Abstract
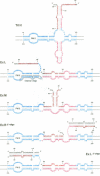
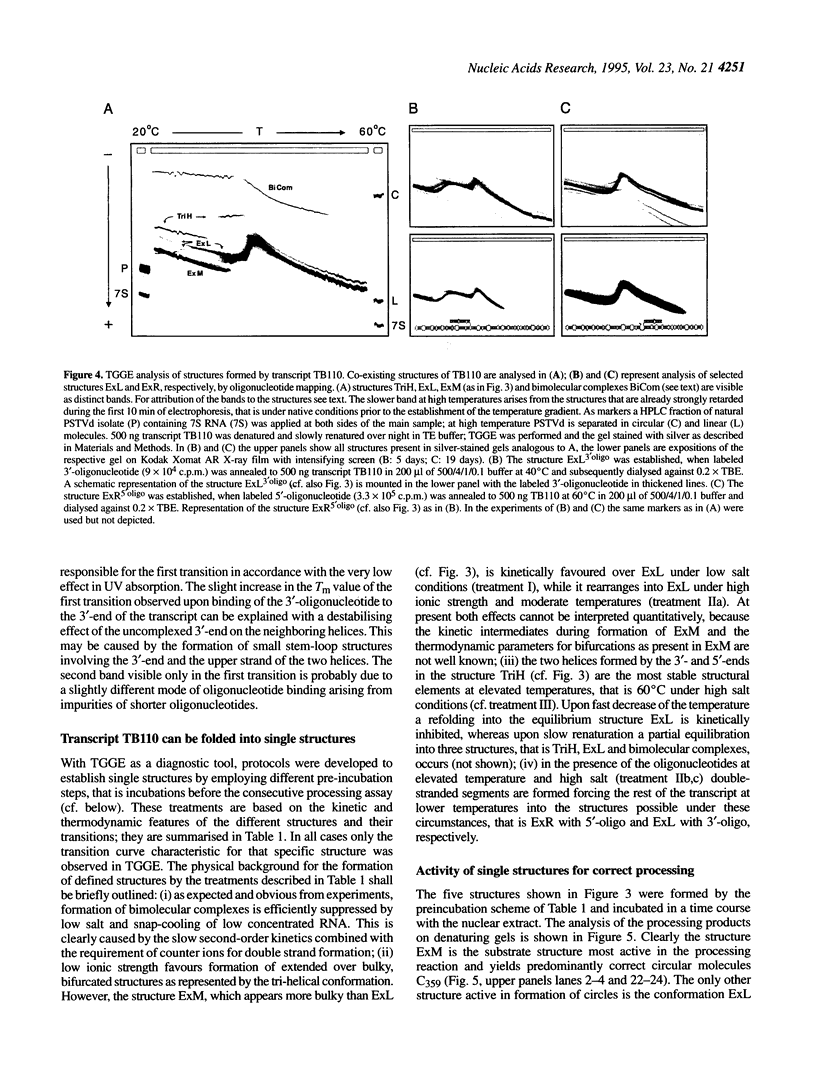
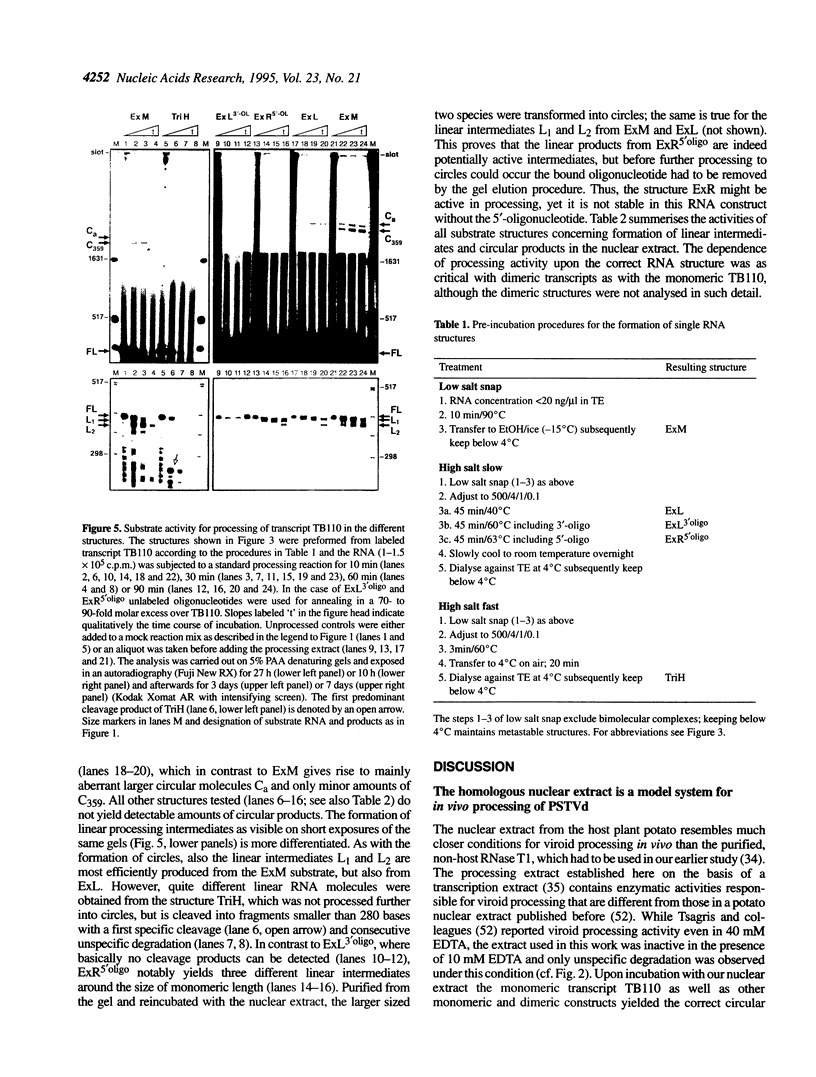
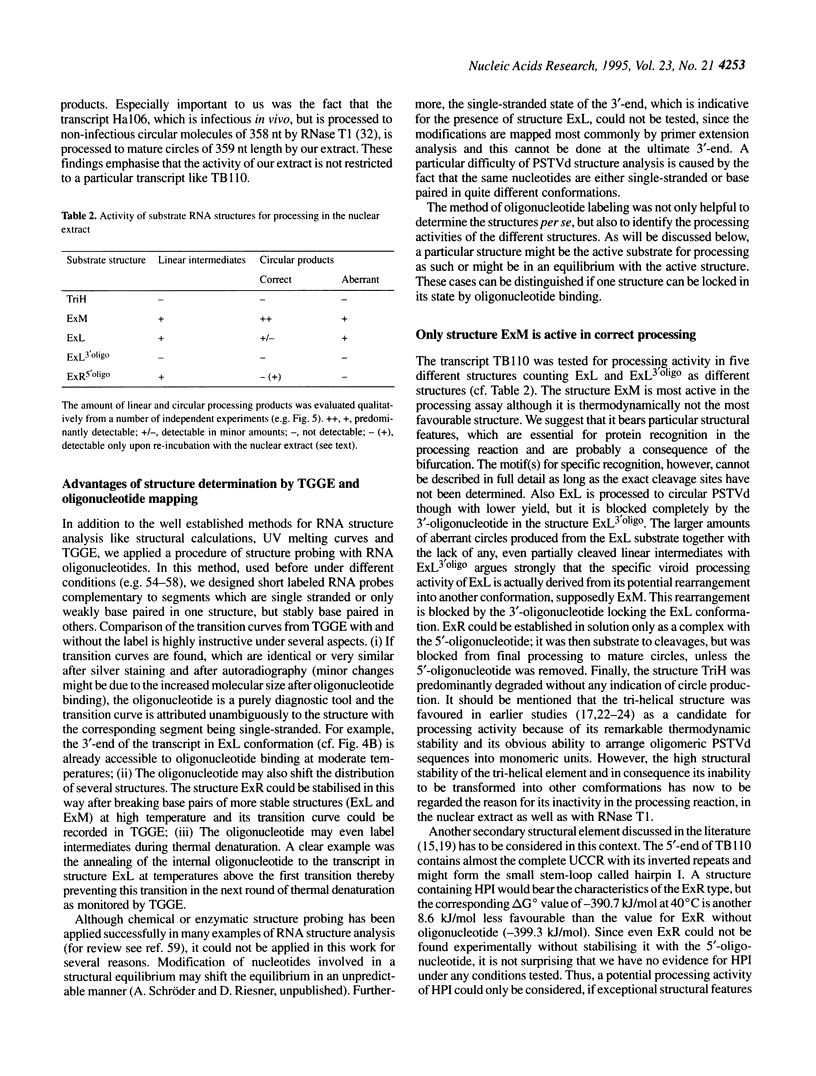
The influence of RNA secondary structure on the substrate activity of a longer-than-unit length transcript for processing to circular viroids was studied in a nuclear extract from potato suspension cells. The nuclear extract was prepared according to a modified procedure for a plant transcription extract. The transcript of the potato spindle tuber viroid (PSTVd) consists of a monomeric molecule with 17 additional nucleotides, thus doubling most of the central conserved region of viroids of the PSTVd-class. The transcript can assume four different secondary structures, which either co-exist as conformers in solution or can be kept as metastable structures after different treatments by temperature and/or ionic strength. The structures were analysed by thermodynamic calculations and temperature-gradient gel electrophoresis and were confirmed by oligonucleotide mapping. Only the so-called extended middle structure was processed to exact viroid circles. In this structure the 5'- and 3'-ends are branching out from the rod-like viroid structure at the loop starting with nucleotide 87. The other structures were processed only if they could be rearranged into the active structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Candresse T., Diener T. O., Owens R. A. The role of the viroid central conserved region in cDNA infectivity. Virology. 1990 Mar;175(1):232–237. doi: 10.1016/0042-6822(90)90203-4. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Viroid processing: a model involving the central conserved region and hairpin I. Proc Natl Acad Sci U S A. 1986 Jan;83(1):58–62. doi: 10.1073/pnas.83.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Baudin F., Mougel M., Romby P., Ebel J. P., Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987 Nov 25;15(22):9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Hammond R. W., Diener T. O., Owens R. A. Infectivity of chimeric viroid transcripts reveals the presence of alternative processing sites in potato spindle tuber viroid. Virology. 1989 Jun;170(2):486–495. doi: 10.1016/0042-6822(89)90440-6. [DOI] [PubMed] [Google Scholar]
