Abstract
We sought to determine the effects of interleukin-2 administered in combination with antiretroviral therapy (ART) on CD4+ T cells in the gut. Lymphocytes from whole blood, colon and terminal ileum of HIV infected adults treated with interleukin-2 and ART or ART alone were examined. There were no differences between groups in the proportion of CD4+ T cells or in expression of CD25 or Ki67 by CD4+T cells in the gut. Although IL-2 administration leads to expansion of peripheral blood CD4+ T cells, there is no alteration in the proportion or activation of CD4+ T cells in the gut mucosa.
Keywords: gastrointestinal tract, mucosa, IL-2, HIV, CD4
INTRODUCTION
Primary HIV and simian immunodeficiency virus (SIV) infections lead to rapid depletion of CD4+ T cells from the gut mucosa 1–3. The majority of gut mucosal CD4+ T cells exhibit an activated effector memory phenotype and are highly susceptible to HIV infection 4. The depletion of CD4+ T cells from the gut is more pronounced than the depletion in the peripheral blood or lymphoid tissues and may be slower to recover following initiation of combination antiretroviral therapy (ART) 5. Loss of these CD4+ T cells may contribute to the disruption of the gut epithelium allowing for translocation of microbial products, which in turn is associated with systemic immune activation in HIV infected patients 6. Recent studies have shown that prolonged treatment with ART leads to restoration of gut mucosal CD4+ T cells and a decrease in the proportion of T cells in cell cycle 7.
Treatment of HIV infected patients with ART leads to control of viral replication to below the limits of quantification of commercially available assays, restoration of peripheral blood CD4+ T cell counts, and reduced morbidity and mortality 8, 9. However, once begun ART must be taken for life, and lifelong ART imposes multiple burdens such as long-term toxicities, drug-drug interactions and drug resistance as a result of non-adherence. Therefore, immune based therapies that restore the CD4 T cell pool have been explored as an adjunct or alternative to ART. One of the best studied of these therapies has been intermittent interleukin-2 (IL-2).
Several phase I and II studies have demonstrated that IL-2 leads to significant and sustained increases in peripheral blood CD4+ T cells in HIV infected patients 10–13. The increased CD4+ T cell numbers were primarily due to peripheral expansion and increased survival 14 of a subset of CD4+ T cells that express the IL-2 receptor alpha chain (CD25) 15 and have a naive or central memory phenotype 14, 16. Furthermore, the increased CD4+ T cell numbers were associated with a long-term decrease in T cell proliferation 16. Despite these promising findings, two large multicenter phase III trials demonstrated recently that the increase in CD4+ T cell counts does not confer clinical benefit 17.
In light of the failure of IL-2 to provide clinical benefit in phase III studies, it is important to understand better how the expanded T cells are distributed and if they are capable of homing after maturation to tissues such as the gut. We conducted this study to measure the degree of immune restoration, the proportion of cycling T cells, and the proportion of CD4+ T cells expressing CD25 in the colon and terminal ileum (TI) of HIV infected patients who received IL2 and ART and compare them to patients who received ART alone as well as to healthy, uninfected participants.
METHODS
Subjects
Biopsies from colon and TI as well as peripheral blood samples were collected from 11 HIV-infected patients treated with ART and intermittent IL-2 (IL-2), 12 HIV-infected patients treated with combination antiretroviral therapy (ART), and10 HIV-uninfected participants (HIV-). Patients in the IL-2 group had previously received intermittent IL-2 therapy as part of other ongoing or completed protocols. All participants provided informed consent for peripheral blood and gastrointestinal tract sampling under an IRB approved NIH Protocol. Plasma viral loads were determined by ultrasensitive bDNA assay (Versant HIV-1 version 3.0, Siemens Corp., New York City). Results from the ART and the HIV-groups have been reported previously in part 7.
Biopsy Processing
Colonic and TI tissue biopsies were collected as previously described 7. Briefly, approximately thirty biopsies were extracted from the gut mucosa at each location, and 16–20 were processed for flow cytometric analysis. The biopsies were weighed, placed in RPMI (Mediatech, Herndon, VA) with 10% heat-inactivated fetal bovine serum, and digested using collagenase (Sigma-Aldrich, St. Louis, MO) and DNAse I (Invitrogen, Carlsbad, CA)or benzoase (Novagen, Madison, WI) and then filtered. After washing, the cell suspension was counted using a Beckman Coulter Counter to obtain the number of total viable cells. Absolute numbers of CD4+and CD8+T-cell per gram of gut tissue were determined as previously described 7.
