Abstract
α-Synuclein is one of the most abundant proteins in presynaptic terminals. Normal expression of α-synuclein is essential for neuronal survival and it prevents the initiation of apoptosis in neurons through covalent cross-linking of cytochrome c released from mitochondria. Exocytosis of α-synuclein occurs with neuronal mitochondrial dysfunction, making its detection in cerebrospinal fluid (CSF) of children after severe traumatic brain injury (TBI) a potentially important marker of injury. Experimental therapeutic hypothermia (TH) improves mitochondrial function and attenuates cell death, and therefore may also affect CSF α-synuclein concentrations. We assessed α-synuclein levels in CSF of 47 infants and children with severe TBI using a commercial ELISA for detection of monomeric protein. 23 patients were randomized to TH based on published protocols where cooling (32–33°C) was initiated within 6–24 h, maintained for 48 h, and then followed by slow rewarming. CSF samples were obtained continuously via an intraventricular catheter for 6 days after TBI. Control CSF (n = 9) was sampled from children receiving lumbar puncture for CSF analysis of infection that was proven negative. Associations of initial Glasgow Coma Scale (GCS) score, age, gender, treatment, mechanism of injury and Glasgow Outcome Scale (GOS) score with CSF α-synuclein were compared by multivariate regression analysis. CSF α-synuclein levels were elevated in TBI patients compared to controls (p = 0.0093), with a temporal profile showing an early, approximately 5-fold increase on days 1–3 followed by a delayed, >10-fold increase on days 4–6 versus control. α-Synuclein levels were higher in patients treated with normothermia versus hypothermia (p = 0.0033), in patients aged <4 years versus ≥4 years (p < 0.0001), in females versus males (p = 0.0007), in nonaccidental TBI versus accidental TBI victims (p = 0.0003), and in patients with global versus focal injury on computed tomography of the brain (p = 0.046). Comparisons of CSF α-synuclein levels with initial GCS and GOS scores were not statistically significant. Further studies are needed to evaluate the conformational status of α-synuclein in CSF, and whether TH affects α-synuclein aggregation.
Key Words: Nonaccidental head injury, Abusive head injury, Synaptic dysfunction, Apoptosis, Mitochondrial injury, Cell death, Oxidative stress, Secondary injury
Introduction
α-Synuclein is a ubiquitous synaptic protein that comprises 0.5–1.0% of the brain within the neurons of the neocortex, basal ganglia and cerebellum [Iwai et al., 1995], and is not found in significant quantities in other organ systems [Giasson et al., 2000; Ischiropoulos, 2003; Scherzer et al., 2008; Uéda et al., 1993]. Within the neuron, it is primarily localized to the presynaptic terminal [Jakes et al., 1994] and constitutively exocytosed via endoplasmic-reticulum- and Golgi-apparatus-independent mechanisms [Lee et al., 2005]. There is also evidence that it is found in glial cells [Mori et al., 2002], but is likely first produced in the neuron and transported to glial cells [Lee et al., 2010; Solano et al., 2000]. Recent reports also suggest that a portion of α-synuclein is present in the mitochondrial membrane in normal neurons [Li et al., 2007] and its exocytosis increases with mitochondrial dysfunction [Lee et al., 2005]. The exact function of α-synuclein itself is unclear, but it likely plays a role in synaptic function and maintenance, given its localization to the presynaptic terminal [Jakes et al., 1994]. It is involved in fatty acid metabolism, particularly in mitochondrial cardiolipin acyl chain composition and production [Ellis et al., 2005]. Recently, an antiapoptotic role for α-synuclein has been elucidated as it undergoes lipid-dependent, covalent heterooligomerization with cytochrome c [Bayir et al., 2009b]. Thus, α-synuclein levels in cerebrospinal fluid (CSF) may reflect the progression of synaptic and mitochondrial dysfunction and neuronal apoptosis – important secondary injury mechanisms after traumatic brain injury (TBI) [Ansari et al., 2008; Gobbel et al., 2007; Reeves et al., 1995; Robertson et al., 2007, 2009; Singh et al., 2006].
Studies in patients with degenerative and infectious disorders of the central nervous system indicate the presence, as well as both increases and decreases in levels of α-synuclein monomers in human CSF [Borghi et al., 2000; El-Agnaf et al., 2003; Mollenhauer et al., 2008; Mukaetova-Ladinska et al., 2008; Öhrfelt et al., 2009] as well as in plasma, where soluble aggregates of the protein have also been identified [El-Agnaf et al., 2003, 2006; Fjorback et al., 2007; Mollenhauer et al., 2008]. Despite evidence that TBI victims demonstrate a neuropathology and clinical propensities for neurodegenerative syndromes [Ikonomovic et al., 2004; Uryu et al., 2007], there has not been a study evaluating α-synuclein levels in CSF after TBI in adults or children. Moreover, there have been no previous studies relating CSF α-synuclein levels to any pediatric disease process. During normal neuronal development, α-synuclein is localized to cytosol and growth cones at 3 days in vitro (DIV), then within axons and growth cones by 10–21 DIV [Quilty et al., 2003]. Furthermore, α-synuclein accumulates within damaged axons at 21 DIV and at later time points after injury to growth-cone-like structures in regenerating neurites, suggesting a role for the protein in regeneration and recovery. Corroborating these data, Newell et al. [1999] and Uryu et al. [2007] have described localization of α-synuclein to axonal swellings post mortem in the traumatized brain in adults. The oxidized form, which is prone to aggregation, has also been identified in the neuronal cytoplasm of TBI victims, and likely reflects postinjury modification [Ikonomovic et al., 2004].
