Abstract
The Major Histocompatibility Complex (MHC) on human chromosome 6 represents the most important genetic locus for a number of common human autoimmune diseases. Specific alleles that differ from closely related alleles by only one or a few amino acids in the peptide binding groove are frequently strongly associated with disease susceptibility, raising the important question of which peptide presentation events are critical in disease initiation and progression. This review will cover a number of topics pertinent to this fundamental question, including MHC linked disease susceptibility to autoimmune diseases, molecular mechanisms for the role of MHC molecules in autoimmune diseases as well as the recognition of self and microbial peptides by self-reactive T cell receptors (TCRs).
Keywords: Autoimmune diseases, MHC, self-antigen, T cells, molecular mimicry
Section 1. MHC genes in autoimmune diseases
1.1 MHC genes in autoimmune diseases: general considerations
Genome-wide scans in a number of human autoimmune diseases have now shown that the MHC region plays a central role in determining disease susceptibility. These genome-wide investigations have uncovered a large number of genes that contribute to disease development, but the contribution of most of these genes to disease risk is small. In contrast, the MHC region on human chromosome 6 stands out as the most prominent susceptibility locus in three common chronic inflammatory human diseases, multiple sclerosis (MS), type 1 diabetes and rheumatoid arthritis [1; 2; 3]. For example, in a genome wide analysis of MS patients the HLA-DRA locus within the MHC class II region gave a p value of 8.94×10−81, while the genes ranked second and third (IL2RA and IL7RA) yielded substantially lower p values (p=2.96×10−8 and p=2.94×10−7, respectively) [1].
However, MHC linked susceptibility represents a complex problem. It has been difficult to precisely define which gene(s) within the MHC are responsible for disease susceptibility due to strong linkage disequilibrium across the region which contains a large number of genes relevant for immune responses, including MHC class I and class II genes, TNF and complement genes, TAP and HLA-DM genes. Linkage disequilibrium means that neighboring genes are frequently inherited as a block, such as HLA-DR and HLA-DQ genes. In MS, the peak in genome-wide scans is centered on this DR-DQ interval and the disease-associated HLA-DRB1*1501 allele occurs in strong linkage disequilibrium with the HLA-DQB1*0602 allele. Comparison of different ethnic groups can help to define the primary genetic association. African populations are characterized by greater haplotypic diversity and distinct patterns of linkage disequilibrium, and in a well-characterized African American data set a selective association of MS susceptibility with HLA-DRB1*15 was shown, independent of the HLA-DQB1*0602 gene [4]. However, another recent study suggested that the combination of these DR and DQ alleles confers risk [5].
It is also becoming increasingly evident that more than a single gene within the MHC locus can contribute to disease risk. In type 1 diabetes, the strongest association is also observed with the MHC class II region, in particular the alleles that encode the beta chains of HLA-DQ2 (DQB1*0201) and HLA-DQ8 (DQB1*0302) [6; 7]. In addition, a contribution of the MHC class I genes HLA-B and HLA-A was shown to be independent of the stronger HLA-DQ association. Amongst these MHC class I genes, HLA-B*39 appears to make the strongest contribution to disease risk [8].
From an immunological, functional perspective protective MHC alleles are of particular interest. In MS, it was recently shown that the MHC class I alleles HLA-A*02 and HLA-B*44 independently reduce susceptibility to MS; HLA-B*44 demonstrated association with a better radiologic outcome, helping to preserve brain volume [9]. The best known example of a protective allele is HLA-DQB1*0602 which confers strong protection against the development of type 1 diabetes. In one of the more recent studies, a 33-fold reduction in disease risk was reported for this allele [10]. Interestingly, several other DR-DQ haplotypes can also protect from the development of type 1 diabetes [11].
Thus, there is a rather complex contribution of the MHC locus to the risk for the development of autoimmunity. It is evident that there is not a single gene or allele that is responsible for the entire disease risk. In addition to primary susceptibility genes, other MHC class I or class II genes can modify disease susceptibility by either increasing or decreasing risk.
1.2 Hypotheses for the role of MHC genes in autoimmune diseases
MHC genes are the most polymorphic genes in the human genome, with an unusually large number of alleles for most MHC class I and class II genes. Autoimmune diseases are typically associated with specific alleles that frequently differ only at one or a few positions from alleles that do not elevate the risk for the particular disease. These polymorphisms are typically located to pockets of the MHC class II peptide binding groove, strongly implicating peptide presentation in disease susceptibility [12]. In experimental animal models such as the experimental autoimmune encephalomyelitis model of MS, MHC class II proteins from susceptible strains present myelin-derived self-peptides to CD4 T cells, such as the myelin basic protein (MBP) Ac-11 peptide in mice with the H-2u haplotype and the proteolipid protein (PLP) 139–151 peptide in mice with the H-2s haplotype [13; 14]. In animal models it has thus been possible to prove that MHC class II genes confer susceptibility through presentation of self-peptides. Direct proof for this hypothesis is obviously difficult to obtain in human diseases because one of the key experiments, the passive transfer of an implicated T cell population, would be unethical. Nevertheless, the overall evidence for a role of disease-associated MHC class II proteins in peptide presentation is very strong.
Within this conceptual framework, several related hypotheses have been proposed on how peptide presentation is related to the development of an autoimmune disease: (1) Disease-associated polymorphisms enable the presentation of key self-peptide(s) in the target organ of the disease, (2) These polymorphisms do result in poor presentation of critical epitopes and thereby favor escape from thymic tolerance mechanisms, (3) Key polymorphisms exert effects on the T cell repertoire important in disease initiation and/or amplification, (4) Protective MHC molecules select regulatory T cells with specificity for the target organ and thereby keep autoimmune inflammation in check, (5) Disease-associated MHC molecules present not only relevant self-peptides, but also microbial peptides that can cause expansion and activation of the relevant self-reactive T cells. It is obvious that these hypotheses are not necessarily mutually exclusive, and indeed possible that there are scenarios where a subset or most apply simultaneously.
