Abstract
Background
The impact of abnormal spirometric findings on risk for incident heart failure among older adults without clinically apparent lung disease is not well elucidated.
Methods
We evaluated the association of baseline lung function with incident heart failure, defined as first hospitalization for heart failure, in 2125 participants of the community-based Health, Aging, and Body Composition Study (age, 73.6±2.9 years; 50.5% men; 62.3% white; 37.7% black) without prevalent lung disease or heart failure. Abnormal lung function was defined either as forced vital capacity (FVC) or forced expiratory volume in 1st second (FEV1) to FVC ratio below lower limit of normal. Percent predicted FVC and FEV1 were also assessed as continuous variables.
Results
During follow-up (median, 9.4years), heart failure developed in 68 of 350 (19.4%) participants with abnormal baseline lung function, as compared to 172 of 1775 (9.7%) participants with normal lung function (hazard ratio [HR], 2.31; 95% confidence interval [CI], 1.74-3.07; P<.001). This increased risk persisted after adjusting for previously identified heart failure risk factors in the Health ABC Study, body mass index, incident coronary heart disease, and inflammatory markers (HR, 1.83; 95% CI, 1.33-2.50; P<.001). Percent predicted (%) FVC and FEV1 had a linear association with heart failure risk (HR, 1.21; 95%CI, 1.11-1.32 and 1.18; 95%CI, 1.10-1.26, per 10% lower %FVC and %FEV1, respectively; both P<.001 in fully adjusted models). Findings were consistent in sex and race subgroups, and for heart failure with preserved or reduced ejection fraction.
Conclusions
Abnormal spirometric findings in older adults without clinical lung disease are associated with increased heart failure risk.
Keywords (MeSH): Elderly, Epidemiology, Heart Failure, Pulmonary Function Test
Impaired lung function, as evaluated with spirometry, is encountered in more than 10% of the general population1; interestingly, 40% to 88% of patients do not have a prior diagnosis of lung disease.1 In retrospective studies from administrative data,2-5 COPD has been associated with risk for cardiovascular disease and heart failure. Also, %FVC has been proposed as a cardiovascular risk factor,6 and %FEV1 has been associated with all-cause and cardiovascular mortality.7, 8 The cardiovascular risk associated with COPD has been partially attributed to systemic inflammation.9 However, in a large cohort study,6 the increased risk in men with impaired lung function could not be entirely explained by the concomitant increase in serum inflammatory markers.
Spirometric parameters have been included in studies investigating predictors of heart failure.10, 11 However, no data exist on the impact of lung function on heart failure risk in the elderly, especially in the absence of clinically apparent lung disease. The association of lung function with heart failure risk in this population segment might be important, considering that the elderly represent a rapidly growing segment and have the highest prevalence of both abnormal lung function1 and heart failure.12 While use of spirometry as a screening tool for early diagnosis of lung disease is still a subject of debate,13, 14 whether detection of lung function abnormalities has the potential to identify elderly at risk for heart failure is unknown.
In the Health ABC Study, a community-based cohort study of well-functioning older adults, a substantial proportion had abnormal spirometric findings at baseline without clinically diagnosed lung disease.15 Using 10-year follow-up data, we investigated the association between lung function and risk for incident heart failure in participants without clinical lung disease or heart failure at baseline, explored for interaction effects of sex and race, and assessed the role of inflammation and incident coronary events.
Methods
Study Population
Health ABC is a community-based study of 3075 well-functioning men and women aged 70 to 79 years at inception. Participants were recruited in 1997-98 from a random sample of white Medicare beneficiaries and all age-eligible black residents in designated zip code areas surrounding Pittsburgh and Memphis. To be eligible, participants had to report no difficulty in walking 1/4 mile or climbing 10 stairs. Exclusion criteria included difficulties with daily activities, cognitive impairment, inability to communicate, intention of moving within 3 years, or participation in a trial involving lifestyle interventions. The institutional review boards at both sites approved the study.
For this analysis, we excluded participants with (1) history of heart failure or inconclusive or missing data on heart failure at baseline (n=140); (2) history of lung disease or inconclusive or missing data on lung disease at baseline (n=343); (3) contraindications to spirometry (n=165) or poor quality spirometric data (n=302). Lung disease was considered present when both a history of asthma or COPD and use of pulmonary medications and/or oral steroids were reported. History alone or medications alone was considered inconclusive evidence. The current analysis included 2125 participants (Figure 1).