The temporal profile of potential CSF neuronal injury markers could have significant ramifications for secondary injury mechanisms and therapeutic interventions. The behavior of α-synuclein in the traumatized brain may depend on several parameters. Age is tied to the progression of synucleinopathies, as well as slower α-synuclein axonal transport [Freichel et al., 2007; Jellinger, 2004; Li et al., 2004; Stéphan et al., 2002; Thiruchelvam et al., 2004]. Age-dependent synaptic density may also influence α-synuclein levels because of the protein's localization to presynaptic terminals. Factors affecting apoptosis, particularly cytochrome-c-mediated caspase 3 activation, may also influence α-synuclein levels and its conformational status. Apoptotic cell death following TBI is well documented [Darwish and Amiridze, 2010; Huang et al., 2009; Jia et al., 2009; Nathoo et al., 2004]. In addition, levels of CSF cytochrome c are increased at 4 days after TBI in females, in nonaccidental head injury victims and in patients with a Glasgow Coma Scale (GCS) score of ≥5 versus 3 or 4 [Satchell et al., 2005]. Injury degree and predicted functional outcome have also correlated with the activation of proapoptotic triggers in humans and animal models [Darwish and Amiridze, 2010; Knoblach et al., 2002; Nathoo et al., 2004]. Based on these previous findings, age, gender, mechanism of injury, time and degree of initial insult may all influence extracellular α-synuclein levels.
Therapeutic hypothermia (TH) has been postulated as a neuroprotective intervention following TBI, though exact mechanisms have yet to be defined [Adelson, 2009; Bayir et al., 2009a; Mansfield et al., 1996]. No study to date has assessed the effect of TH on synaptic dysfunction after TBI, though TH has been shown to preserve certain synaptic properties. In particular, it decreases misfolding and aggregation of proteins in postsynaptic densities of hippocampal neurons after global cerebral ischemia [Capani et al., 2009; Dong et al., 2001]. Hypothermic conditions have also been reported to attenuate the constitutive release of α-synuclein from the presynaptic terminal [Lee et al., 2005]. Whether this mediates the effects of TH on the injured brain is important but unknown.
The current study was aimed at investigating the effect of TBI on CSF α-synuclein levels compared to controls. Patients were randomized to normothermia or TH after admission. Additional goals were to determine the effects of age, gender, mechanism of injury, initial GCS score, 6-month Glasgow Outcome Scale (GOS) score and TH on CSF α-synuclein levels in TBI patients.
Patients and Methods
Patients
This study was approved by the institutional review board of the Children's Hospital of Pittsburgh and informed consent was obtained from parents for sample collection. All patients with severe TBI (GCS score <8) admitted to the Children's Hospital of Pittsburgh are treated with a ventricular catheter insertion, and CSF is drained continuously to limit intracranial hypertension. CSF (n = 155) from 47 infants and children with severe TBI enrolled at our center in 3 concurrent randomized controlled trials assessing the effect and safety of moderate TH in severe TBI in infants and children was utilized for analysis. The general paradigm for patients treated with TH involved cooling to 32–33°C (within either 6 or 24 h following injury) for 48 h, and then gradual rewarming. The details of the study protocols and results of 2 of these trials on clinical outcome have been previously reported [Adelson et al., 2005]. Briefly, patients receiving TH were cooled to 32–33°C for a period of 48 h. The core temperature was measured by rectal probe, and patients in the normothermia arm were also maintained at 36.5–37.5°C for the initial 48-hour study period, and if the patient's initial core temperature was below 36°C, the patient was passively warmed. Neuromuscular blockade and sedation were implemented to prevent shivering in both groups during the study period. Predetermined enrollment criteria were closed head injury with a GCS score of ≤8, age <13 (multicenter trial) or 18 years (single-center trials). Patients were excluded if they had a normal head computed tomography scan, penetrating brain injury, brain death, uncorrectable coagulopathy (prothrombin time/partial thromboplastin time >16/40 s), prolonged hypotension (>15 min), and there was a failure to obtain informed consent within 6 h of injury (the 2 multicenter trials), or a failure to obtain informed consent within 24 h of admission to the Children's Hospital of Pittsburgh (single-center trial). Patient management was in accordance with guidelines for the management of severe pediatric TBI (GCS scores ≤8) [Adelson et al., 2003]. Barbiturates and mechanical ventilation to a PaCO2 of <35 were used as second-tier therapies for refractory intracranial hypertension. Control CSF was excess CSF obtained via lumbar puncture from infants and children who were later proven not to have meningitis based on cell count, culture and Gram stain. CSF samples were centrifuged for 10 min at 5,000 g and stored at −70°C until the time of analysis. Clinical data collected included demographic information, mechanism of injury, GCS score at presentation, survival status and GOS at 6-month follow-up if available.
CSF Collection and ELISA
CSF α-synuclein content was measured by a commercial ELISA kit (Invitrogen Corp., Carlsbad, Calif., USA). The detection limits of the assay were 0.2–15 ng/ml. An optimal dilution was determined and the ELISA was performed according to the manufacturer's instructions.
Statistical Analysis
All values are expressed as means ± standard errors of the mean unless otherwise noted. A nonparametric Wilcoxon test was used to compare daily mean and peak synuclein levels in the cases to the levels in the control population, pairwise comparisons of levels between days after injury, as well as between peak values in focal as opposed to global brain injury. Multivariable longitudinal regression models were used to compare the association between CSF α-synuclein levels and age, gender, time after injury, nonaccidental head injury, treatment (randomization to TH or normothermia) and initial GCS score. Because of multiple observations for each person, the assumption of independent observations necessary for standard regression models was violated. Therefore, generalized estimating equation models and regression models that control for correlation within individuals were used. A logistic regression model was used to estimate the association of peak synuclein levels with outcome as measured by the GOS. Categorical data between groups were assessed using Fisher's exact test. Patients were compared on the basis of age as being less than 4 years or greater than or equal to it, based on data from the National Traumatic Coma Databank that describes unfavorable outcome in TBI patients less than 4 years of age [Levin et al., 1992]. Patient injury as a result of nonaccidental head injury was also considered a factor, given differences in biochemical markers of brain injury seen between accidental TBI and nonaccidental head injury patients [Beers et al., 2007]. p < 0.05 was considered statistically significant.