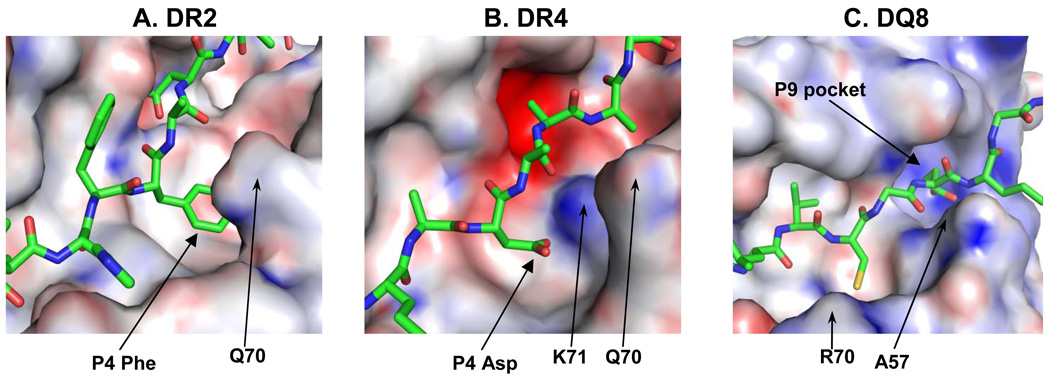
With respect to the first hypothesis, it is apparent that key polymorphisms drastically change the peptide binding specificity of the respective MHC class II molecule (Figure 1). Good examples are the DR β71 position relevant in several HLA-DR associated autoimmune diseases. DR β71 has a positive charge in common rheumatoid arthritis associated alleles (DRB1*0101, DRB1*0401 and DRB1*0404) and the P4 pocket of the binding site therefore has a preference for an acidic peptide side chain, although other amino acids can also be accommodated (Figure 1B) [15; 16]. In the pemphigus vulgaris associated DRB1*0402 allele, however, position DR β71 and the neighboring β70 position both have a negative charge, obviously interfering with binding of peptides with an acidic side chain at P4 [17]. In the MS associated DRB1*1501 allele, DR β71 is an alanine and the P4 pocket assumes a hydrophobic character (Figure 1A) [18]. The instigating peptide(s) in these diseases are not known with certainty, but candidate self-peptides that bind to the respective HLA-DR proteins have been identified and shown to be recognized by human T cells.
Figure 1. Structural features of MHC class II molecules associated with susceptibility to autoimmune diseases.
A. One of the characteristic features of the MS associated HLA-DR2 (DRA, DRB1*1501) molecule is the large hydrophobic P4 pocket that accommodates a phenylalanine residue (P4 Phe) of the bound MBP (85–99) peptide in this structure. A key polymorphic residue, DRβ 71, is a small amino acid (alanine) which provides the room for the large peptide side chain in this pocket (the neighboring amino acid Q70 is highlighted). The bound peptide is shown as a stick model. A positive charge is indicated by a blue surface on the MHC molecule, a negative charge by a red surface.
B. The rheumatoid arthritis associated DR4 (DRB1*0401) molecule has a P4 pocket with very different characteristics. A key polymorphic amino acid, DRβ71, is a lysine (K71), which makes the pocket smaller than in DR2 and gives it a positive charge. As a consequence, this pocket has a preference for negatively charged amino acids, like the aspartic acid residue (P4 Asp) of the bound type II collagen peptide.
C. The type 1 diabetes associated DQ8 molecule has a positively charged P9 pocket because the key polymorphic residue, DQβ 57, lacks a charge (an alanine, A57). This pocket therefore has a positive charge, indicated by the blue color.
The second hypothesis is based on an interesting recent finding concerning insulin, a key autoantigen in the pathogenesis of type 1 diabetes in NOD mice [19]. The human and mouse MHC class II proteins associated with susceptibility to type 1 diabetes share a very interesting structural property: mouse I-Ag7 and human DQ2 and DQ8 proteins all lack an aspartic acid at position 57 of the I-A and DQ β chains, respectively, while an aspartic acid is common among alleles not associated with the disease [7; 20]. The presence of a serine (I-Ag7) or an alanine (DQ2 and DQ8) at this position results in a positively charged P9 pocket because the charge of Arg76 from the α chain is unopposed (Figure 1C) [21; 22]. The Kappler lab recently defined the binding frame for a key epitope in NOD mice, the insulin B9-23 peptide, by locking the binding frame through a disulfide bond. Surprisingly, they found that an arginine was present at the P9 position, even though a positive charge would obviously be unfavorable in the already positively charged P9 pocket [23]. Consequently, this insulin peptide is bound with a very low affinity by I-Ag7. At the present time, it is not yet known whether this mechanism is more general or restricted to this particularly interesting case.
The third hypothesis, an effect of a disease associated polymorphism on the T cell repertoire, was discovered using HLA-DQ8 as an example. DQ8 not only confers susceptibility to type 1 diabetes, but also to celiac disease, an inflammatory disease of the small intestine caused by ingestion of gliadins in wheat. The positive charge of the P9 pocket was shown to affect the resulting T cell repertoire in that negatively charged residues were present at particular positions of the CDR3β loop of the TCR, even though this loop is unlikely to directly contact the P9 pocket [24]. Glutamine residues in gliadins are deamidated to negatively charged glutamic acid by transglutaminase, an enzyme induced by intestinal inflammation [25]. DQ8 restricted T cells were found to respond to native gluten peptides with a glutamine at P9, but deamidation could enhance the T cell response, a mechanism that may amplify an early inflammatory response in this disease [24].
With respect to the fourth hypothesis, it has been postulated that protective MHC class II proteins promote the selection of regulatory T cells with specificity for the target organ in the thymus and/or activate regulatory T cells in the target organ. Such a mechanism may explain the strong protective effect of the DQB1*0602 allele from type 1 diabetes, but no definitive data to prove this hypothesis are available. This mechanism is attractive because protection by DQB1*0602 is dominant.
The fifth hypothesis, the presentation of both self-peptides and microbial peptides that activate self-reactive T cells will be discussed in detail in the next section. Before covering this topic, we will discuss how self-reactive TCRs recognize self-peptide/MHC complexes.
1.3 TCR recognition of self-peptides that bind MHC with very low affinity
How do self-reactive TCRs recognize their target peptide-MHC complexes? A number of crystal structures are now available which show significant structural alterations in all cases that have been studied to date. These structural alterations either result in suboptimal interactions of the peptide with the MHC molecule and/or suboptimal recognition of the self-peptide/MHC complex by the TCR. It is thought that these structural alterations facilitate escape from negative selection in the thymus.
