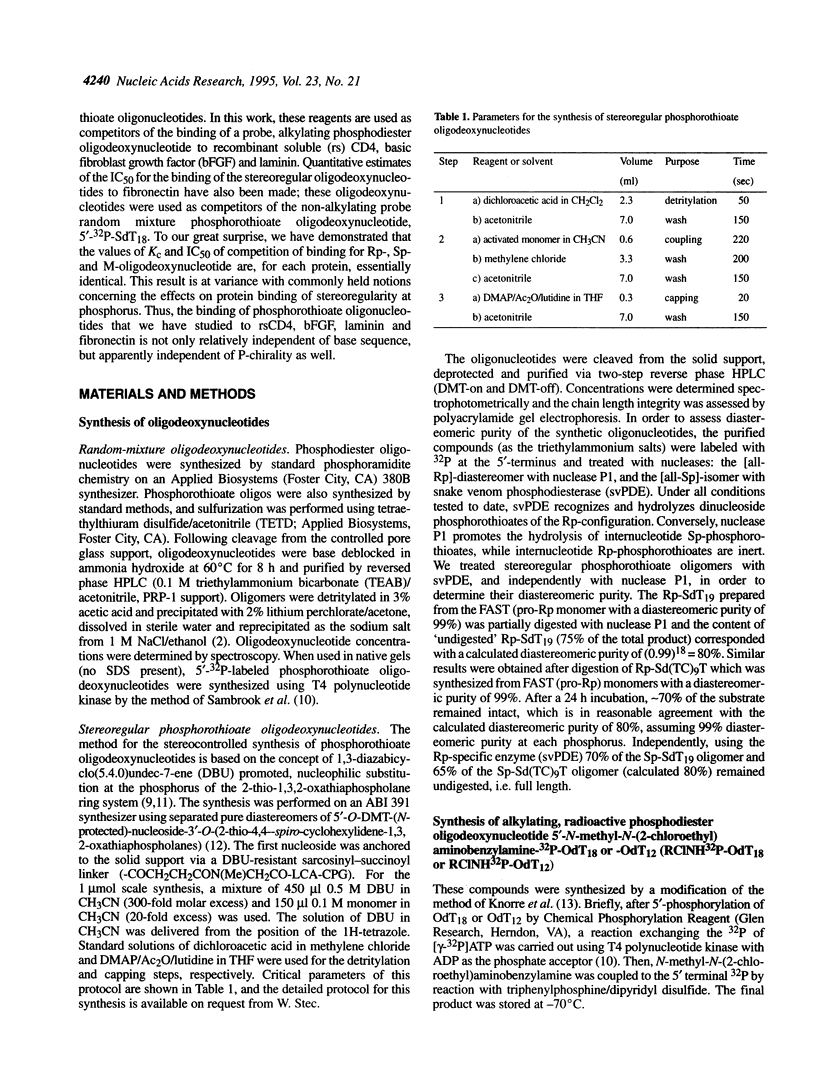
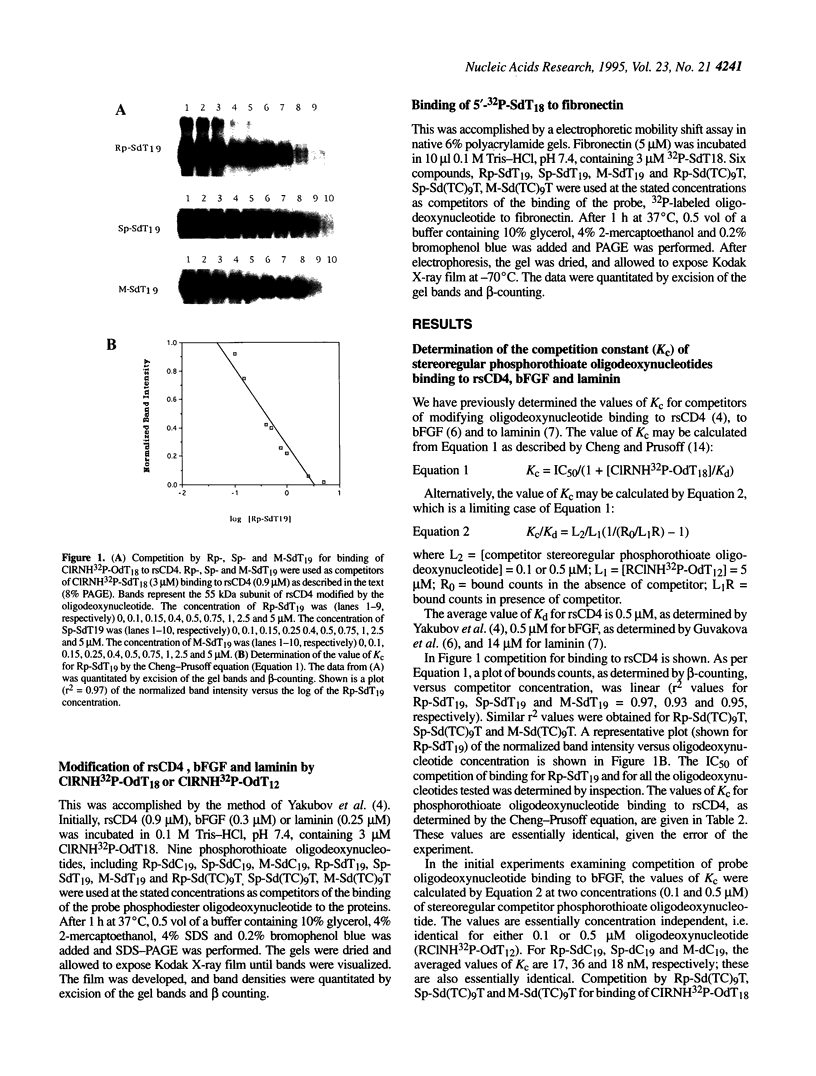
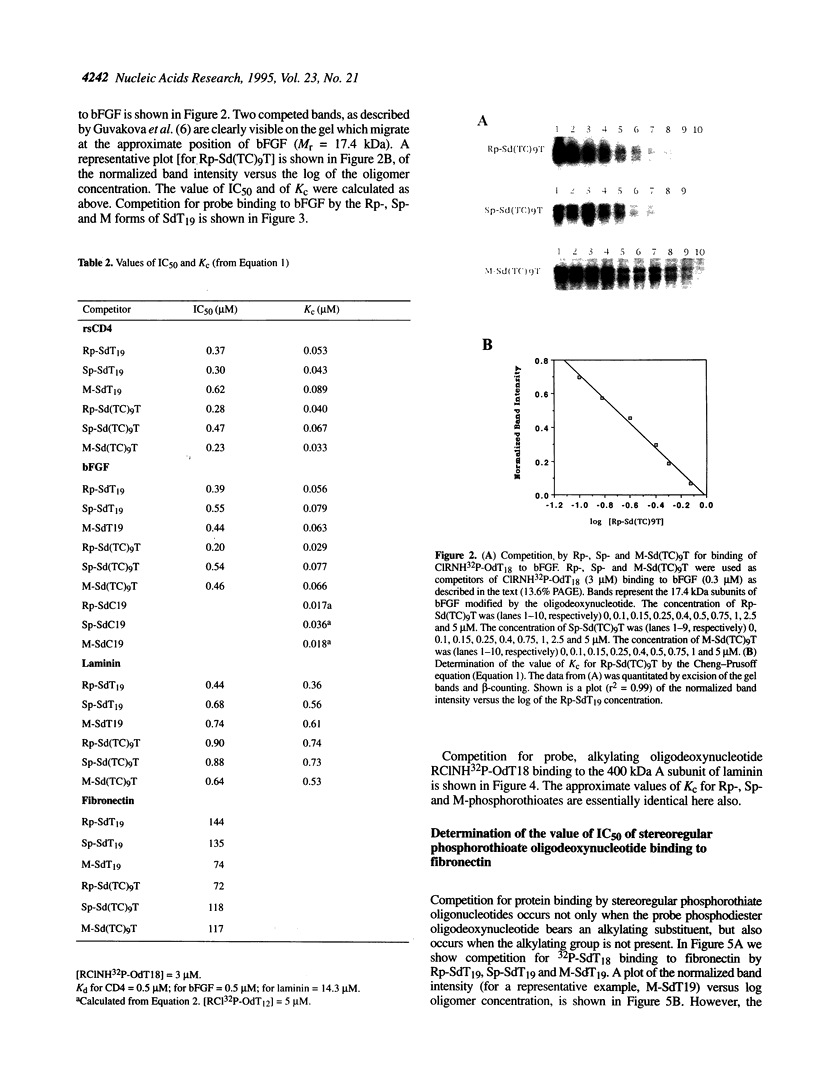
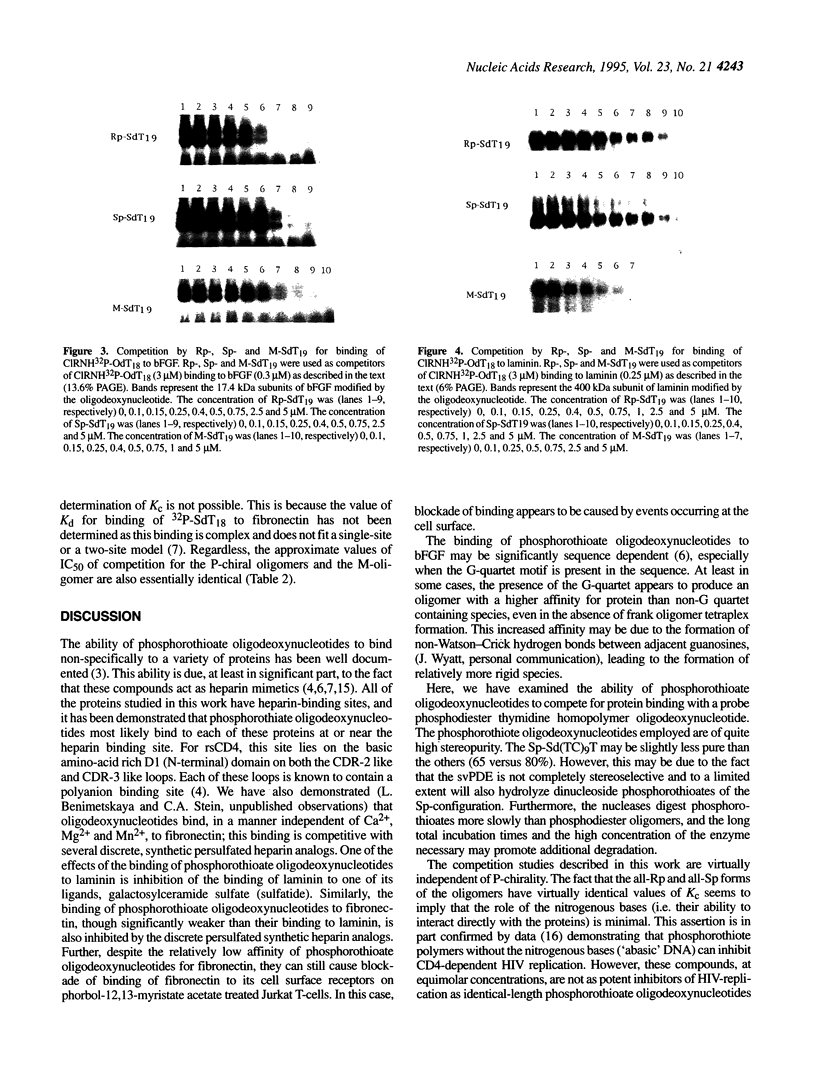
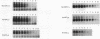Abstract
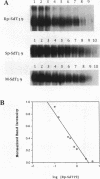
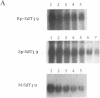
Antisense oligodeoxynucleotides can selectively inhibit the expression of individual genes and thus have potential applications in anticancer and antiviral therapy. A critical prerequisite to their use as therapeutic agents is the understanding of their non-specific interactions with biological structures, e.g. proteins. In this study we examined the interactions of P-chiral phosphorothioate oligodeoxynucleotides with several proteins. The Rp- and Sp- diastereomers, and racemic machine-made mixtures, or M-oligodeoxynucleotides were used independently as competitors of the binding of a probe, phosphodiester oligodeoxynucleotide bearing a 5' alkylating moiety, to rsCD4, bFGF and laminin. These oligodeoxynucleotides were also used as competitors of the binding of a non-alkylating probe M-phosphorothioate