Figure 1.
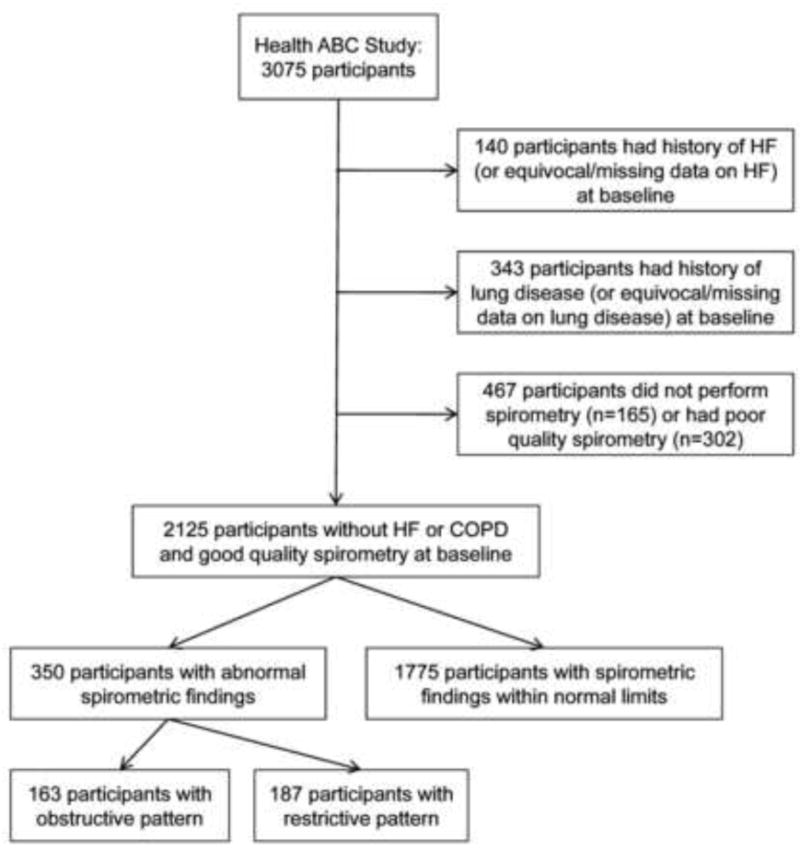
Flowchart of participant selection for the current analysis.
Risk Factor Definitions
Cardiovascular disease status (including heart failure) at baseline was based on ICD 9-CM codes as reported by Medicare Services for the years 1995-1998; self-reported history; and medications. History of coronary revascularization, electrocardiographic evidence of myocardial infarction, or history of myocardial infarction or angina accompanied by use of antianginal medications was considered evidence of coronary heart disease. Diabetes was defined as history of diabetes or use of antidiabetic medications. Hypertension was defined as use of antihypertensive medications and self-reported history or physician diagnosis of hypertension. Smoking was classified as current, past (if ≥100 lifetime cigarettes), or never. Left ventricular hypertrophy was determined from electrocardiography.16 Incident coronary heart disease was defined as (1) hospitalization for adjudicated myocardial infarction or angina, or (2) coronary revascularization.
Pulmonary Function
Spirometry was performed with a horizontal dry rolling seal spirometer (SensorMedics Corporation, Yorba Linda, CA).15 Contraindications were recent abdominal, thoracic, or ophthalmological surgery, recent myocardial infarction, or other recent cardiac illness. A 4-point scoring system was used to grade quality for FVC and FEV1 curves; a score of 2-4 indicates that the American Thoracic Society quality criteria for acceptability and reproducibility were met.17 This analysis includes only FVC and FEV1 values with quality score of 2 or higher.