Results
Patient Demographics
The characteristics of the TBI patients and controls are shown in table 1. Demographics were available for 8 control patients. The age ranged from 4 weeks to 16 years for the TBI patients, and from 5 weeks to 14 years for the controls. Twenty-three patients were randomized to TH and 24 patients were randomized to normothermia. There were proportionally more males in the control group than in the TBI group; however, this was not statistically significant (p = 0.239). The initial GCS score of the TBI patients was used as part of the statistical analysis, and 8 patients initially presented with a GCS score of >8; however, these patients’ level of consciousness deteriorated and met the study criteria within the enrollment period. Aside from the aforementioned, there neither were significantly different clinical or demographic attributes between TBI patients and controls, nor between TH patients and those treated with normothermia.
Table 1.
Clinical and demographic characteristics of study population
| TBI patients | Hypothermia | Normothermia | Controls | |
|---|---|---|---|---|
| Number | 47 | 23 (49) | 24 (51) | 9 |
| Age, years | 7.40 ± 4.99 | 7.04 ± 5.00 | 7.22 ± 5.08 | 4.50 ± 4.58 |
| Female gender | 18 (38) | 8 (35) | 10 (42) | 1 (13) |
| Mechanism | ||||
| Nonaccidental TBI | 11 (23) | 5 (22) | 6 (25) | |
| Fall | 5 (11) | 3 (13) | 2 (8) | |
| MVI | 14 (30) | 8 (35) | 6 (25) | |
| Pedestrian struck by auto | 5 (11) | 2 (9) | 3 (13) | |
| Other | 12 (25) | 5 (22) | 7 (29) | |
| Initial GCS score | ||||
| 3–4 | 9 (19) | 5 (22) | 4 (17) | |
| 5–8 | 30 (64) | 15 (65) | 15 (62) | |
| >8 | 8 (17) | 3 (13) | 5 (21) | |
| GOS score | ||||
| 1-2 | 23 (64) | 13 (65) | 10 (62) | |
| 3-5 | 13 (36) | 7 (35) | 6 (38) |
Values are means ± SD, percentages in parentheses. MVI = Motor vehicle injury.
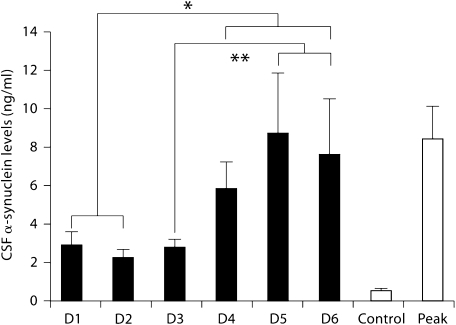
TBI patients demonstrated mean daily α-synuclein levels that were significantly higher than that of controls (fig. 1). CSF mean α-synuclein levels ranged from 2.203 to 8.734 ng/ml in TBI patients, and peak CSF α-synuclein levels during the period of observation averaged 8.381 ± 1.70 ng/ml, which is significantly higher compared to controls (0.463 ± 0.10 ng/ml). TBI patients demonstrated an approximately 5-fold increase in CSF synuclein levels on days 1–3 after injury compared to controls. A delayed elevation >10-fold compared to controls was observed on days 3–5, and regression analysis revealed that there was an effect of time after injury on α-synuclein levels (p = 0.0010). Post hoc analysis demonstrated that values for postinjury days 1 and 2 were significantly lower than those for days 4, 5 and 6. For day 3, the value was also significantly lower than for days 5 and 6.
Fig. 1.
α-Synuclein levels for all TBI patients, inclusive of both those receiving TH and normothermia, during the first 6 days (D1–6) of admission compared to controls and the mean peak level during the period of observation. Levels for D1–6 and peak levels for TBI patients were significantly higher than those for control. ∗ Levels for D4–6 were significantly higher than those for D1 or D2. ∗∗ Levels for D5 and D6 were also significantly higher than D3 levels. Bars: mean values for given day. Error bars: standard errors of the mean. D = Day.
Hypothermia
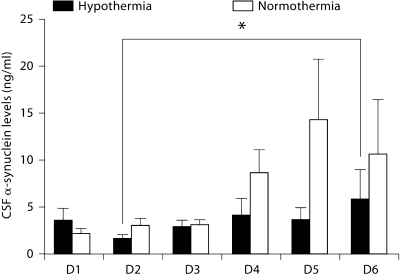
There were no significantly different clinical parameters or demographic characteristics between patients treated with TH or normothermia. TBI patients randomized to TH demonstrated significantly lower levels of CSF α-synuclein overall (p = 0.0033) (fig. 2). To evaluate whether there is a differential time course by treatment, we performed individual pairwise comparisons between days among patients treated with TH. There was no statistically significant difference in patients treated with hypothermia with the exception of day 2 versus day 6 after injury (p = 0.043).
Fig. 2.
α-Synuclein levels for all TBI patients treated with TH versus normothermia. TBI patients randomized to TH demonstrated significantly lower levels of CSF α-synuclein overall. There was a differential time course in patients treated with hypothermia. ∗ Levels demonstrated a significant elevation in day 6 (D6) above D2. D = Day.