A particularly interesting case is the N-terminal epitope of MBP (Ac1-11) which causes severe EAE in mice with the H-2u haplotype [13]. This peptide binds with a very low affinity to I-Au and has a half-life of <15 minutes [26]. In contrast, a C-terminal MBP peptide (residues 121–140) binds stably, with a long half-life of 144 hours [26]. Structural studies showed that the Ac1-11 peptide only partially fills the I-Au binding site. Furthermore, the fourth residue of the MBP peptide is placed in a structurally incompatible pocket (P6 pocket) of the I-Au molecule; substitution of this peptide residue dramatically increases its affinity for I-Au [27]. Structures of the tri-molecular complex have now been determined for three TCRs that recognize this MBP Ac1-11 peptide [28; 29]. These structures showed that TCR interactions with this self-peptide were also impaired by partial occupancy of the binding groove. In all three cases, only the two CDR3 loops made contacts to the peptide, but not the CDR1 loops which typically also contribute to peptide specificity. Partial occupancy of the peptide binding groove therefore not only affected the stability of the peptide-MHC complex, but also reduced potential peptide contacts with TCRs. Interestingly, this MBP epitope is only immunodominant in mice that express MBP. In MBP deficient mice, a cluster of epitopes in the C-terminal region becomes immunodominant because these peptides have a substantially higher affinity for I-Au, as discussed above [26]. MBP Ac1-11 specific T cells therefore appear to escape negative selection based on the abnormalities of the tri-molecular complex discussed above.
Recently, an even more drastic situation was reported for the chromogranin A peptide which is the autoantigen for the BDC2.5 and related T cell clones that cause type 1 diabetes upon transfer into young NOD mice. Experiments with mimic peptides had shown that a tryptophan residue at the P5 position of the peptide is essential for TCR recognition. In the chromogranin A peptide, the 14 amino acid epitope actually starts with the P5 position which usually sits in the center of the peptide-MHC binding groove [30]. A structure is not yet available, but it is thought that half of the peptide binding groove is empty because P5 tryptophan is the first residue. Contacts between the C-terminal part of the peptide and I-Ag7 outside of the groove appear to stabilize binding of this epitope, but its binding affinity is nevertheless low. This chromogranin A peptide appears to be generated in a pre-processed form in the secretory granules of pancreatic beta cells [30], and the peptide should thus be able to bind at the cell surface without a requirement for peptide processing in an endosomal compartment.
1.4 Self-reactive T cell receptors with unusual binding topologies
However, not all self-peptides bind to their peptide-MHC target with a low affinity. When peptides are bound with an intermediate or high affinity, other structural abnormalities have been observed in the tri-molecular complex.
Two crystal structures of human MBP-specific TCRs from MS patients have been reported, and in both cases abnormalities in the topology of TCR binding were observed. The Ob.1A12 TCR originated from a patient with relapsing-remitting MS and is specific for the MBP (85–99) peptide bound to HLA-DR2 (DRB1*1501), a HLA-DR molecule strongly associated with susceptibility to the disease [31]. Transgenic mice that co-expressed this human TCR and HLA-DR2 developed a spontaneous inflammatory and demyelinating disease in the CNS, demonstrating that this TCR has the potential to be pathogenic [32]. The crystal structure showed a highly unusual TCR binding topology in which the TCR was shifted towards the peptide N-terminus and the DRβ chain helix. As a result, the CDR3 loops were not positioned over the central P5 residue, but rather over the P2 histidine (Figure 2A, B). Furthermore, the TCR showed an unusual counterclockwise rotation that changed the interaction of the TCRβ chain with the MHC molecule [33].
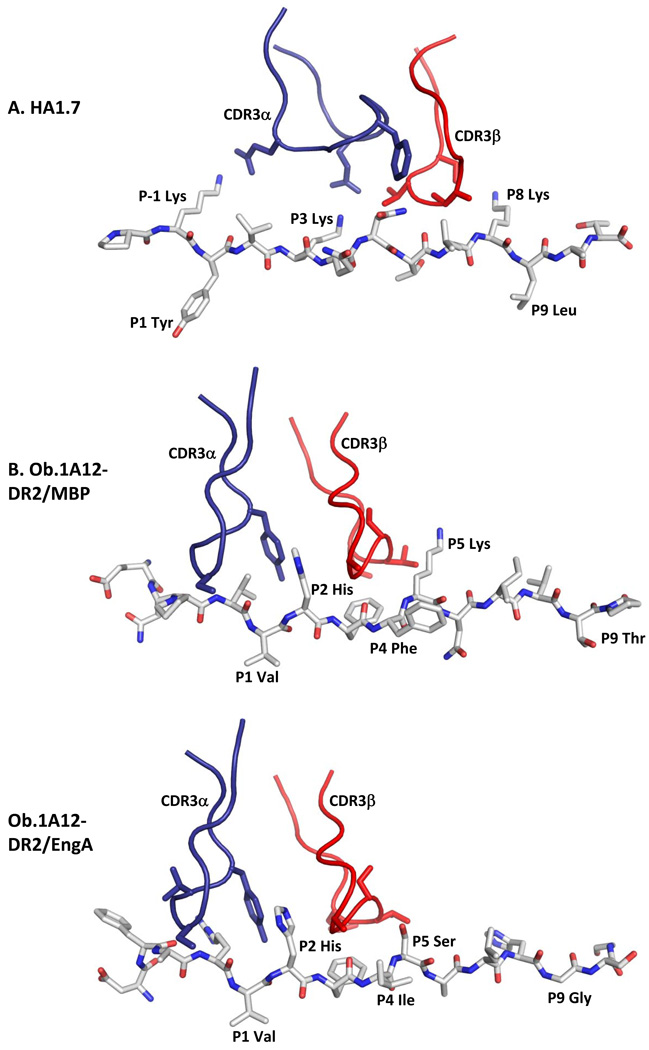
Figure 2. Ob.1A12 TCR with MBP and engA peptides.
A. Recognition of the influenza hemagglutinin (306–318) peptide by the human HA1.7 TCR. Shown are the TCR CDR3 loops, CDR3α in blue and CDR3β in red, as well as the peptide as a stick model. Peptide residues representing key TCR contacts are labeled, as well as peptide residues occupying key pocket of the MHC molecule (HLA-DR1).
B. Recognition of the self-peptide MBP (85–99) by the Ob.1A12 TCR isolated from a patient with relapsing-remitting MS. The CDR3 loops of this TCR are focused over the P2 peptide position (P2 His), rather than the P5 position as for HA1.7 TCR. Key TCR contacts of Ob.1A12 TCR are P2 His, P3 Phe and P5 Lys (ENPVVHFFKNIVTPR), key HLA-DR2 binding residues are P1 Val and P4 Phe.