Abnormal spirometric parameters were defined according to age-, sex-, and race-specific normalized reference values of NHANES III and predicted values for FVC and FEV1 were calculated from the NHANES III equations.18 Abnormal lung function was defined as %FVC or FEV1/FVC below the lower limit of normal (5th percentile), and further classified into obstructive (when FEV1/FVC was below lower limit of normal) and restrictive (when FEV1/FVC was above and %FVC was below lower limit of normal).19
Inflammatory Markers
Interleukin-6 (IL-6), tumour necrosis factor alpha (TNF-α), C-reactive protein (CRP) were available in 1906 of 2125 (89.7%) participants and were measured in duplicate by ELISA in the Core Laboratory at the University of Vermont.20
Outcome
Participants were asked to report all hospitalizations and every 6 months they were contacted to elicit information about interim events. All first admissions with an overnight stay confirmed to be related to heart failure by independent adjudication were classified as incident heart failure. Briefly, heart failure was confirmed if, in addition to physician diagnosis of heart failure, there was documentation of (1) symptoms (e.g. shortness of breath, orthopnea) and signs (e.g. edema, pulmonary crackles); (2) supporting imaging findings (e.g. pulmonary oedema on chest radiography); or (3) medical therapy for heart failure, including at least a diuretic and a vasodilator and/or digitalis. Left ventricular ejection fraction (LVEF) at heart failure diagnosis was not prospectively evaluated. Thus, information on LVEF was based on echocardiography or left ventriculography reports at time of heart failure diagnosis; LVEF data were available in 187 of 240 (77.9%) participants with incident heart failure.
Statistical Analysis
The association of abnormal lung function (treated as a binary variable) and continuous spirometric parameters with heart failure risk was assessed with proportional hazards models. Possible interaction of lung function and spirometric variables with sex and race was evaluated using appropriate interaction terms. The functional form (i.e. linear vs. non-linear) of continuous variables was explored with fractional polynomials.21 The proportionality of hazards was assessed with the Schoenfeld residuals and smoothed hazard plots.
In multivariate proportional hazards models, we controlled for body mass index (BMI), which has been shown to be associated with lung function,22 previously identified predictors of heart failure in Health ABC (age, history of coronary heart disease, smoking, systolic blood pressure, heart rate, creatinine, fasting glucose, albumin, and electrocardiographic left ventricular hypertrophy),16 and incident coronary heart disease as a time-varying covariate. Finally, we controlled for inflammatory markers in the subset of participants (n=1906 of 2125; 89.7%) with available data. In secondary analyses, we assessed (1) the impact of obstructive versus restrictive pattern on heart failure risk and (2) the association of lung function and spirometric parameters with risk for heart failure with reduced versus preserved LVEF, using a cut-off point of 40%.
Complete data on covariates were available in 2072 of 2125 (97.5%) participants in the main analysis cohort and in 1869 of 1906 (98.1%) in the subset with inflammatory markers available. Thus, we repeated multivariable analyses in five datasets imputed with chained equations23 and obtained estimates by combining the imputed datasets.24 Both the outcome and time to event were included in the equations (in addition to the covariates) for calculation of missing values. Analyses were performed with STATA 10 (StataCorp, College Station, TX). A two-sided P<.05 value was considered significant.
Results
Participant Characteristics and Outcomes
The participant characteristics according to baseline lung function are shown in Table 1. After a median follow-up of 9.4 years (interquartile range, 7.4-9.4 years), heart failure developed in 240 (11.3%) participants (14.1 per 1000 person-years; 95% CI, 12.4-16.0).
Table 1. Baseline participant characteristics.
| Characteristic | Normal Spirometric Findings (n=1775) |
Abnormal Spirometric Findings (n=350) |
P value |
|---|---|---|---|
| Age, years | 73.6±2.9 | 73.2±3.0 | .006 |
| Males, % | 47.6 | 65.7 | <.001 |
| Whites, % | 61.8 | 65.1 | .25 |
| Smoking status | |||
| Current, % | 7.8 | 17.4 | <.001 |
| Past, % | 44.2 | 52.6 | |
| Body mass index, kg/m2 | 27.3±4.6 | 26.8±4.8 | .08 |
| Systolic blood pressure, mmHg | 136±20 | 137±20 | .18 |
| Diastolic blood pressure, mmHg | 71±11 | 72±11 | .28 |
| Heart rate, beats/minute | 64±10 | 67±12 | .001 |
| Fasting glucose, mg/dl | 103±32 | 107±34 | .003 |
| Serum creatinine, g/dl | 1.04±0.36 | 1.09±0.42 | .06 |
| Serum albumin, mg/dl | 4.00±0.31 | 3.98±0.31 | .19 |
| Hypertension, % | 50.6 | 60.0 | .002 |
| Diabetes mellitus, % | 16.9 | 13.1 | .06 |
| Coronary heart disease, % | 16.0 | 19.6 | .10 |
| Left ventricular hypertrophy, % | 10.7 | 11.7 | .57 |
| Inflammatory markers | |||
| Interleukin-6, pg/ml* | 1.68 (1.16, 2.51) | 2.20 (1.50, 1.36) | <.001 |
| Tumour necrosis factor α, pg/ml* | 3.11 (2.40, 4.05) | 3.20 (2.51, 4.01) | .21 |
| C-reactive protein, μg/ml* | 1.56 (0.95, 2.84) | 1.81 (1.12, 3.53) | <.001 |
| Spirometric parameters | |||
| FEV1, % predicted | 102±16 | 69±14 | - |
| FVC, % predicted | 99±14 | 76±16 | - |
| FEV1 to FVC ratio | 77±5 | 68±10 | - |
FEV1=Forced Expiratory Volume in 1st second; FVC=Forced Vital Capacity
P values were derived with the Mann-Whitney test for continuous variables and the Fisher exact test for categorical variables.