Age and Gender
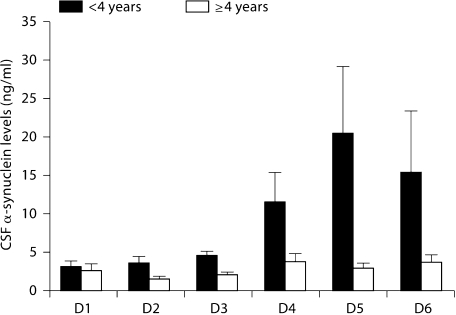
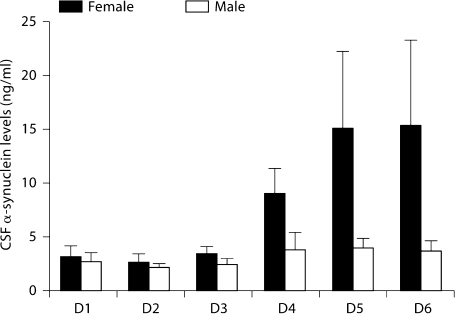
Patients younger than 4 years as well as females demonstrated significantly higher α-synuclein levels (p < 0.0001 and p = 0.0007, respectively) (fig. 3, 4). Interdependence between age and nonaccidental head injury was not found in multivariate regression analysis of the data despite the higher representation of young patients in the nonaccidental head injury group as noted above.
Fig. 3.
α-Synuclein levels for all TBI patients <4 years old versus those 4 years or older. Patients <4 years of age demonstrated significantly higher α-synuclein levels. D = Day.
Fig. 4.
α-Synuclein levels for all female versus male TBI patients. Females demonstrated significantly higher α-synuclein levels. D = Day.
Nonaccidental Head Injury
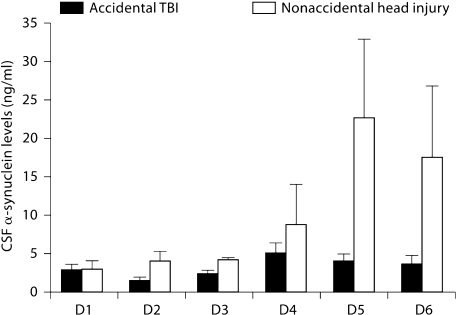
Nonaccidental head injury victims also demonstrated α-synuclein levels significantly elevated above those of accidental TBI patients (p = 0.0003) (fig. 5). The representation of patients <4 years of age in the nonaccidental head injury population was significantly higher than that in the accidental TBI group (p = 0.002), as might be expected. The presence of nonaccidental head injury did not influence the time course of the rise in α-synuclein levels (p = 0.8489).
Fig. 5.
α-Synuclein levels for all trauma patients admitted with a diagnosis of accidental TBI versus nonaccidental head injury. Nonaccidental head injury victims demonstrated α-synuclein levels significantly elevated above those of accidental TBI patients. D = Day.
GCS and Injury Severity
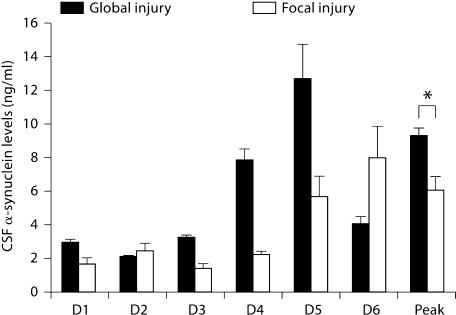
There was no association of the severity of injury as measured by the GCS with synuclein levels. α-Synuclein CSF levels were significantly higher in patients who sustained global brain injury as opposed to focal injury, as assessed by computed tomography of the brain during the patients’ admission (p = 0.046) (fig. 6).
Fig. 6.
α-Synuclein levels for all TBI patients who sustained global brain injury versus focal brain injury. ∗ Peak levels for patients with global injury were higher than levels for patients who had focal injury. D = Day.
Outcome
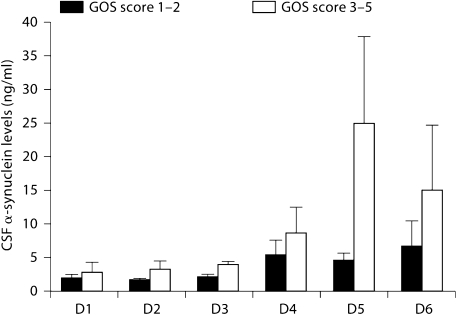
With regard to the GOS, the odds ratio of a higher peak CSF α-synuclein level being associated with either good outcome (GOS score 1 or 2) or poor outcome (GOS 3–5) was not significant. Patients who had a GOS score of 1–2 at the 6-month follow-up had a mean peak CSF α-synuclein level of 6.209 ± 1.76 ng/ml, and those with a GOS score of 3–5 had a level of 15.760 ± 6.409 ng/ml (fig. 7). Regression analysis showed that for a 1 ng/ml increase in peak synuclein in the TBI patients, the odds of having a poor outcome increase by 4.8%, but this was not significant (p = 0.1339).
Fig. 7.
α-Synuclein levels for all TBI patients with 6-month GOS score of 2 or lower versus 3 or greater. There was no statistically significant association of GOS with synuclein levels. D = Day.
Discussion
Our study shows that α-synuclein is significantly elevated in CSF after TBI, and patients who were treated with TH manifest lower α-synuclein levels. We demonstrated that nonaccidental head injury is a significant risk factor for elevated levels of CSF α-synuclein, as well as age and gender, and that these effects were not interrelated. Regression analysis did not demonstrate a significant effect of initial GCS score or 6-month GOS score on α-synuclein levels.