C. Recognition of a cross-reactive microbial peptide by Ob.1A12 TCR. This TCR cross-reacts with the engA peptide of E. coli and Hemophilus influenzae (DFARVHFISALHGSG) which shares the P2 His and P3 Phe residues with the MBP peptide (bold, underlined). The peptide also has hydrophobic anchor residues for the P1 and P4 pockets (P1 Val and P4 Ile), but is otherwise quite distinct from the MBP peptide. Nevertheless, the binding of Ob.1A12 TCR to the two peptides is very similar (compare B and C).
The 3A6 TCR also showed a shift towards the peptide N-terminus so that the CDR3 loops were again centered over the P2 residue, as well as a shift towards the DRβ chain helix [34]. However, this TCR did not have the same unusual counterclockwise rotation as Ob.1A12 TCR. The N-terminal position may represent a suboptimal TCR binding site because both TCRs bound with very low affinity to their self-peptide/MHC complex. It is thought that these unusual binding modes facilitated escape from negative selection, analogous to murine T cells specific for the MBP Ac1-11 peptide.
Section 2. Activation of self-reactive T cells by microbial peptides
2.1 The discovery of TCR poly-specificity
The idea that microbial peptides drive the initial clonal expansion of self-reactive T cells has been vigorously pursued by the field because the context of an infection provides appropriate secondary signals, including signals through innate immune receptors and cytokine receptors, required for differentiation into self-reactive effector T cell populations with pathogenic potential. In contrast, antigen presentation in the absence of such inflammatory signals is known to induce T cell tolerance [35]. In early studies, the sequence of the entire self-peptide was used to search databases for microbial peptides with substantial linear sequence identity/similarity. Using this approach, Fujinami and Oldstone identified a hepatitis B peptide in which six consecutive amino acids were identical to the encephalitogenic T cell epitope of rabbits. Immunization with this hepatitis B peptide induced histological signs of EAE, providing first experimental support for the concept of molecular mimicry [36]. Molecular mimicry refers to the activation of self-reactive T cells by a microbial peptide with sequence similarity to the self-peptide.
At that time, T cells were thought to be highly specific for a particular antigen and such cross-reactivity was thought to be a rather rare occurrence. However, characterization of the structural requirements for peptide binding by MHC class II molecules demonstrated that these binding motifs were in fact quite degenerate [37]. This meant that for each peptide residue positioned in a particular pocket of the peptide binding groove several amino acids were compatible for binding. For example, the P1 pocket of the MS associated HLA-DR2 molecule required an aliphatic (valine, leucine, isoleucine, methionine) or a small aromatic (phenylalanine) amino acid [31]. Also, only a limited number of peptide positions were found to be important for TCR recognition [31; 38].
Both MHC binding and TCR recognition motifs were identified for human T cell clones that recognized the MBP (85–99) peptide bound to HLA-DR2 [31]. Definition of these dual structural requirements for T cell activation enabled a novel approach for the identification of microbial peptides which activate self-reactive T cells [39]. In an initial study, 129 microbial peptides were synthesized and tested against a panel of seven T cell clones from MS patients. A total of eight microbial peptides were identified that could activate particular MBP-specific T cell clones, seven viral and one bacterial peptide. Interestingly, only one of these peptides had obvious sequence similarity with the MBP self-peptide, while the other peptides were quite distinct from MBP and each other [39]. Activating microbial peptides were discovered for three of the seven clones tested in this initial study; stimulatory peptides for two of the remaining clones were identified in a follow-up study [40]. This report thus showed that a T cell clone can recognize a number of different peptides with limited similarity to each other. This concept was later coined poly-specificity to describe two salient features: the recognition of multiple peptides by a TCRs and the specificity with which such recognition occurs [41]. Furthermore, a MBP specific T cell clone that recognized the MBP and an EBV DNA polymerase peptide was shown to be activated by EBV transformed B cells in which expression of the DNA polymerase had been induced [39]. These results indicated that activation of self-reactive T cell cells by microbial peptides is not necessarily a rare event and that multiple peptides/pathogens could be involved in the clonal expansion of self-reactive T cells specific for one self-peptide.
A similar line of reasoning was used by Evavold and colleagues to identify endogenous ligands for murine T cells specific for a hemoglobin peptide. The hemoglobin (64–78) peptide was found to have two prominent MHC anchors and one primary TCR contact residue. This TCR contact residue had to be preserved, but many other changes in the peptide were tolerated (albeit with reduction in potency). Search of a rather small subset of rodent proteins (n=3,178) led to identification of two endogenous peptides that activated one of these T cell clones; one of the peptides was an agonist, the other a partial agonist [42].
Synthetic combinatorial peptide libraries have emerged as a powerful tool for the identification of multiple distinct peptide ligands for human/murine CD4 and CD8 T cell clones [43; 44]. For each position, a set of 20 libraries is synthesized in which one of the 20 naturally occurring amino acids occupies a defined position while all other positions are synthesized with amino acid mixtures. All positions of a 9-mer peptide can thus be interrogated with a set of 180 mixtures (9×20). Incorporation of defined amino acids in the active mixtures permitted synthesis of super-agonist peptide ligands for a variety of T cell clones that are active at substantially lower peptide concentrations than the peptide used to isolate the T cell clone. Furthermore, protein databases searches led to the identification of alternative self or microbial peptides for these clones [45].
These libraries are composed of complex mixtures. Individual peptides are present at concentrations that are far too low to induce T cell activation. The estimated number of stimulating ligands differs by several orders of magnitude depending on the activity assumed for an individual peptide, for example 2×103 versus 2×106 stimulatory peptides for a 9-mer library depending on whether peptides are assumed to have an average activity of 10 pM or 10 nM. Since both weak and strong ligands are likely to contribute to activation, the precise number cannot be determined with certainty. It is, however, evident that the number of peptides contributing to T cell activation by such libraries is large. The specificity of TCR recognition is also reflected by this analysis because the fraction of stimulatory peptide ligands in these highly complex libraries is still likely to be small.
2.2 How do TCRs recognize multiple different peptides?
Intriguing studies on antibodies have shown that antibody multi-specificity can be mediated by conformational diversity. Linus Pauling predicted in 1940 that antibodies exist as an ensemble of pre-existing antibody conformations from which specific binding configurations are selected [46]. Particularly interesting are the combined structural and kinetic studies on an antibody selected for binding to the hapten dinitrophenol [47]. This hapten was recognized with a high degree of specificity because closely related analogs were not bound. However, this antibody also bound two completely unrelated haptens (alizarin red and furazolidone) as well as a protein antigen. Structural studies identified two conformations of the antigen binding site prior to ligand binding with entirely unrelated CDR3 loop (L3 and H3) conformations, as well as two additional conformations when either a hapten or the protein antigen was bound. The hapten-bound antibody showed a deep binding pocket, while the protein-bound antibody had a rather flat binding surface. The hapten-bound state was related to one of the free antibody conformations, suggesting that hapten binding occurred through this pre-existing isomer [47].