Baseline serum concentrations of interleukin-6, tumour necrosis factor α, and C-reactive protein were available in 2020 (95.1%), 1996 (93.9%), and 2104 (99.0%) of 2125 participants, respectively; values are expressed as median (interquartile range) because of highly skewed distributions.
Baseline Lung Function and Incident Heart Failure
Among the 1775 participants with normal lung function, heart failure developed in 172 (9.7%) participants (11.8 per 1000 person-years). In comparison, among the 350 participants with abnormal lung function, heart failure developed in 68 (19.4%) participants (27.1 per 1000 person-years). In models controlling for BMI, predictors of heart failure in the Health ABC Study,16 and incident coronary heart disease, participants with abnormal lung function were still at higher risk (Table 2). In the subset of participants with available inflammatory markers (1906 of 2125; 89.7%), addition of IL-6, TNF-α, and CRP did not attenuate this association (Table 2). The proportional hazards assumption was valid in all models. Figure 2 shows the annualized heart failure risk according to lung function. The increased heart failure risk persisted over time without attenuation. The estimates were not altered in analyses performed in five imputed datasets (data not shown).
Table 2. Abnormal lung function and risk for heart failure (overall, with reduced and preserved ejection fraction).
| Overall heart failure | Heart failure with reduced ejection fraction | Heart failure with preserved ejection fraction | ||||
|---|---|---|---|---|---|---|
| HR (%CI) | P value | HR (%CI) | P value | HR (%CI) | P value | |
| Unadjusted | 2.31 (1.74-3.06) | <.001 | 2.28 (1.46-3.57) | <.001 | 2.20 (1.39-3.49) | <.001 |
| Model 1* | 1.83 (1.35-2.46) | <.001 | 1.61 (0.99-2.60) | .054 | 1.90 (1.17-3.08) | .009 |
| Model 2† | 1.83 (1.36-2.47) | <.001 | 1.61 (0.99-2.61) | .053 | 1.89 (1.17-3.08) | .01 |
| Model 3‡ | 1.83 (1.33-2.50) | <.001 | 1.71 (1.03-2.84) | .037 | 1.75 (1.05-2.90) | .032 |
Adjusted for body mass index, previously identified predictors of heart failure in the Health ABC Study (age, history of coronary artery disease, smoking status, systolic blood pressure, heart rate, left ventricular hypertrophy by electrocardiography, and creatinine, fasting glucose, and albumin levels)
Adjusted for the above parameters plus incident coronary heart disease
Adjusted for the above parameters plus inflammatory markers (interleukin-6, tumour necrosis factor α, and C-reactive protein)
CI= confidence interval; HR=hazard ratio
Figure 2.
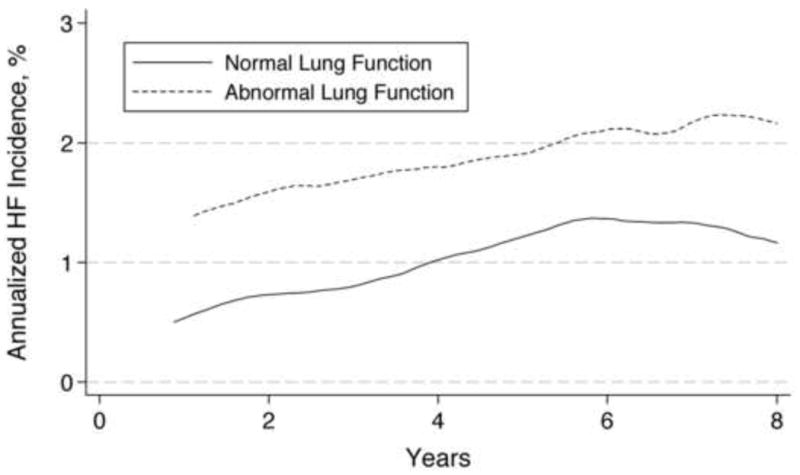
Annualized risk of incident heart failure for participants with normal versus abnormal lung function tests at baseline, adjusted for predictors of heart failure and body mass index. Smoothed hazard estimate was obtained using an Epanechnikov kernel. Although the absolute annualized risk increases from year 1 to year 5 before reaching a plateau in both groups, the relative risk remains stable over time.