Flow cytometry
Immunophenotypic analyses were performed on whole blood and cells extracted from the gut biopsiesas described elsewhere 18. The following monoclonal antibodies were used for staining: CD3, CD4, CD8, CD27, CD45RO, CD25, and Ki67conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin-chlorophyll-protein or allophycocyanin (Becton Dickinson (BD) Pharmingen, San Jose, CA). Samples were acquired using a FACSCalibur flow cytometer (BD Pharmingen, San Jose, CA). The data were analyzed using FlowJo software (Tree Star, Inc., San Carlos, CA).
Statistical Analyses
Values are expressed as medians with interquartile ranges. Kruskal-Wallis and Mann Whitney tests were used for between group comparisons. Given the exploratory nature of the study, all P values<0.05 are shown. Analyses were performed using Prism v5.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Participant characteristics
Clinical characteristics of the three groups are shown in the Table. Patients in the ART and IL-2 groups had initiated ART during chronic infection. Patients in the IL-2 group had received a median of seven cycles of IL-2 administered at doses ranging from 3.0 to 7.5 IU twice daily for 5 days by subcutaneous injection and the median time since the last IL-2 cycle was four months. All participants in the ART andIL-2 groups were on regimens containing three or more antiretrovirals, at least one of which was a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor. Two participants in the ART group were on regimens containing both a protease inhibitor and a non-nucleoside reverse transcriptase inhibitor. No participants were taking entry-inhibitors, CCR5 antagonists or integrase inihibitors. All participants were asymptomatic at the time of biopsy.
Table 1.
Clinical characteristics of study participants.a
| HIV- (n=10) | ART (n=12) | IL-2 (n=11) | P values (<0.05) | |
|---|---|---|---|---|
| Age (years) | 46 (40–50) | 48 (42–50) | 46 (41–49) | |
| CD4+ T cells/μL | 773 (496–1,359) | 535 (384–608) | 701 (626–971) | P=0.018b |
| % CD4+ T cells | 45 (38–49) | 32 (23–38) | 37 (29–45) | P=0.007c |
| CD8+ T cells/μL | 399 (313–443) | 703 (520–988) | 890 (781–1118) | P<0.001c,d |
| % CD8+ T cells | 20 (19–25) | 42 (36–53) | 46 (39–48) | P<0.001c,d |
| CD4:CD8 ratio | 2.13 (1.59–2.58) | 0.72 (0.45–1.04) | 0.83 (0.66–1.05) | P<0.001c.d |
| Nadir CD4+ T cells/μL | N/A | 187 (36–270) | 204 (32–267) | |
| Plasma HIV RNA (copies/mL) | N/A | <50 | <50 | |
| Years on ART | N/A | 8 (5–10) | 8 (6–10) | |
| Number of IL-2 cycles | N/A | N/A | 7 (6–10) | |
| Months since last IL-2 cycle | N/A | N/A | 4 (3–8) |
Median values with interquartile range in parentheses
ART vs IL-2;
HIV-vs ART;
HIV-vs IL-2
ART, antiretroviral therapy; IL-2, interleukin-2; N/A, not applicable
Immunologic response to IL-2 therapy
The absolute number of CD4+ T cells in the IL-2 group (701 cells/μL) was higher than the ART group (535 cells/μL, P=0.018) and similar to the HIV-group (773 cells/μL, P=0.699) (Table). In the IL-2 group, CD4+ T cell counts at the time of sampling were a median of 51% (22–70%) higher compared to a time just prior to their most recent IL-2 cycle.