During positive selection in the thymus, T cells need to recognize endogenous thymic self-peptides; failure to recognize such peptides on the surface of cortical thymic epithelial cells results in apoptosis. The crystal structure of the 2C TCR bound to the H-2Kb/dEV8 self-peptide complex showed important principles for TCR recognition of such low affinity ligands [48]. The interface between the TCR and the peptide exhibited extremely poor shape complementarity and the central hydrophobic TCR pocket formed by the two CDR3 loops was not occupied by a peptide side chain. These structural features were consistent with the low affinity of this complex (~100 µM). This structure also showed large differences in the position of TCR loops, in particular the CDR3 loops, between bound and free TCRs. Large differences in the position of three CDRs were observed, in particular for the CDR3α loop [48].
A large conformational change for the CDR3β loop was also described for the KB5-C20 TCR between the free and bound structures [49]. Particularly interesting is the case of the BM3.3 TCR which recognizes two different peptides with no sequence identity bound to H-2Kb. There was a large difference in the conformation of the CDR3α loop that enabled recognition of these two peptides [50]. In general, the CDR3 loops are longer and more flexible that the germline-encoded CDR1 and CDR2 loops which contact the MHC helices, explaining how multiple conformations of the CDR3 loops can enable recognition of different peptides [48; 51].
Even more distinct binding solutions are possible when a TCR recognizes two different peptide-MHC complexes. An excellent example is the comparison of 2C TCR bound to the H-2Kb-dEV8 peptide complex and the allo-reactive H-2Ld-QL9 peptide complex [52]. The dEV8 and QL9 peptides are unrelated in sequence and the MHC α1–α2 domains of H-2Kb and H-2Ld differ by 31 residues, of which 14 polymorphic residues are located on the MHC helices. The 2C binding orientations on the syngeneic and the allogeneic peptide-MHC were found to be highly divergent. Comparison of the binding mode for the H-2Ld-QL9 and H-2Kb-dEV8 complexes showed a counterclockwise rotation of the Vβ domain by 15° and the Vα domain by 30°; also the 2C TCR was translated laterally towards the peptide C-terminus by approximately 3Å. These rotational and translational shifts resulted in alternative contacts of 2C TCR with these two peptide-MHC complexes. In addition, the shape complementarity was significantly higher for the H-2Ld-QL9 complex (Sc = 0.7) compared to the H-2Kb-dEV8 (Sc = 0.41). Thus, a TCR can recognize different peptide-MHC complexes using different binding orientations that result in distinct interface chemistries.
Cross-reactivity can also be the result of structural mimicry of a binding hotspot shared by self and microbial antigens. This principle was illustrated using the Ob.1A12 TCR which was already discussed above. This TCR is activated by the MBP (85–99) peptide bound to HLA-DR2 as well as several microbial peptides, including a peptide from the engA protein expressed by a variety of bacteria, including E. coli and H. influenzae. The MBP and engA peptides share the two principal TCR contact residues of Ob.1A12 TCR, P2 His and P3 Phe. Sequence identity in this binding hotspot resulted in a very similar binding topology in crystal structures with either MBP or engA peptides (Figure 2B, C) [53].
2.3 TCR crossreactivity and immunopathology
Several different experimental autoimmune diseases have been induced by immunization with microbial peptides, indicating that crossreactive T cell populations can be pathogenic. EAE has been induced in different strains of mice and in Lewis rats with mimicry peptides of MBP and myelin oligodendrocyte glycoprotein [54; 55; 56; 57; 58]. Such experiments have also been performed in humanized mice that express HLA-DR2 and the Ob.1A12 TCR, and the E. coli/H. influenzae engA peptide was shown to induce CNS inflammation and demyelination [53]. Clinical disease was also induced by three other bacterial mimic peptides in transgenic mice that co-expressed HLA-DR2 and the Ob.1A12 TCR. [59].
An important question is whether an autoimmune disease can also result from infection with pathogens that carry such T cell epitopes. To address this question, Olson et al. [60] generated recombinant Theiler’s viruses in which the candidate sequences were placed into the leader segment of the virus. The first recombinant virus carried the sequence of the PLP (139–151) peptide which is immunodominant in SJL mice; infection with this virus resulted in the rapid development of CNS inflammation and vigorous CD4 T cell responses to the PLP peptide. It is important to note that the wild-type Theiler’s virus also induced CNS pathology, but disease onset was significantly later (day 30, rather than day 10) permitting the two disease states to be distinguished. Also, tolerance induction with the PLP (139–151) peptide prevented induction of the early disease process by the PLP (139–151) expressing virus, but not the late disease caused by wild-type Theiler’s virus [61]. Importantly, CNS autoimmunity was not only induced by a virus that carried the self peptide, but also by a recombinant virus that expressed a mimic peptide from Hemophilus influenzae which stimulated PLP (139–151) specific T cells [60]. The recombinant virus with the H. influenzae epitope induced a Vβ19+ T cell population that was not expanded by PLP (139–151), indicating that microbial mimics can induce distinct self-reactive T cell populations [62]. Recombinant viruses that expressed other PLP (139–151) mimic peptide also induced CNS inflammation [63].
The diverse nature of the viral/bacterial peptides that stimulate autoreactive T cell clones suggests that different infectious agents could initiate autoimmunity by molecular mimicry. However, it is important to keep in mind that a number of other mechanisms could also result in the activation of autoreactive T cells. The diverse nature of the mimicry peptides and the ubiquitous presence of some of these pathogens may make it difficult to establish a direct epidemiological link between infectious agents and the occurrence of certain autoimmune diseases. In particular, the temporal relationship between an infection and development of an autoimmune process may in many cases not be clear because of the time that frequently elapses until clinical symptoms become obvious and a diagnosis is made. Such epidemiological relationships may be more readily established for autoimmune disorders with a rapid disease onset since early diagnosis can greatly increase the likelihood of establishing a link with a preceding infection.
Such a relationship has been established between an inflammatory, demyelinating disease of the peripheral nervous system (Guillain-Barré syndrome) and preceding infections [64]. Patients with this disease acutely develop severe symptoms and rapid diagnosis permitted isolation of Campylobacter jejuni from approximately a third of new cases, compared to 2% of household controls. A number of studies have demonstrated that patients with GBS develop antibodies specific for lipopolysaccharides (LPS) of certain strains of Camplyobacter jejuni which crossreact with gangliosides from peripheral nerves. Gangliosides are membrane-anchored glycosphingolipids with a hydrophilic extracellular oligosaccharide. The outer polysaccharide moieties of LPS from certain strains of Campylobacter bear striking structural similarities with gangliosides found in peripheral nerves [65].