The increased heart failure risk associated with abnormal lung function was more pronounced in women compared to men and in white compared to black participants in adjusted models (Figure 3); however, this differential association did not reach statistical significance (P=.32 and P=.24 for the interaction terms for sex and race, respectively).
Figure 3.
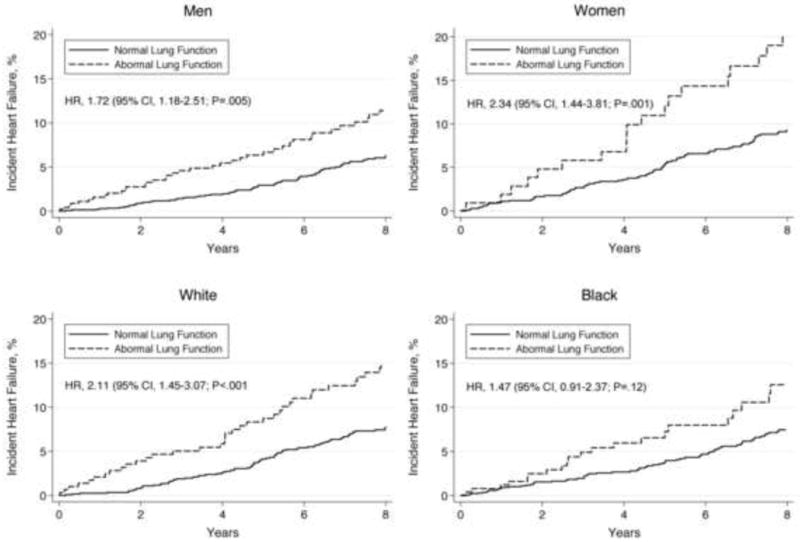
Kaplan-Meier rates of incident heart failure by baseline lung function status in sex and race subgroups, adjusted for predictors of heart failure and body mass index.
Obstructive vs. Restrictive Baseline Pattern and Incident Heart Failure
Compared to participants with normal lung function at baseline, those with obstructive pattern (n=163) had HR 1.44 (95% CI, 0.92-2.26; P=.11) and those with restrictive pattern (n=187) had HR 2.12 (95% CI, 1.50-3.01; P<.001) for incident heart failure in models adjusted for predictors of heart failure and BMI (P=.15 for the comparison of patterns).
Continuous Spirometric Parameters and Incident Heart Failure
Both %FVC and %FEV1 were associated with heart failure risk in unadjusted analyses (Table 3, Figure 4) and in models controlling for predictors of heart failure, BMI, and incident coronary heart disease (Table 3); FEV1/FVC ratio was marginally significant. The strength of association was comparable for %FVC and %FEV1. These associations persisted when IL-6, TNF-α, and CRP were added in the model (HR, 1.21; 95% CI, 1.11-1.32; P<.001; and HR, 1.18; 95% CI, 1.09-1.26; P<.001, per 10% lower %FVC and %FEV1, respectively). There was no evidence of non-linear association of %FVC or %FEV1 with risk. Hazards were proportional in all models. The estimates were not altered in analyses done in five imputed datasets (data not shown).
Table 3. Baseline spirometric parameters and risk for heart failure.