This is the first study to examine CSF α-synuclein levels in TBI. We observed an initial 5-fold increase on days 1–3 followed by a >10-fold increase on days 4–6 in α-synuclein levels in TBI patients compared to controls. This could reflect different mechanisms involved in the progression of TBI pathophysiology. Initial increases could be due to a disruption of tissue after the initial insult with the release of intracellular components into the extracellular space; hence, levels for the first 3 days are elevated over those of controls. Subsequently, the notable rise on day 4 and after may be related to secondary injury mechanisms and therefore amenable to therapeutic interventions. As such, it is possible that significant tissue injury may provoke a release of α-synuclein from glial cells as well, though the largest contribution of soluble α-synuclein is likely from injured neurons [Mori et al., 2002; Solano et al., 2000]. It is also possible that α-synuclein has a protective antiapoptotic effect that accompanies elevated levels of cytochrome c, as demonstrated in previous studies [Hashimoto et al., 1999; Satchell et al., 2005]. Another possibility is that CSF synuclein levels are elevated as a result of synaptic dysfunction and altered transport mechanisms following TBI.
Effect of Hypothermia
TH attenuates proteolysis, inflammation and apoptosis among numerous other biochemical processes in the aftermath of TBI in various animal models [Büki et al., 1999; Huang et al., 2009; Jia et al., 2009; Lotocki et al., 2006]. Secondary injury pathways after TBI are promising avenues of investigation, whether they can directly improve outcome or widen therapeutic windows for other interventions. In addition to reducing early astrocyte c-Jun N-terminal kinase activation [Huang et al., 2009; Lotocki et al., 2006], TH decreases X-linked inhibitor of apoptosis protein cleavage in experimental TBI [Lotocki et al., 2006], and reduces the expression of proapoptotic tissue inhibitor of metalloproteinase-3 expression [Jia et al., 2009]. Our study demonstrates that TH has negative effects on α-synuclein release into the CSF after TBI. This is supported by previous observations that TH attenuates constitutive α-synuclein release from the synapse [Lee et al., 2005]. There are several possibilities as to the underlying mechanisms behind these results. One is that α-synuclein release is increased after TBI, as is the case in other neuronal injury mechanisms [Lee et al., 2005, 2010], and TH reduces its transport and exocytosis. Alternatively, α-synuclein release may be blunted because of a neuroprotective effect from TH against secondary injury mechanisms. A third possible mechanism is that α-synuclein production is upregulated following TBI and TH attenuates its levels. Whether the effects of TH on CSF α-synuclein levels are mediated directly, or are the consequence of other phenomena that can result from TH such as serum electrolyte changes, blood pressure and fluid balance changes, altered immunity and others, remains to be discovered. A larger study population with more frequent α-synuclein sampling would be instrumental in accomplishing this. Finally, mitochondrial dysfunction, inclusive and exclusive of cytochrome c release, is also an important factor in secondary injury following TBI [Robertson et al., 2009; Uzan et al., 2006]. TH is a promising intervention protecting mitochondrial viability in animal models of ischemic injury [Canevari et al., 1999].
Effect of Age
Severe TBI in the 0- to 4-year-old patient has been associated with a 1-year postinjury mortality rate approaching 62% and a poor GOS score at the 6-month follow-up [Levin et al., 1992]. Factors such as the mechanism of injury, relative size of the patient to the injury source as well as synaptic density [Gill et al., 2009; Glantz et al., 2007; Tsujimoto, 2008] may play a role in the significance of the initial insult. Moreover, animal models of pediatric and young adult TBI have described progressive decreases in synaptic proteins in injured cortex and hippocampus suggestive of synaptic dysfunction [Ansari et al., 2008; Gobbel et al., 2007]. Given that synaptic proliferation is exuberant by the age of 2 years in children [Johnston et al., 2009], the synaptic pathology following severe TBI is perhaps more significant in the young than in older populations. In this study, TBI patients <4 years of age had greater elevation in CSF α-synuclein than patients ≥4 years of age. This possibly reflects increased extracellular α-synuclein dispersion after injury after cell lysis, disrupted cell membrane integrity or disrupted regulation of normal exocytosis. Another potential reason for decreased release of α-synuclein from the injured neuron is reduced axonal transport of the protein in older individuals, as seen in a transgenic mouse model [Li et al., 2004]. The age dependence of this phenomenon is unknown, however, and requires further investigation.
Effect of Gender
Memory function following moderate-to-severe pediatric TBI has been demonstrated to be significantly worse in males than in females [Donders and Hoffman, 2002; Donders and Woodward, 2003]. This complements other literature that also describes protective effects of female gender after TBI [Bayir et al., 2004; Berry et al., 2009; O'Connor et al., 2003; Ost et al., 2008], though this has been controversial [Davis et al., 2006; Ponsford et al., 2008]. Mechanisms to explain this have included the extrinsic effect of circulating sex steroids [Berry et al., 2009] as well as greater antioxidant capacity [Du et al., 2004]. There has not been a study to date delineating the behavior of α-synuclein after TBI between males and females; however, work done by Satchell et al. [2005] described a relationship between female gender and increased CSF levels of cytochrome c as an indicator of apoptosis.