2.4 Protective role of TCR cross-reactivity in chronic viral infections
These results pose another important question: can TCR cross-reactivity also be beneficial, for example in fighting infectious agents? Before discussing recent studies on this issue, it is necessary to introduce some basic concepts on thymic selection. Intriguing studies by Huseby and colleagues showed that mice in which negative selection in the thymus was severely limited harbored substantial numbers of T cells that were highly cross-reactive [66]. They utilized mice that expressed only a single MHC class II molecule with a covalently tethered peptide. Negative selection was severely impaired in these mice because only a single I-Ab + peptide combination was present in the thymus. Many of the CD4 T cells in these mice were highly cross-reactive against many analogs of an immunizing peptide and also resistant to many mutations on the MHC helices. As a consequence, these CD4 T cells were self-reactive when confronted with APC from wild-type mice and allo-reactive to many MHC class II alleles. The crystal structure of a highly cross-reactive TCR (YAe62) showed a shrinking, increasingly hydrophobic TCR ligand surface involving fewer TCR amino acids compared to TCRs from WT mice [67]. The reduced interaction surface with both MHC and peptide explained the high degree of peptide and MHC cross-reactivity of this TCR.
The central concept from these studies was recently shown to be relevant for understanding why particular MHC class I alleles, namely HLA-B57 and HLA-B27, confer improved protection from HIV. Rare individuals infected with HIV (‘elite controllers”) maintain very low levels of HIV RNA without therapy, and certain HLA class I alleles are markedly enriched in elite controllers, with the highest association observed for HLA-B57. They found that HLA-B57 selects a naïve T cell repertoire in which a larger fraction of CD8 T cells recognized a viral epitope, and that these T cells were also more cross-reactive to mutants of the targeted epitopes [68]. Such cross-reactivity towards mutants of the original viral epitope is biologically important because the RNA genome of HIV undergoes rapid mutation, leading to the emergence of escape mutants. Such epitope loss is one of the principal reasons for progression to AIDS.
Why are CD8 T cells selected by HLA-B57 more cross-reactive? Accurate prediction algorithms for binding of peptides to this allele showed that it bound a smaller number of self-peptides from the human proteome (~70,000 of 107 unique peptide sequences), while a non-protective allele (HLA-B*0701) presented a significantly larger number of self-peptides (180,000). A similar correlation was observed for the protective allele in macaques (Mamu-B*17) which binds 4–13 times fewer self-peptides than three other alleles which confer less protection. Modeling data suggested that a reduced number of self-peptides presented in the thymus results in more cross-reactive T cells [68]. Indeed, peripheral blood mononuclear cells from patients expressing HLA-B57 contained CTL that were more cross-reactive to various HIV epitopes and their point mutants than those of HLA-B8 positive patients [69]. HLA-B8 is associated with rapid progression to disease and is predicted to bind a greater diversity of self-peptides that HLA-B57. Also, in people with a protective HLA allele, the initial T cell response to HIV is dominated by T cells restricted by the protective HLA allele [70]. Two mechanisms thus improve protection by HLA-B57 against HIV: there is a larger precursor frequency of HIV specific T cells in the repertoire of subjects that express HLA-B57 and these T cells recognize point mutants of HIV epitopes more effectively, making them more resistant to viral escape. Interestingly, HLA-B57 is also protective against hepatitis C virus (HCV), another virus with a high mutation rate, suggesting that this principle may be general [71].
This higher degree of TCR cross-reactivity is also related to a higher risk for the development of autoimmune diseases. HLA-B57 has been associated with autoimmune psoriasis and hypersensitivity reactions [72; 73]. HLA-B27 is also associated with improved protection from HIV and CD8 T cells restricted by this allele appear to have a higher degree of cross-reactivity [68]. HLA-B27 is associated with a substantially increased risk for a chronic inflammatory joint disease, ankylosing spondylitis [74].
These results show that TCR cross-reactivity, while increasing the risk for autoimmune diseases, can improve protection from rapidly mutating viruses that establish chronic infection. It will be interesting to determine whether MHC class II molecules that confer an increased risk for particular autoimmune diseases have similar properties to HLA-B57 and HLA-B27.
Table 1.
Proposed mechanisms for MHC-linked susceptibility to autoimmune diseases
| (1) Disease-associated polymorphisms enable the presentation of key self-peptide(s) in the target organ of the disease |
| (2) Disease-associated polymorphisms result in poor presentation of critical epitopes and thereby favor escape from thymic tolerance mechanisms |
| (3) Key polymorphisms exert effects on the T cell repertoire important in disease initiation and/or amplification |
| (4) Protective MHC molecules select regulatory T cells with specificity for the target organ |
| (5) Disease-associated MHC molecules present not only relevant self-peptides, but also microbial peptides that enable expansion and activation of relevant self-reactive T cells |
Acknowledgements
We would like to thank collaborators, postdoctoral fellows and students who have contributed to studies described here. This work was supported by NIH grants to K.W.W. (PO1 AI045757 and R01 AI054520). D.S. was supported by a fellowship from the National Multiple Sclerosis Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009 doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKay K, Eyre S, Myerscough A, Milicic A, Barton A, Laval S, Barrett J, Lee D, White S, John S, Brown MA, Bell J, Silman A, Ollier W, Wordsworth P, Worthington J. Whole-genome linkage analysis of rheumatoid arthritis susceptibility loci in 252 affected sibling pairs in the United Kingdom. Arthritis Rheum. 2002;46:632–639. doi: 10.1002/art.10147. [DOI] [PubMed] [Google Scholar]