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| HR† (95% CI) | P | HR† (95% CI) | P | |
| Total study population (n=2125) | ||||
| % predicted FEV1 | 1.26 (1.18-1.34) | <.001 | 1.20 (1.12-1.28) | <.001 |
| % predicted FVC | 1.33 (1.23-1.44) | <.001 | 1.24 (1.14-1.34) | <.001 |
| FEV1 to FVC ratio | 1.35 (1.14-1.60) | <.001 | 1.21 (1.00-1.46) | .05 |
| Men (n=1074) | ||||
| % predicted FEV1 | 1.27 (1.17-1.39) | <.001 | 1.20 (1.09-1.31) | <.001 |
| % predicted FVC | 1.40 (1.25-1.56) | <.001 | 1.28 (1.13-1.43) | <.001 |
| FEV1 to FVC ratio | 1.27 (1.02-1.58) | .04 | 1.16 (0.91-1.48) | .24 |
| Women (n=1051) | ||||
| % predicted FEV1 | 1.23 (1.12-1.36) | <.001 | 1.22 (1.11-1.35) | <.001 |
| % predicted FVC | 1.25 (1.12-1.40) | <.001 | 1.25 (1.12-1.40) | <.001 |
| FEV1 to FVC ratio | 1.42 (1.08-1.85) | .01 | 1.32 (1.00-1.74) | .05 |
| White (n=1324) | ||||
| % predicted FEV1 | 1.27 (1.16-1.38) | <.001 | 1.23 (1.12-1.35) | <.001 |
| % predicted FVC | 1.37 (1.23-1.53) | <.001 | 1.29 (1.15-1.45) | <.001 |
| FEV1 to FVC ratio | 1.38 (1.09-1.75) | .008 | 1.27 (0.99-1.64) | .06 |
| Black (n=801) | ||||
| % predicted FEV1 | 1.26 (1.15-1.38) | <.001 | 1.17 (1.07-1.29) | .001 |
| % predicted FVC | 1.31 (1.18-1.46) | <.001 | 1.21 (1.08-1.35) | .001 |
| FEV1 to FVC ratio | 1.33 (1.05-1.69) | .02 | 1.15 (0.88-1.49) | .31 |
| Heart failure with reduced ejection fraction (n=96) | ||||
| % predicted FEV1 | 1.23 (1.12-1.37) | <.001 | 1.16 (1.05-1.29) | .006 |
| % predicted FVC | 1.26 (1.11-1.43) | <.001 | 1.17 (1.03-1.33) | .01 |
| FEV1 to FVC ratio | 1.49 (1.15-1.93) | .002 | 1.31 (0.98-1.75) | .065 |
| Heart failure with preserved ejection fraction (n=91) | ||||
| % predicted FEV1 | 1.22 (1.10-1.35) | <.001 | 1.18 (1.05-1.32) | .004 |
| % predicted FVC | 1.34 (1.18-1.52) | <.001 | 1.25 (1.10-1.43) | .001 |
| FEV1 to FVC ratio | 0.95 (0.69-1.29) | .73 | 0.90 (0.65-1.26) | .55 |
Adjusted for body mass index, previously identified predictors of heart failure in the Health ABC Study (age, history of coronary artery disease, smoking status, systolic blood pressure, heart rate, left ventricular hypertrophy by electrocardiography, and creatinine, fasting glucose, and albumin levels) and incident coronary heart disease.
Hazard ratios refer to 10% percent decrease of the parameter.
CI= confidence interval; HR=hazard ratio; FEV1=forced expiratory volume in 1st sec; FVC=forced vital capacity
Figure 4.
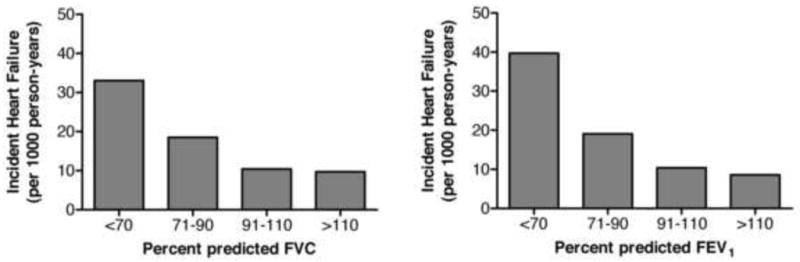
Unadjusted incident heart failure rates according to baseline forced expiratory volume in 1st sec (FEV1) and forced vital capacity (FVC) values. Rates are expressed in cases per 1000 person-years.
Results were similar for sex- and race-specific analyses (Table 3). The interaction effects of sex and race on spirometric parameters were nonsignificant in fully adjusted models (P=.82 and P=.74 for the effect of sex and P=.42 and P=.50 for the effect of race on %FVC and %FEV1, respectively).
Among participants with normal lung function (n=1775), both %FVC and %FEV1 were still associated with heart failure risk in models adjusted for predictors of heart failure and BMI (HR, 1.14; 95% CI, 1.02-1.29; P=.02, and 1.12; 95% CI, 1.01-1.23; P=.03, per 10% lower %FVC and %FEV1, respectively).