- Hecker R., Wang Z. M., Steger G., Riesner D. Analysis of RNA structures by temperature-gradient gel electrophoresis: viroid replication and processing. Gene. 1988 Dec 10;72(1-2):59–74. doi: 10.1016/0378-1119(88)90128-x. [DOI] [PubMed] [Google Scholar]
- Henco K., Sänger H. L., Riesner D. Fine structure melting of viroids as studied by kinetic methods. Nucleic Acids Res. 1979 Jul 11;6(9):3041–3059. doi: 10.1093/nar/6.9.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C., Flores R. Plus and minus RNAs of peach latent mosaic viroid self-cleave in vitro via hammerhead structures. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3711–3715. doi: 10.1073/pnas.89.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Krupp G. RNA synthesis: strategies for the use of bacteriophage RNA polymerases. Gene. 1988 Dec 10;72(1-2):75–89. doi: 10.1016/0378-1119(88)90129-1. [DOI] [PubMed] [Google Scholar]
- LeCuyer K. A., Crothers D. M. Kinetics of an RNA conformational switch. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3373–3377. doi: 10.1073/pnas.91.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbach H. P., Sänder H. L. Continuous replication of potato spindle tuber viroid (PSTV) in permanent cell cultures of potato and tomato. Biosci Rep. 1981 Jan;1(1):79–87. doi: 10.1007/BF01115152. [DOI] [PubMed] [Google Scholar]
- Petersen I., Ohgaki H., Ludeke B., Kleihues P. Direct DNA sequencing following SSCP analysis. Anal Biochem. 1994 May 1;218(2):478–479. doi: 10.1006/abio.1994.1216. [DOI] [PubMed] [Google Scholar]
- Pinard R., Payant C., Brakier-Gingras L. Mutations at positions 13 and/or 914 in Escherichia coli 16S ribosomal RNA interfere with the initiation of protein synthesis. Biochemistry. 1995 Jul 25;34(29):9611–9616. doi: 10.1021/bi00029a038. [DOI] [PubMed] [Google Scholar]