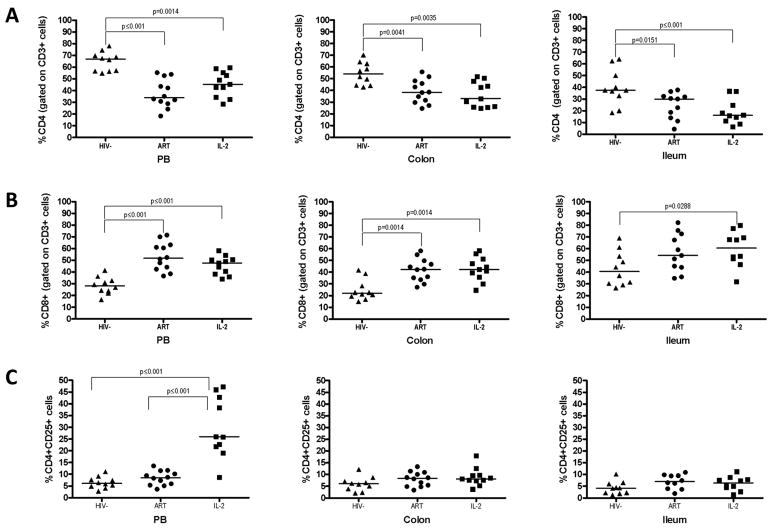
In both the colon and TI, there were no differences in the proportions of CD4+ T cells between the IL-2 group and the ART group (colon: 33 vs 38%, P=0.559; TI: 16 vs 30%, P=0.342) (figure A). The proportions of CD4+ T cells were lower at both gastrointestinal sites in the IL-2 and ART groups compared to the HIV-control group (colon: 54%; P=0.004 for both comparisons; TI: 38%; vs ART, P=0.015; vs IL-2, P<0.001) (figure A). A similar pattern was seen in the peripheral blood. The proportion of CD8+ T cells was also similar at the gastrointestinal sites of the IL-2 and ART groups (colon: 42 vs 42%, P=0.758; TI: 54 vs 61%, P=0.597) (figure B). In the colon, the proportion of CD8+ T cells was lower in the control group compared to both the IL-2 group and the ART group (22%, P=0.001 for both comparisons). Again a similar pattern was seen in peripheral blood.
Figure.
The proportions of CD4 (A) and CD8 (B) T cells in peripheral blood, colon and ileum are shown as a percentage of CD3+ T cells. The proportions of CD4+CD25+ T cells are shown in (C). Black bars indicated median values.
There were no differences in the absolute numbers of CD4+ or CD8+ T cells in the gastrointestinal sites between the IL-2 group and the ART group (data not shown).
CD25 expression and cell cycling following IL-2 therapy
In the IL-2 group, peripheral blood CD4+ T cells expressed significantly higher levels of CD25 (26%) compared to the ART group or HIV-group (ART: 8.52%, HIV-: 6.13%; P<0.001 for both comparisons). However, at the gastrointestinal sites there were no differences between groups in CD25 expression on CD4+ T cells (figure C).
The proportion of cycling (Ki67+) CD4+ T cells in the colon in the IL-2 group (3.9%) was not different compared to the ART group (3.0%; P=0.086) and higher than the HIV-group (2.2%; P=0.006). Expression of Ki67 by CD8+ T cells in the colon was higher in the IL-2 group (4.4%) compared to the ART group (2.6%; P=0.033) and not different from the HIV-group (3.3%; P=0.397). In the ileum, Ki67 expression by CD4+ T cells was not different in the IL2 group (2.2%) compared to the ART group (3.9%; P=0.622) or the HIV-group (1.9%; P=0.065). There were no differences in Ki67 expression by CD8+ T cells among the groups in the ileum. Expression of Ki67 by CD4+ or CD8+ T cells in the peripheral blood was not different between the three groups (data not shown).
DISCUSSION
In contrast to its known effect of increasing CD4+ T cell counts in the peripheral blood, this study showed no additional effect of IL-2 treatment compared to ART alone, on the proportion of CD4+ T cells in the gut mucosa. In addition, there was no difference in CD25 expression on CD4+ T cells or proportion of cycling CD4+ T cells in the gut.
IL-2, a cytokine secreted by activated lymphocytes, plays a critical role in the homeostasis of lymphocytes by regulating their proliferation, differentiation and survival 19. Given that IL-2 administration had no effect on the proportion of CD4+ T cell in the gut, it seems that it is not having an impact onCD4+ T cell homeostasis in that site. Additionally, CD4+CD25+ T cells do not appear to be homing to the gut or maturing into effectors that can migrate to the gut tissue in response to local stimuli, although it should be noted that the function of these cells was not examined in this study and might also be differentially affected by IL-2. Interestingly, Ki67 expression was higher in CD8+ T cells in the colon in the IL-2 group than the ART group, although the proportions of CD8+ T cells overall were not different. The reasons for this are unclear and warrant further studies, including examination of additional markers of T cell activation and apoptosis as well as gut viral load.