Heart Failure with Reduced vs. Preserved Ejection Fraction
Data on post-heart failure LVEF were available in 187 of 240 (77.9%) participants with incident heart failure. Mean LVEF in these participants was 41±16%; 96 of 187 (51.3%) had LVEF ≤40% and 91 of 187 (48.7%) had LVEF >40% at heart failure diagnosis. Abnormal lung function was associated with higher risk for heart failure with preserved and reduced LVEF in models adjusted for BMI, predictors for heart failure, incident coronary heart disease and inflammatory markers (Table 2). Finally, % FVC and %FEV1 were significantly associated with risk for heart failure with reduced and preserved ejection fraction in unadjusted and adjusted models (Table 3).
Discussion
In this study, we observed increased heart failure incidence among older persons with lung function alterations but without clinically apparent lung disease. This increased heart failure risk persisted after controlling for multiple heart failure risk factors, incident coronary heart disease, and inflammatory markers, and did not attenuate over time. When analysed as continuous variables, both FVC and FEV1 had a linear association with heart failure risk in adjusted models. These findings were consistent in sex and race subgroups.
Several mechanisms might contribute to development of heart failure in older persons with abnormal lung function in the absence of established lung disease. First, low-grade systemic inflammation is present both in patients with chronic lung diseases25 and in patients with abnormal lung volumes without established lung disease6; notably, the latter is also supported by our findings. Inflammation may lead to heart failure through direct myocardial damage26 and accelerated atherosclerosis,27 and inflammatory pathways have been associated with heart failure risk in epidemiologic studies.28 Second, impaired lung function is characterized by oxidative stress,29 which has been shown to directly affect myocardial function.30, 31 Finally, changes in respiratory mechanics and intrathoracic pressure can reduce cardiac output by affecting preload and afterload.32, 33 Because mechanisms like inflammation and oxidative stress are also associated with coronary heart disease,34 it is possible that heart failure risk is primarily mediated through incident coronary heart disease. However, controlling for inflammatory markers and incident coronary heart disease did not alter the associations with heart failure risk in our study, suggesting a more direct cardio-pulmonary interaction. Notably, in a recent report,35 percentage emphysema and FEV1/FVC were associated with left ventricular filling. Interestingly, in concordance with the impaired ventricular filling hypothesis, in our study the association of impaired lung function with heart failure risk was stronger for heart failure with preserved LVEF; however, post-heart failure LVEF was not prospectively evaluated in Health ABC and thus these findings should be interpreted with caution.
In our analyses, restrictive pattern of abnormal lung function demonstrated a trend towards stronger association with heart failure risk than obstructive pattern. Considering that fewer participants had obstructive as compared to restrictive spirometry pattern, the lack of statistical significance in the former group might represent lack of power. Although both spirometric patterns are encountered among heart failure patients,36 restrictive spirometric pattern is more common37; notably, this pattern is met in 10-12% of older persons.38 One may therefore contend that undiagnosed heart failure or asymptomatic left ventricular dysfunction at baseline is primarily driving our findings. However, we found that the excessive risk for heart failure associated with abnormal baseline lung function was constant over time. Because it is unlikely that heart failure will remain undiagnosed for many years, it is improbable that the observed association can be entirely ascribed to undetected heart failure.
What are the practical implications of these findings? Considering the association of %FEV1 and %FVC with heart failure risk, it is possible that spirometry might be used to predict heart failure risk in the elderly. Indeed, the Framingham investigators suggested that FVC can be used to predict heart failure risk11, and use of %FEV1 has been proposed as a marker with broad clinical utility in risk assessment and possible prevention of both respiratory (COPD and lung cancer) and cardiovascular diseases.39 Whether spirometry is a cost-effective tool for screening purposes in the elderly needs further study. However, whenever abnormal spirometric findings are encountered in elderly patients, a comprehensive assessment for heart failure risk may be warranted.16
Our study has several limitations. Echocardiography was not performed at baseline and therefore participants with asymptomatic structural cardiac abnormalities may have been included in our study. Diagnosis of heart failure was based on hospitalization and thus absolute heart failure incidence is likely underestimated. Hospitalization rates for heart failure may be selectively higher among participants with impaired lung function because of reduced capacity to compensate heart failure, leading to more severe presentation. Participants with undiagnosed lung disease at baseline may have been included in the analyses. Specific data on left- versus right-sided heart failure were not available. However, isolated right ventricular failure is far less common than left ventricular failure even among patients with established COPD.40 Finally, Health ABC included only older adults of white or black race; therefore, these findings may not apply to younger adults or other races.