Nonaccidental Head Injury
Nonaccidental TBI is a significant cause of head injury in children <4 years of age [Gao et al., 2007; Langlois et al., 2006]. A number of CSF biochemical markers examining inflammatory and excitatory cascades are more pronounced in nonaccidental TBI than in other TBI mechanisms [Bell et al., 1997; Lai et al., 2004; Ruppel et al., 2001], and nonaccidental TBI victims fare more poorly in measures of functional and cognitive testing when matched for injury severity and demographics [Beers, 2007]. Furthermore, Satchell et al. [2005] also found that cytochrome c elevation in nonaccidental TBI patients exceeded levels in accidental TBI patients, suggesting nonaccidental TBI patients are at higher risk for apoptotic cell death following injury. Similarly, in this study, CSF α-synuclein levels were higher in victims of nonaccidental TBI. One might argue that lower CSF levels of both cytochrome c and synuclein would be expected due to sequestration of cytochrome c by α-synuclein during apoptosis forming intracellular aggregates. Clearly, factors affecting production, clearance and interactions with other cellular components for both proteins are involved. Ultimately, increases may correlate more closely with severity of tissue damage following TBI and resultant cell lysis or disrupted permeability. The initial presenting GCS score for each group was not significantly different, however. It is possible that α-synuclein levels are more closely tied to secondary injury magnitude than the degree of initial impact. Lastly, the increases in cytochrome c and α-synuclein seen several days after injury may also reflect accumulation of the proteins in excess of available lipids for aggregation to occur.
GCS and GOS
In the current study, we did not find a correlation between CSF α-synuclein levels and injury severity or global outcome. Several studies in neurodegenerative diseases have also failed to show a consistent correlation between increased serum α-synuclein levels and the presence of disease symptoms/signs [El-Agnaf et al., 2006; Lee et al., 2006; Li et al., 2007; Mollenhauer and Trenkwalder, 2009]. Furthermore, an inverse correlation between CSF α-synuclein levels and disease severity has been shown for patients with Alzheimer's disease and Parkinson's disease [Öhrfelt et al., 2009; Mollenhauer et al., 2008], suggesting the possibility that α-synuclein levels correlate with synaptic integrity and thus show a decline over time with neurodegeneration. Supporting a role for α-synuclein and synaptic density, our study demonstrated higher α-synuclein levels in patients younger than 4 years. However, further studies evaluating other measures of synaptic function in addition to α-synuclein are required to assess this assumption.
Limitations of the Study
Despite the heterogeneity of TBI and how it is affected in terms of mechanism, demographic data and clinical characteristics, this study was able to demonstrate significant elevations in α-synuclein in pediatric TBI patients and several important subgroups. As with other studies investigating children with TBI, it is difficult to obtain control CSF. The demographics of the control CSF donors differed from those of the study population, and the control CSF collection method also differed from that for the trauma patient CSF. It is likely that protein levels in continuously drained CSF would be lower than in lumbar-puncture-derived CSF. Corroborating this, it has been reported that continuously collected CSF demonstrates lower concentrations of neuronal injury markers compared to samples obtained intermittently [Shore et al., 2004]. α-Synuclein exists in a number of different conformational states including aggregated and oligomeric states [Uversky and Eliezer, 2009] in the adult brain after TBI [Ikonomovic et al., 2004; Uryu et al., 2003, 2007], and soluble α-synuclein aggregates have been detected in plasma of patients with Parkinson's disease [El-Agnaf et al., 2006]. The assay we used in this study for the evaluation of α-synuclein in CSF was primarily designed for detection of the monomeric protein, using a monoclonal antibody that identifies amino acid residues 115–125 in the C-terminal domain. It has been shown that nitration and phosphorylation of several amino acid residues in the C-terminal domain of α-synuclein facilitates its oligomerization [Giasson et al., 2000; Uryu et al., 2003]. We previously showed that α-synuclein monomers and its cross-linked complexes with cytochrome c and cardiolipin can be detected by Western blot using an antibody that recognizes the C-terminal 111–131 residues [Bayir et al., 2009b]. The immunoreactivity of monomers to the detection antibody appears grossly similar to that of α-synuclein-cytochrome c-cardiolipin oligomers formed in the presence of hydrogen peroxide. Therefore, it is possible that α-synuclein oligomers were detected by the ELISA we used; however, it is more likely that insoluble aggregated forms would largely be found in tissue, as opposed to soluble monomers in CSF.
Conclusions
In summary, we conclude that CSF α-synuclein is significantly elevated in pediatric TBI, and levels for postinjury days 4–6 are higher than for days earlier in the hospitalization. Levels are significantly higher in victims of nonaccidental TBI, in those under 4 years of age and in female patients. Lastly, elevations in α-synuclein after TBI are also significantly attenuated in patients receiving TH after injury.
Acknowledgements
This study was supported by grants from the National Institutes of Health (NS061817, NS30318, T32 HD040686-10).