- Rakowski A. G., Symons R. H. Infectivity of linear monomeric transcripts of citrus exocortis viroid: terminal sequence requirements for processing. Virology. 1994 Sep;203(2):328–335. doi: 10.1006/viro.1994.1491. [DOI] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Rigden J. E., Rezaian M. A. In vitro synthesis of an infectious viroid: analysis of the infectivity of monomeric linear CEV. Virology. 1992 Jan;186(1):201–206. doi: 10.1016/0042-6822(92)90074-y. [DOI] [PubMed] [Google Scholar]
- Roberts M. W., Okita T. W. Accurate in vitro transcription of plant promoters with nuclear extracts prepared from cultured plant cells. Plant Mol Biol. 1991 May;16(5):771–786. doi: 10.1007/BF00015070. [DOI] [PubMed] [Google Scholar]
- Rosenbaum V., Riesner D. Temperature-gradient gel electrophoresis. Thermodynamic analysis of nucleic acids and proteins in purified form and in cellular extracts. Biophys Chem. 1987 May 9;26(2-3):235–246. doi: 10.1016/0301-4622(87)80026-1. [DOI] [PubMed] [Google Scholar]
- Schmitz M., Steger G. Base-pair probability profiles of RNA secondary structures. Comput Appl Biosci. 1992 Aug;8(4):389–399. doi: 10.1093/bioinformatics/8.4.389. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Baumstark T., Mörchen M., Tabler M., Tsagris M., Sänger H. L., Riesner D. Structural requirements for viroid processing by RNase T1. J Mol Biol. 1992 Oct 5;227(3):719–737. doi: 10.1016/0022-2836(92)90220-e. [DOI] [PubMed] [Google Scholar]
- Steger G., Tabler M., Brüggemann W., Colpan M., Klotz G., Sänger H. L., Riesner D. Structure of viroid replicative intermediates: physico-chemical studies on SP6 transcripts of cloned oligomeric potato spindle tuber viroid. Nucleic Acids Res. 1986 Dec 22;14(24):9613–9630. doi: 10.1093/nar/14.24.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G. Thermal denaturation of double-stranded nucleic acids: prediction of temperatures critical for gradient gel electrophoresis and polymerase chain reaction. Nucleic Acids Res. 1994 Jul 25;22(14):2760–2768. doi: 10.1093/nar/22.14.