In conclusion, we observed a significant association between abnormal spirometric findings and heart failure risk among older persons. These findings were consistent in sex- and race-based subgroups and persisted after controlling for incident coronary heart disease and inflammatory markers. The practical implication of our findings, including the possible clinical use of spirometry to detect individuals at increased risk for heart failure, needs further study.
Acknowledgments
Funding Sources: This work was supported in part by the Intramural Research Program of the National Institute of Aging, National Institutes of Health, Bethesda MD [Grants N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106]; and by PHS [Grant UL1 RR025008] from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
Dr. Kritchevsky is also supported by National Heart, Lung, and Blood Institute grant R01-HL-7-4104.
This work was also partially funded through an Emory University Heart and Vascular Board grant entitled ‘Novel Risk Markers and Prognosis Determination in Heart Failure’.
Footnotes
Conflict of interest for all authors: None declared
All authors had access to the data and provide substantial input for this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Archives of internal medicine. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 2.Sidney S, Sorel M, Quesenberry CP, Jr, et al. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 3.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Annals of epidemiology. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 5.Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991-1999. COPD. 2005;2:35–41. doi: 10.1081/copd-200050671. [DOI] [PubMed] [Google Scholar]
- 6.Engstrom G, Lind P, Hedblad B, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation. 2002;106:2555–2560. doi: 10.1161/01.cir.0000037220.00065.0d. [DOI] [PubMed] [Google Scholar]
- 7.Schunemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 8.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ (Clinical research ed. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. discussion 715-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, D'Agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Archives of internal medicine. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics -- 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–1161. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 14.Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:529–534. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 15.Waterer GW, Wan JY, Kritchevsky SB, et al. Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc. 2001;49:1032–1038. doi: 10.1046/j.1532-5415.2001.49205.x. [DOI] [PubMed] [Google Scholar]
- 16.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident Heart Failure Prediction in the Elderly: The Health ABC Heart Failure Score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Serum resistin concentrations and risk of new onset heart failure in older persons: the health, aging, and body composition (Health ABC) study. Arterioscler Thromb Vasc Biol. 2009;29:1144–1149. doi: 10.1161/ATVBAHA.109.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–5528. doi: 10.1002/sim.3148. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997;111:891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 23.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Barnard J, Rubin B. Small-sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–955. [Google Scholar]
- 25.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114:758–762. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 26.Bryant D, Becker L, Richardson J, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 27.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ (Clinical research ed. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults: The Health, Aging, and Body Composition Study. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2009.12.045. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. American journal of respiratory and critical care medicine. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 30.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. Journal of the American College of Cardiology. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 31.Blaustein AS, Schine L, Brooks WW, et al. Influence of exogenously generated oxidant species on myocardial function. The American journal of physiology. 1986;250:H595–599. doi: 10.1152/ajpheart.1986.250.4.H595. [DOI] [PubMed] [Google Scholar]
- 32.van den Hout RJ, Lamb HJ, van den Aardweg JG, et al. Real-time MR imaging of aortic flow: influence of breathing on left ventricular stroke volume in chronic obstructive pulmonary disease. Radiology. 2003;229:513–519. doi: 10.1148/radiol.2292020559. [DOI] [PubMed] [Google Scholar]
- 33.Peters J, Fraser C, Stuart RS, et al. Negative intrathoracic pressure decreases independently left ventricular filling and emptying. Am J Physiol. 1989;257:H120–131. doi: 10.1152/ajpheart.1989.257.1.H120. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part III: biomarkers of oxidative stress and angiogenic growth factors. Circulation. 2006;113:e289–292. doi: 10.1161/CIRCULATIONAHA.105.595546. [DOI] [PubMed] [Google Scholar]
- 35.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waxman AB. Pulmonary function test abnormalities in pulmonary vascular disease and chronic heart failure. Clin Chest Med. 2001;22:751–758. doi: 10.1016/s0272-5231(05)70063-0. [DOI] [PubMed] [Google Scholar]
- 37.Faggiano P. Abnormalities of pulmonary function in congestive heart failure. Int J Cardiol. 1994;44:1–8. doi: 10.1016/0167-5273(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 38.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254:540–547. doi: 10.1111/j.1365-2796.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 39.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30:616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 40.Rutten FH, Cramer MJ, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]


