waterhouse-Friderichsen sydrome. J Exp Med. 1965;1(121):247–260. doi: 10.1084/jem.121.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loisa P, Rinne T, Kaukinen S. Adrenocortical function and multiple organ failure in severe sepsis. Acta Anaesthiol Scand. 2002;46:145–151. doi: 10.1034/j.1399-6576.2002.460204.x. [DOI] [PubMed] [Google Scholar]
- 66.Dybdal N, McFarlane D. Endocrine and metabolic diseases. In: Smith B, editor. Large Animal Internal Medicine. 4th ed. Mosby; Philadelphia, PA: 2009. p. 1345. [Google Scholar]
- 67.Guillamondegui O, Gunter O, Patel S, Fleming S, Cotten B, Morris J. Acute adrenal insufficiency may affect outcome in the trauma patient. Am Surg. 2009;75(4):287–290. [PubMed] [Google Scholar]
- 68.Henzen C, Kobza R, Schwaller-Protzmann B, Stulz P, Briner V. Adrenal function during coronary artery bypass grafting. Eur J Endocrinol. 2003;148(6):663–668. doi: 10.1530/eje.0.1480663. [DOI] [PubMed] [Google Scholar]
- 69.Aneja R, Carcillo J. What is the rationale for hydrocortisone treatment in children with infection-related adrenal insufficiency and septic shock. Arch Dis Child. 2007;92:165–169. doi: 10.1136/adc.2005.088450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elsouri N, Bander J, Guzman J. Relative adrenal insufficiency in patients with septic shock; a close look at practice patterns. Journal of Critical Care. 2006;21:73–78. doi: 10.1016/j.jcrc.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez J, Escorell A, Zabalza M, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology. 2006;44(5):1288–1295. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- 72.Pizarro C, Troster E, Damiani D, Carcillo J. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med. 2005;33(4):855–859. doi: 10.1097/01.ccm.0000159854.23324.84. [DOI] [PubMed] [Google Scholar]
- 73.McGavin M, Zachary J. Pathologic Basis of Veterinary Disease. 4th ed. Mosby Elsevier; St. Louis: 2007. [Google Scholar]
- 74.De Jong F, Mallios C, Jansen C, Scheck P, Lamberts S. Etomidate suppresses adrenocortical function by inhibition of 11-beta-hydroxylation. J Clin Endocrin Metab. 1984;59(6):1143–1147. doi: 10.1210/jcem-59-6-1143. [DOI] [PubMed] [Google Scholar]
- 75.Beishuizen A, Thijs L. Endotoxin and the hypothalamic-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9(1):3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 76.Gaillard R. Interactions between the hypothalamic-pituitary-adrenal axis and the immunological system. In: Gaillard R, editor. The ACTH Axis: Pathogenesis, Diagnosis, and Treatment. Kluwer Academic Publishers; Boston: 2003. pp. 109–135. [Google Scholar]
- 77.Judd A, Call G, Barney M, et al. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann NY Acad Sci. 2000;917:628–637. doi: 10.1111/j.1749-6632.2000.tb05428.x. [DOI] [PubMed] [Google Scholar]
- 78.Sharshar T, Gray F, Lorin de la Grandmaison G, et al. Apoptosis of neurons in cardiovascular autonomic centers triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–1805. doi: 10.1016/s0140-6736(03)14899-4. [DOI] [PubMed] [Google Scholar]
- 79.Aubry J, Turnbull A, Pozzoli G, Rivier C, Vale W. Endotoxin decreases corticotropin-releasing factor receptor 1 messenger ribonucleic acid levels in the rat pituitary. Endocrinology. 1997;138(4):1621–1626. doi: 10.1210/endo.138.4.5050. [DOI] [PubMed] [Google Scholar]
- 80.Qahwash I, Cassar C, Radcliff R, Smith G. Bacterial lipopolysaccharide-induced coordinate downregulation of arginine vasopressin receptor V3 and corticotropin-releasing factor receptor 1 messenger ribonucleic acids in the anterior pituitary of endotoxemic steers. Endocrine. 2002;18:13–20. doi: 10.1385/ENDO:18:1:13. [DOI] [PubMed] [Google Scholar]
- 81.Liu L, Sun B, Tian Y, Lu B, Wang J. Changes of pulmonary glucocorticoid receptor and phospholipase A2 in sheep with acute lung injury. Am Rev Respir Dis. 1993;148:878–881. doi: 10.1164/ajrccm/148.4_Pt_1.878. [DOI] [PubMed] [Google Scholar]
- 82.Couetil L, Hoffman A. Adrenal insufficiency in a neonatal foal. J Am Vet Med Assoc. 1998;212:1594–1596. [PubMed] [Google Scholar]
- 83.Gold J, Divers T, Barton M, et al. Plasma adrenocorticotropin, cortisol, and adrenocorticotropin/cortisol ratios in septic and normal-term foals. J Vet Intern Med. 2007;21:791–796. doi: 10.1892/0891-6640(2007)21[791:pacacr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 84.Hurcombe S, Toribio R, Slovis N, et al. Blood arginine vasopressin, adrenocorticotropin hormone, and cortisol concentrations at admission in septic and critically ill foals and their association with survival. J Vet Intern Med. 2008;22(3):639–647. doi: 10.1111/j.1939-1676.2008.0090.x. [DOI] [PubMed] [Google Scholar]
- 85.Hart K, Slovis N, Barton M. Hypothalamic-pituitary-adrenal axis dysfunction in hospitalized neonatal foals. J Vet Intern Med. 2009;23:901–912. doi: 10.1111/j.1939-1676.2009.0323.x. [DOI] [PubMed] [Google Scholar]
- 86.Wong D, Vo D, Alcott C, Peterson A, Sponseller B, Hsu W. Baseline plasma cortisol and ACTH concentrations and response to low dose ACTH stimulation testing in ill foals. J Am Med Assoc. 2009;234(1):126–132. doi: 10.2460/javma.234.1.126. [DOI] [PubMed] [Google Scholar]
- 87.Fernandez E, Watterberg K. Relative adrenal insufficiency in the preterm and term infant. J Perinatol. 2009;29(Suppl 2):S44–S49. doi: 10.1038/jp.2009.24. [DOI] [PubMed] [Google Scholar]
- 88.Hart K, Barton M, Vandenplas M, Hurley D. Effects of low-dose hydrocortisone therapy on immune function in neonatal horses. 2010. Submitted - In Review. [DOI] [PMC free article] [PubMed]
- 89.Dowling P, Williams M, Clark T. Adrenal insufficiency associated with long-term anabolic steroid administration in a horse. J Am Vet Med Assoc. 1994;203(8):1166–1169. [PubMed] [Google Scholar]
- 90.Baker H, Baker I, Epstein V, Hudson B. Effect of stress on steroid hormone levels in racehorses. Aust Vet J. 1982;58(2):70–71. doi: 10.1111/j.1751-0813.1982.tb02692.x. [DOI] [PubMed] [Google Scholar]
- 91.Unwin R, Capasso G. The renal tubular acidoses. J R Soc Med. 2000;94:221–225. doi: 10.1177/014107680109400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arroyo L, Stampfli H. Equine renal tubular disorders. Vet Clin Equine. 2007;23(3):631–639. doi: 10.1016/j.cveq.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Styne D, Grumbach M. Puberty: Ontogeny, Neuroendocrinology, Physiology, and Disorders. In: Kronenberg H, Melmed S, Polonsky K, Larsen P, editors. Williams Textbook of Endocrinology. 11th ed. Saunders: Elsevier, Inc.; Philadelphia: 2008. pp. 969–1166. [Google Scholar]