References
- Adelson PD. Hypothermia following pediatric traumatic brain injury. J Neurotrauma. 2009;26:429–436. doi: 10.1089/neu.2008.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HEM, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, Warden CR, Wright DW. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1. Introduction. Pediatr Crit Care Med. 2003;4(suppl):S2–S4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, Cassidy LD, Chang Y, Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–754. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- Bayir H, Adelson PD, Wisniewski SR, Shore P, Lai Y, Brown D, Janesko-Feldman KL, Kagan VE, Kochanek PM. Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Crit Care Med. 2009a;37:689–695. doi: 10.1097/CCM.0b013e318194abf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Kapralov AA, Jiang J, Huang Z, Tyurina YY, Tyurin VA, Zhao Q, Belikova NA, Vlasova II, Maeda A, Zhu J, Na H-M, Mastroberardino P-G, Sparvero LJ, Amoscato AA, Chu CT, Greenamyre JT, Kagan VE. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome c: protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009b;284:15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- Beers SR, Berger RP, Adelson PD. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma. 2007;24:97–105. doi: 10.1089/neu.2006.0055. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Wisniewski SR, Whalen MJ, DeKosky ST. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma. 1997;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A. The effect of gender on patients with moderate to severe head injuries. J Trauma. 2009;67:950–953. doi: 10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M. Full length α-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- Büki A, Koizumi H, Povlishock JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol. 1999;159:319–328. doi: 10.1006/exnr.1999.7139. [DOI] [PubMed] [Google Scholar]
- Canevari L, Console A, Tendi EA, Clark JB, Bates TE. Effect of postischaemic hypothermia on the mitochondrial damage induced by ischaemia and reperfusion in the gerbil. Brain Res. 1999;817:241–245. doi: 10.1016/s0006-8993(98)01278-5. [DOI] [PubMed] [Google Scholar]
- Capani F, Saraceno GE, Botti V, Aon-Bertolino L, de Oliveira DM, Barreto G, Galeano P, Giraldez-Alvarez LD, Coirini H. Protein ubiquitination in postsynaptic densities after hypoxia in rat neostriatum is blocked by hypothermia. Exp Neurol. 2009;219:404–413. doi: 10.1016/j.expneurol.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Darwish R, Amiridze N. Detectable levels of cytochrome c and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocrit Care. 2010;12:337–341. doi: 10.1007/s12028-009-9328-3. [DOI] [PubMed] [Google Scholar]
- Davis DP, Douglas DJ, Smith W, Sise MJ, Vilke GM, Holbrook TL, Kennedy F, Eastman AB, Velky T, Hoyt DB. Traumatic brain injury outcomes in pre- and post-menopausal females versus age-matched males. J Neurotrauma. 2006;23:140–148. doi: 10.1089/neu.2006.23.140. [DOI] [PubMed] [Google Scholar]
- Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16:491–499. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- Donders J, Woodward HR. Gender as a moderator of memory after traumatic brain injury in children. J Head Trauma Rehabil. 2003;18:106–115. doi: 10.1097/00001199-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Dong H, Moody-Corbett F, Colbourne F, Pittman Q, Corbett D. Electrophysiological properties of CA1 neurons protected by postischemic hypothermia in gerbils. Stroke. 2001;32:788–795. doi: 10.1161/01.str.32.3.788. [DOI] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38670. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, Cookson MR, Hardy J, Allsop D. α-Synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barceló-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol Cell Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback AW, Varming K, Jensen PH. Determination of α-synuclein concentration in human plasma using ELISA. Scand J Clin Lab Invest. 2007;67:431–435. doi: 10.1080/00365510601161497. [DOI] [PubMed] [Google Scholar]
- Freichel C, Neumann M, Ballard T, Müller V, Woolley M, Ozmen L, Borroni E, Kretzschmar HA, Haass C, Spooren W, Kahle PJ. Age-dependent cognitive decline and amygdala pathology in α-synuclein transgenic mice. Neurobiol Aging. 2007;28:1421–1435. doi: 10.1016/j.neurobiolaging.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gao W-M, Chadha MS, Berger RP, Omenn GS, Allen DL, Pisano M, Adelson PD, Clark RSB, Jenkins LW, Kochanek PM. A gel-based proteomic comparison of human cerebrospinal fluid between inflicted and non-inflicted pediatric traumatic brain injury. J Neurotrauma. 2007;24:43–53. doi: 10.1089/neu.2006.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gill JR, Goldfeder LB, Armbrustmacher V, Coleman A, Mena H, Hirsch CS. Fatal head injury in children younger than 2 years in New York City and an overview of the shaken baby syndrome. Arch Pathol Lab Med. 2009;133:619–627. doi: 10.5858/133.4.619. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbel GT, Bonfield C, Carson-Walter EB, Adelson PD. Diffuse alterations in synaptic protein expression following focal traumatic brain injury in the immature rat. Childs Nerv Syst. 2007;23:1171–1179. doi: 10.1007/s00381-007-0345-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Takeda A, Hsu LJ, Takenouchi T, Masliah E. Role of cytochrome c as a stimulator of α-synuclein aggregation in Lewy body disease. J Biol Chem. 1999;274:28849–28852. doi: 10.1074/jbc.274.41.28849. [DOI] [PubMed] [Google Scholar]
- Huang T, Solano J, He D, Loutfi M, Dietrich WD, Kuluz JW. Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26:1535–1545. doi: 10.1089/neu.2008.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VMY, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Oxidative modifications of α-synuclein. Ann NY Acad Sci. 2003;991:93–100. doi: 10.1111/j.1749-6632.2003.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-Aβ component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Lewy body-related α-synucleinopathy in the aged human brain. J Neural Transm. 2004;111:1219–1235. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- Jia F, Jiang JY, Liang YM, Mao Q (2009): The effect of hypothermia on the expression of TIMP-3 after traumatic brain injury in rats. J Neurotrauma, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Dev. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Nikolaeva M, Huang X, Fan L, Krajewski S, Reed JC, Faden AI. Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J Neurotrauma. 2002;19:1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- Lai Y, Kochanek PM, Adelson PD, Janesko K, Ruppel RA, Clark RSB. Induction of the stress response after inflicted and non-inflicted traumatic brain injury in infants and children. J Neurotrauma. 2004;21:229–237. doi: 10.1089/089771504322972022. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE (2006): Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control.