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Tzortzakaki S., Tsagris M. Processing of linear longer-than-unit-length potato spindle tuber viroid RNAs into infectious monomeric circular molecules by a G-specific endoribonuclease. Virology. 1992 Oct;190(2):746–753. doi: 10.1016/0042-6822(92)90912-9. [DOI] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Mühlbach H. P., Sänger H. L. Linear oligomeric potato spindle tuber viroid (PSTV) RNAs are accurately processed in vitro to the monomeric circular viroid proper when incubated with a nuclear extract from healthy potato cells. EMBO J. 1987 Aug;6(8):2173–2183. doi: 10.1002/j.1460-2075.1987.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Sänger H. L. Ribonuclease T1 generates circular RNA molecules from viroid-specific RNA transcripts by cleavage and intramolecular ligation. Nucleic Acids Res. 1991 Apr 11;19(7):1605–1612. doi: 10.1093/nar/19.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Baller J., Doty P. Complementary oligonucleotide binding to the anticodon loop of fMet-transfer RNA. Nature. 1970 Feb 7;225(5232):508–510. doi: 10.1038/225508a0. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Forster A. C., Symons R. H. Infectivity and in vitro mutagenesis of monomeric cDNA clones of citrus exocortis viroid indicates the site of processing of viroid precursors. Nucleic Acids Res. 1985 Aug 26;13(16):5843–5856. doi: 10.1093/nar/13.16.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller J. W., Hill W. E. Probing dynamic changes in rRNA conformation in the 30S subunit of the Escherichia coli ribosome. Biochemistry. 1992 Mar 17;31(10):2748–2757. doi: 10.1021/bi00125a015. [DOI] [PubMed] [Google Scholar]
- Zarrinkar P. P., Williamson J. R. Kinetic intermediates in RNA folding. Science. 1994 Aug 12;265(5174):918–924. doi: 10.1126/science.8052848. [DOI] [PubMed] [Google Scholar]