In interpreting the recent phase III studies of IL-2 administration in combination with ART two hypotheses have emerged to explain the lack of clinical benefit of increased peripheral CD4+ T cell counts: 1) the CD4+ T cells induced by IL-2 are not functional or 2) the CD4+ T cells are functional but the negative effects of interleukin-2 negate the benefits the expanded cells 17. Subsequently it has been shown that IL-2 administration induced transient increases in high-sensitivity C-reactive protein and D-dimer suggesting increased inflammation and/or thrombosis as a possible mechanism to explain some of the adverse clinical events seen in these trials 20. It is also possible that expanded CD4+CD25+ T cells were not maturing or differentiating into effectors able to home to gut or other tissue. Although IL-2 has been shown to decrease CD4+ T cell cycling in the periphery long-term 16, in the gut there was no difference in the proportion of cycling CD4+ T cells between the IL-2 treated and ART only groups.
This study shows that IL-2 administration does not affect the proportion of gut CD4+ T cells nor does it have an effect on the cycling of CD4+ T cells or proportion of CD4+CD25+ T cells in the gut. Given these findings and the lack of clinical benefit of IL-2, it may be worth examining the effects of other immune based therapies currently being developed on gut or other mucosal T cell populations to evaluate homing and maturation potential of newly expanded T lymphocytes.
Acknowledgments
The authors would like to acknowledge the staff of the OP8 clinic and the GI suite at the NIH Clinical Center for their assistance with patient recruitment and care. Special thanks to all study participants who volunteered for research gut biopsy procedures.
This research was supported in part by the Intramural Program of the NIH, NIAID and Critical Care Medicine Department. Additionally, this project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Note: These results were presented in abstract form at the 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia, July 2007.
DISCLOSURE
Potential conflicts of interest: The US government has been granted a use patent for intermittent IL-2 therapy, including J. A. Kovacs as an inventor. All other authors declare no competing financial interests.
References
- 1.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol Nov. 2003;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998 Apr 17;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000 Aug 18;14(12):1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 5.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med Dec. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 7.Ciccone EJ, Read SW, Mannon PJ, et al. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol Mar. 2010;3(2):172–181. doi: 10.1038/mi.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 9.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006 Aug 5;368(9534):451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs JA, Vogel S, Albert JM, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996 Oct 31;335(18):1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 11.Davey RT, Jr, Chaitt DG, Albert JM, et al. A randomized trial of high- versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J Infect Dis Apr. 1999;179(4):849–858. doi: 10.1086/314678. [DOI] [PubMed] [Google Scholar]
- 12.Levy Y, Capitant C, Houhou S, et al. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: a randomised controlled trial. ANRS 048 study group. Lancet. 1999 Jun 5;353(9168):1923–1929. doi: 10.1016/s0140-6736(98)07345-0. [DOI] [PubMed] [Google Scholar]
- 13.Davey RT, Jr, Murphy RL, Graziano FM, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: A randomized controlled trial. Jama. 2000 Jul 12;284(2):183–189. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs JA, Lempicki RA, Sidorov IA, et al. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. J Clin Invest Aug. 2005;115(8):2139–2148. doi: 10.1172/JCI23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sereti I, Martinez-Wilson H, Metcalf JA, et al. Long-term effects of intermittent interleukin 2 therapy in patients with HIV infection: characterization of a novel subset of CD4(+)/CD25(+) T cells. Blood. 2002 Sep 15;100(6):2159–2167. [PubMed] [Google Scholar]
- 16.Sereti I, Anthony KB, Martinez-Wilson H, et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004 Aug 1;104(3):775–780. doi: 10.1182/blood-2003-12-4355. [DOI] [PubMed] [Google Scholar]
- 17.Abrams D, Levy Y, Losso MH, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009 Oct 15;361(16):1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sereti I, Herpin B, Metcalf JA, et al. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. Aids. 2001 Sep 28;15(14):1765–1775. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 19.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 20.Porter BO, Shen J, Kovacs JA, et al. Interleukin-2 cycling causes transient increases in high-sensitivity C-reactive protein and D-dimer that are not associated with plasma HIV-RNA levels. AIDS. 2009 Sep 24;23(15):2015–2019. doi: 10.1097/QAD.0b013e32832d72c6. [DOI] [PMC free article] [PubMed] [Google Scholar]