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Lee G, Park HJ, Bang OY, Joo IS, Huh K. The plasma α-synuclein levels in patients with Parkinson's disease and multiple system atrophy. J Neural Transm. 2006;113:1435–1439. doi: 10.1007/s00702-005-0427-9. [DOI] [PubMed] [Google Scholar]
- Levin HS, Aldrich EF, Saydjari C, Eisenberg HM, Foulkes MA, Bellefleur M, Luerssen TG, Jane JA, Marmarou A, Marshall LF, Young HF. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992;31:435–444. doi: 10.1227/00006123-199209000-00008. [DOI] [PubMed] [Google Scholar]
- Li QX, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Culvenor JG, Horne MK. Plasma α-synuclein is decreased in subjects with Parkinson's disease. Exp Neurol. 2007;204:583–588. doi: 10.1016/j.expneurol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Li W, Hoffman PN, Stirling W, Price DL, Lee MK. Axonal transport of human α-synuclein slows with aging but is not affected by familial Parkinson's disease-linked mutations. J Neurochem. 2004;88:401–410. doi: 10.1046/j.1471-4159.2003.02166.x. [DOI] [PubMed] [Google Scholar]
- Lotocki G, de Rivero Vaccari JP, Perez ER, Alonso OF, Curbelo K, Keane RW, Dietrich WD. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Mansfield RT, Schiding JK, Hamilton RL, Kochanek PM. Effects of hypothermia on traumatic brain injury in immature rats. J Cereb Blood Flow Metab. 1996;16:244–252. doi: 10.1097/00004647-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Ng J, Schulz-Schaeffer W, Kretzschmar HA, McLean PJ, Trenkwalder C, Sarracino DA, Vonsattel J-P, Locascio JJ, El-Agnaf OMA, Schlossmacher MG. Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213:315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trenkwalder C. Neurochemical biomarkers in the differential diagnosis of movement disorders. Mov Disord. 2009;24:1411–1426. doi: 10.1002/mds.22510. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of α-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- Mukaetova-Ladinska EB, Milne J, Andras A, Abdel-All Z, Cerejeira J, Greally E, Robson J, Jaros E, Perry R, McKeith IG, Brayne C, Xuereb J, Cleghorn A, Doherty J, Mcintosh G, Milton I. Alpha- and gamma-synuclein proteins are present in cerebrospinal fluid and are increased in aged subjects with neurodegenerative and vascular changes. Dement Geriatr Cogn Disord. 2008;26:32–42. doi: 10.1159/000141039. [DOI] [PubMed] [Google Scholar]
- Nathoo N, Narotam PK, Agrawal DK, Connolly CA, van Dellen JR, Barnett GH, Chetty R. Influence of apoptosis on neurological outcome following traumatic cerebral contusion. J Neurosurg. 2004;101:233–240. doi: 10.3171/jns.2004.101.2.0233. [DOI] [PubMed] [Google Scholar]
- Newell KL, Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, Hyman BT. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol. 1999;58:1263–1268. doi: 10.1097/00005072-199912000-00007. [DOI] [PubMed] [Google Scholar]
- O'Connor CA, Cernak I, Vink R. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J Neurotrauma. 2003;20:533–541. doi: 10.1089/089771503767168465. [DOI] [PubMed] [Google Scholar]
- Öhrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, Zetterberg H. Cerebrospinal fluid α-synuclein in neurodegenerative disorders: a marker of synapse loss? Neurosci Lett. 2009;450:332–335. doi: 10.1016/j.neulet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Ost M, Nylén K, Csajbok L, Blennow K, Rosengren L, Nellgård B. Apolipoprotein E polymorphism and gender difference in outcome after severe traumatic brain injury. Acta Anaesthesiol Scand. 2008;52:1364–1369. doi: 10.1111/j.1399-6576.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- Ponsford JL, Myles PS, Cooper DJ, Mcdermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SA. Gender differences in outcome in patients with hypotension and severe traumatic brain injury. Injury. 2008;39:67–76. doi: 10.1016/j.injury.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Quilty MC, Gai WP, Pountney DL, West AK, Vickers JC. Localization of α-, β-, and γ-synuclein during neuronal development and alterations associated with the neuronal response to axonal trauma. Exp Neurol. 2003;182:195–207. doi: 10.1016/s0014-4886(03)00108-0. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp Brain Res. 1995;106:248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M, Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Scafidi S, McKenna MC, Fiskum G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppel RA, Kochanek PM, Adelson PD, Rose ME, Wisniewski SR, Bell MJ, Clark RS, Marion DW, Graham SH. Excitatory amino acid concentrations in ventricular cerebrospinal fluid after severe traumatic brain injury in infants and children: the role of child abuse. J Pediatr. 2001;138:18–25. doi: 10.1067/mpd.2001.110979. [DOI] [PubMed] [Google Scholar]
- Satchell MA, Lai Y, Kochanek PM, Wisniewski SR, Fink EL, Siedberg NA, Berger RP, DeKosky ST, Adelson PD, Clark RSB. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab. 2005;25:919–927. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B, Bresnick EH, Schlossmacher MG. GATA transcription factors directly regulate the Parkinson's disease-linked gene α-synuclein. Proc Natl Acad Sci USA. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, Bayir H, Jackson EK, Kochanek PM. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004;21:1113–1122. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Solano SM, Miller DW, Augood SJ, Young AB, Penney JB., Jr Expression of α-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson's disease. Ann Neurol. 2000;47:201–210. [PubMed] [Google Scholar]
- Stéphan A, Davis S, Salin H, Dumas S, Mallet J, Laroche S. Age-dependent differential regulation of genes encoding APP and α-synuclein in hippocampal synaptic plasticity. Hippocampus. 2002;12:55–62. doi: 10.1002/hipo.10006. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human α-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14:345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Chen X-H, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM-Y, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Giasson BI, Longhi L, Martinez D, Murray I, Conte V, Nakamura M, Saatman K, Talbot K, Horiguchi T, McIntosh T, Lee VM-Y, Trojanowski JQ. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol. 2003;184:214–224. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Eliezer D. Biophysics of Parkinson's disease: structure and aggregation of α-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan M, Erman H, Tanriverdi T, Sanus GZ, Kafadar A, Uzun H. Evaluation of apoptosis in cerebrospinal fluid of patients with severe head injury. Acta Neurochir (Wien) 2006;148:1157–1164. doi: 10.1007/s00701-006-0887-1. [DOI] [PubMed] [Google Scholar]