oligodeoxynucleotide, 5'-32P-SdT18 to fibronectin. The average values of and quantitative estimates for the IC50 of competition and the constant of competition (Kc) of Rp-, Sp- and M-stereoisomers of several homo- and heteropolymer oligodeoxynucleotides were determined and compared. Surprisingly, in the proteins we studied, the values of IC50 and Kc for the Rp-, Sp- and M-oligodeoxynucleotides were essentially identical. Thus, the ability of the phosphorothioate oligodeoxynucleotides we employed, to bind to the proteins studied in this work, is virtually independent of P-chirality. Our results also imply that the role of the purine and pyrimidine bases in oligodeoxynucleotide-protein interactions, as well as the nature of the contact points (sulfur versus oxygen) between the oligomer and the protein, may be relatively unimportant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Guvakova M. A., Yakubov L. A., Vlodavsky I., Tonkinson J. L., Stein C. A. Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J Biol Chem. 1995 Feb 10;270(6):2620–2627. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- Iyer R. P., Uznanski B., Boal J., Storm C., Egan W., Matsukura M., Broder S., Zon G., Wilk A., Koziolkiewicz M. Abasic oligodeoxyribonucleoside phosphorothioates: synthesis and evaluation as anti-HIV-1 agents. Nucleic Acids Res. 1990 May 25;18(10):2855–2859. doi: 10.1093/nar/18.10.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled Z., Rideout D., O'Driscoll K. R., Petrylak D., Cacace A., Patel R., Chiang L. C., Rotenberg S., Stein C. A. Effects of suramin-related and other clinically therapeutic polyanions on protein kinase C activity. Clin Cancer Res. 1995 Jan;1(1):113–122. [PubMed] [Google Scholar]
- Knorre D. G., Vlassov V. V., Zarytova V. F., Karpova G. G. Nucleotide and oligonucleotide derivatives as enzyme and nucleic acid targeted irreversible inhibitors. Chemical aspects. Adv Enzyme Regul. 1985;24:277–299. doi: 10.1016/0065-2571(85)90082-2. [DOI] [PubMed] [Google Scholar]
- Koziolkiewicz M., Stec W. J. Application of phosphate-backbone-modified oligonucleotides in the studies on EcoRI endonuclease mechanism of action. Biochemistry. 1992 Oct 6;31(39):9460–9466. doi: 10.1021/bi00154a019. [DOI] [PubMed] [Google Scholar]
- Stec W. J., Grajkowski A., Koziolkiewicz M., Uznanski B. Novel route to oligo(deoxyribonucleoside phosphorothioates). Stereocontrolled synthesis of P-chiral oligo(deoxyribonucleoside phosphorothioates). Nucleic Acids Res. 1991 Nov 11;19(21):5883–5888. doi: 10.1093/nar/19.21.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cleary A. M., Yakubov L., Lederman S. Phosphorothioate oligodeoxynucleotides bind to the third variable loop domain (v3) of human immunodeficiency virus type 1 gp120. Antisense Res Dev. 1993 Spring;3(1):19–31. doi: 10.1089/ard.1993.3.19. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Tonkinson J. L., Yakubov L. Phosphorothioate oligodeoxynucleotides--anti-sense inhibitors of gene expression? Pharmacol Ther. 1991 Dec;52(3):365–384. doi: 10.1016/0163-7258(91)90032-h. [DOI] [PubMed] [Google Scholar]
- Yakubov L., Khaled Z., Zhang L. M., Truneh A., Vlassov V., Stein C. A. Oligodeoxynucleotides interact with recombinant CD4 at multiple sites. J Biol Chem. 1993 Sep 5;268(25):18818–18823. [PubMed] [Google Scholar]