- 4.Oksenberg JR, Barcellos LF, Cree BA, Baranzini SE, Bugawan TL, Khan O, Lincoln RR, Swerdlin A, Mignot E, Lin L, Goodin D, Erlich HA, Schmidt S, Thomson G, Reich DE, Pericak-Vance MA, Haines JL, Hauser SL. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet. 2004;74:160–167. doi: 10.1086/380997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lincoln MR, Ramagopalan SV, Chao MJ, Herrera BM, Deluca GC, Orton SM, Dyment DA, Sadovnick AD, Ebers GC. Epistasis among HLA-DRB1, HLA-DQA1, and HLA-DQB1 loci determines multiple sclerosis susceptibility. Proc Natl Acad Sci U S A. 2009;106:7542–7547. doi: 10.1073/pnas.0812664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 7.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 8.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healy BC, Liguori M, Tran D, Chitnis T, Glanz B, Wolfish C, Gauthier S, Buckle G, Houtchens M, Stazzone L, Khoury S, Hartzmann R, Fernandez-Vina M, Hafler DA, Weiner HL, Guttmann CR, De Jager PL. HLA B*44: protective effects in MS susceptibility and MRI outcome measures. Neurology. 75:634–640. doi: 10.1212/WNL.0b013e3181ed9c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Auwera BJ, Schuit FC, Weets I, Ivens A, Van Autreve JE, Gorus FK. Relative and absolute HLA-DQA1-DQB1 linked risk for developing type I diabetes before 40 years of age in the Belgian population: implications for future prevention studies. Hum Immunol. 2002;63:40–50. doi: 10.1016/s0198-8859(01)00362-7. [DOI] [PubMed] [Google Scholar]
- 11.Hermann R, Turpeinen H, Laine AP, Veijola R, Knip M, Simell O, Sipila I, Akerblom HK, Ilonen J. HLA DR-DQ-encoded genetic determinants of childhood-onset type 1 diabetes in Finland: an analysis of 622 nuclear families. Tissue Antigens. 2003;62:162–169. doi: 10.1034/j.1399-0039.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 12.Wucherpfennig KW. Insights into autoimmunity gained from structural analysis of MHC-peptide complexes. Curr Opin Immunol. 2001;13:650–656. doi: 10.1016/s0952-7915(01)00274-6. [DOI] [PubMed] [Google Scholar]
- 13.Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 14.Kuchroo VK, Sobel RA, Yamamura T, Greenfield E, Dorf ME, Lees MB. Induction of experimental allergic encephalomyelitis by myelin proteolipid-protein-specific T cell clones and synthetic peptides. Pathobiology. 1991;59:305–312. doi: 10.1159/000163668. [DOI] [PubMed] [Google Scholar]
- 15.Hammer J, Gallazzi F, Bono E, Karr RW, Guenot J, Valsasnini P, Nagy ZA, Sinigaglia F. Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med. 1995;181:1847–1855. doi: 10.1084/jem.181.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dessen A, Lawrence CM, Cupo S, Zaller DM, Wiley DC. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity. 1997;7:473–481. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- 17.Wucherpfennig KW, Yu B, Bhol K, Monos DS, Argyris E, Karr RW, Ahmed AR, Strominger JL. Structural basis for major histocompatibility complex (MHC)-linked susceptibility to autoimmunity: charged residues of a single MHC binding pocket confer selective presentation of self-peptides in pemphigus vulgaris. Proc Natl Acad Sci U S A. 1995;92:11935–11939. doi: 10.1073/pnas.92.25.11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987;84:2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 22.Corper AL, Stratmann T, Apostolopoulos V, Scott CA, Garcia KC, Kang AS, Wilson IA, Teyton L. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 23.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci U S A. 107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hovhannisyan Z, Weiss A, Martin A, Wiesner M, Tollefsen S, Yoshida K, Ciszewski C, Curran SA, Murray JA, David CS, Sollid LM, Koning F, Teyton L, Jabri B. The role of HLA-DQ8 beta57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature. 2008;456:534–538. doi: 10.1038/nature07524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 26.Harrington CJ, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, Beeson C, Goverman J. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- 27.He XL, Radu C, Sidney J, Sette A, Ward ES, Garcia KC. Structural snapshot of aberrant antigen presentation linked to autoimmunity: the immunodominant epitope of MBP complexed with I-Au. Immunity. 2002;17:83–94. doi: 10.1016/s1074-7613(02)00340-0. [DOI] [PubMed] [Google Scholar]
- 28.Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction 'codon'. Nat Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- 29.Maynard J, Petersson K, Wilson DH, Adams EJ, Blondelle SE, Boulanger MJ, Wilson DB, Garcia KC. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wucherpfennig KW, Sette A, Southwood S, Oseroff C, Matsui M, Strominger JL, Hafler DA. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994;179:279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen LS, Andersson EC, Jansson L, Krogsgaard M, Andersen CB, Engberg J, Strominger JL, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L. A humanized model for multiple sclerosis using HLA-DR2 and a human T- cell receptor. Nat Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 33.Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat Immunol. 2005;6:490–496. doi: 10.1038/ni1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. Embo J. 2005;24:2968–2979. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 37.Sinigaglia F, Hammer J. Defining rules for the peptide-MHC class II interaction. Curr Opin Immunol. 1994;6:52–56. doi: 10.1016/0952-7915(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 38.Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93–103) J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 39.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hausmann S, Martin M, Gauthier L, Wucherpfennig KW. Structural features of autoreactive TCR that determine the degree of degeneracy in peptide recognition. J Immunol. 1999;162:338–344. [PubMed] [Google Scholar]
- 41.Wucherpfennig KW, Allen PM, Celada F, Cohen IR, De Boer R, Garcia KC, Goldstein B, Greenspan R, Hafler D, Hodgkin P, Huseby ES, Krakauer DC, Nemazee D, Perelson AS, Pinilla C, Strong RK, Sercarz EE. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19:216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evavold BD, Sloan-Lancaster J, Wilson KJ, Rothbard JB, Allen PM. Specific T cell recognition of minimally homologous peptides: evidence for multiple endogenous ligands. Immunity. 1995;2:655–663. doi: 10.1016/1074-7613(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 43.Nino-Vasquez JJ, Allicotti G, Borras E, Wilson DB, Valmori D, Simon R, Martin R, Pinilla C. A powerful combination: the use of positional scanning libraries and biometrical analysis to identify cross-reactive T cell epitopes. Mol Immunol. 2004;40:1063–1074. doi: 10.1016/j.molimm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Pinilla C, Martin R, Gran B, Appel JR, Boggiano C, Wilson DB, Houghten RA. Exploring immunological specificity using synthetic peptide combinatorial libraries. Curr Opin Immunol. 1999;11:193–202. doi: 10.1016/s0952-7915(99)80033-8. [DOI] [PubMed] [Google Scholar]
- 45.Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus SE, McFarland HF, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- 46.Pauling L. A theory of the structure and process of formation of antibodies. J. Am. Chem. Soc. 1940;62:2643. [Google Scholar]
- 47.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 48.Garcia KC, Degano M, Pease LR, Huang M, Peterson PA, Teyton L, Wilson IA. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 49.Reiser JB, Gregoire C, Darnault C, Mosser T, Guimezanes A, Schmitt-Verhulst AM, Fontecilla-Camps JC, Mazza G, Malissen B, Housset D. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 50.Reiser JB, Darnault C, Gregoire C, Mosser T, Mazza G, Kearney A, van der Merwe PA, Fontecilla-Camps JC, Housset D, Malissen B. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat Immunol. 2003;4:241–247. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 51.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2 [comment] Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 52.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 53.Harkiolaki M, Holmes SL, Svendsen P, Gregersen JW, Jensen LT, McMahon R, Friese MA, van Boxel G, Etzensperger R, Tzartos JS, Kranc K, Sainsbury S, Harlos K, Mellins ED, Palace J, Esiri MM, van der Merwe PA, Jones EY, Fugger L. T cell-mediated autoimmune disease due to low-affinity crossreactivity to common microbial peptides. Immunity. 2009;30:348–357. doi: 10.1016/j.immuni.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Ufret-Vincenty RL, Quigley L, Tresser N, Pak SH, Gado A, Hausmann S, Wucherpfennig KW, Brocke S. In vivo survival of viral antigen-specific T cells that induce experimental autoimmune encephalomyelitis. J Exp Med. 1998;188:1725–1738. doi: 10.1084/jem.188.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grogan JL, Kramer A, Nogai A, Dong L, Ohde M, Schneider-Mergener J, Kamradt T. Cross-reactivity of myelin basic protein-specific T cells with multiple microbial peptides: experimental autoimmune encephalomyelitis induction in TCR transgenic mice. J Immunol. 1999;163:3764–3770. [PubMed] [Google Scholar]
- 56.Gautam AM, Liblau R, Chelvanayagam G, Steinman L, Boston T. A viral peptide with limited homology to a self peptide can induce clinical signs of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:60–64. [PubMed] [Google Scholar]
- 57.Lenz DC, Lu L, Conant SB, Wolf NA, Gerard HC, Whittum-Hudson JA, Hudson AP, Swanborg RH. A Chlamydia pneumoniae-specific peptide induces experimental autoimmune encephalomyelitis in rats. J Immunol. 2001;167:1803–1808. doi: 10.4049/jimmunol.167.3.1803. [DOI] [PubMed] [Google Scholar]
- 58.Mokhtarian F, Zhang Z, Shi Y, Gonzales E, Sobel RA. Molecular mimicry between a viral peptide and a myelin oligodendrocyte glycoprotein peptide induces autoimmune demyelinating disease in mice. J Neuroimmunol. 1999;95:43–54. doi: 10.1016/s0165-5728(98)00254-9. [DOI] [PubMed] [Google Scholar]
- 59.Greene MT, Ercolini AM, DeGutes M, Miller SD. Differential induction of experimental autoimmune encephalomyelitis by myelin basic protein molecular mimics in mice humanized for HLA-DR2 and an MBP(85–99)-specific T cell receptor. J Autoimmun. 2008;31:399–407. doi: 10.1016/j.jaut.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson JK, Croxford JL, Calenoff MA, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. J Clin Invest. 2001;108:311–318. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson JK, Eagar TN, Miller SD. Functional activation of myelin-specific T cells by virus-induced molecular mimicry. J Immunol. 2002;169:2719–2726. doi: 10.4049/jimmunol.169.5.2719. [DOI] [PubMed] [Google Scholar]
- 62.Ercolini AM, Miller SD. Molecular mimics can induce novel self peptide-reactive CD4+ T cell clonotypes in autoimmune disease. J Immunol. 2007;179:6604–6612. doi: 10.4049/jimmunol.179.10.6604. [DOI] [PubMed] [Google Scholar]
- 63.Ercolini AM, Ludovic Croxford J, Degutes M, Miller SD. Cross-reactivity between peptide mimics of the immunodominant myelin proteolipid protein epitope PLP139-151: comparison of peptide priming in CFA vs. viral delivery. J Neuroimmunol. 2007;186:5–18. doi: 10.1016/j.jneuroim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barre syndrome [see comments] N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 65.Moran AP. Structure and conserved characteristics of Campylobacter jejuni lipopolysaccharides. J Infect Dis. 1997;176 Suppl 2:S115–S121. doi: 10.1086/513781. [DOI] [PubMed] [Google Scholar]
- 66.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 67.Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, Pereyra F, Carrington M, Walker BD, Chakraborty AK. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 465:350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turnbull EL, Lopes AR, Jones NA, Cornforth D, Newton P, Aldam D, Pellegrino P, Turner J, Williams I, Wilson CM, Goepfert PA, Maini MK, Borrow P. HIV-1 epitope-specific CD8+ T cell responses strongly associated with delayed disease progression cross-recognize epitope variants efficiently. J Immunol. 2006;176:6130–6146. doi: 10.4049/jimmunol.176.10.6130. [DOI] [PubMed] [Google Scholar]
- 70.Altfeld M, Kalife ET, Qi Y, Streeck H, Lichterfeld M, Johnston MN, Burgett N, Swartz ME, Yang A, Alter G, Yu XG, Meier A, Rockstroh JK, Allen TM, Jessen H, Rosenberg ES, Carrington M, Walker BD. HLA Alleles Associated with Delayed Progression to AIDS Contribute Strongly to the Initial CD8(+) T Cell Response against HIV-1. PLoS Med. 2006;3:e403. doi: 10.1371/journal.pmed.0030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thio CL, Gao X, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O'Brien SJ, Karacki P, Astemborski J, Carrington M, Thomas DL. HLA-Cw* 04 and hepatitis C virus persistence. J Virol. 2002;76:4792–4797. doi: 10.1128/JVI.76.10.4792-4797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhalerao J, Bowcock AM. The genetics of psoriasis: a complex disorder of the skin and immune system. Hum Mol Genet. 1998;7:1537–1545. doi: 10.1093/hmg/7.10.1537. [DOI] [PubMed] [Google Scholar]
- 73.Chessman D, Kostenko L, Lethborg T, Purcell AW, Williamson NA, Chen Z, Kjer-Nielsen L, Mifsud NA, Tait BD, Holdsworth R, Almeida CA, Nolan D, Macdonald WA, Archbold JK, Kellerher AD, Marriott D, Mallal S, Bharadwaj M, Rossjohn J, McCluskey J. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822–832. doi: 10.1016/j.immuni.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 74.Brown MA. Genetics of ankylosing spondylitis. Curr Opin Rheumatol. 22:126–132. doi: 10.1097/BOR.0b013e3283364483. [DOI] [PubMed] [Google Scholar]