Abstract
Recent work on odor hedonics in schizophrenia has indicated that patients display abnormalities in hedonic judgments of odors in comparison to healthy comparison participants. In the current study, identification accuracy for pleasant, neutral, and unpleasant odors in individuals with schizophrenia and healthy controls was examined. Thirty-three schizophrenia patients (63% male) and thirty-one healthy volunteers (65% male) were recruited. The groups were well matched on age, sex, and smoking status. Participants were administered the University of Pennsylvania Smell Identification Test, which was subsequently divided into 16 pleasant, 15 neutral, and 9 unpleasant items. Analysis of identification z-scores for pleasant, neutral, and unpleasant odors revealed a significant diagnosis by valence interaction. Post-hoc analysis revealed that schizophrenia participants made more identification errors on pleasant and neutral odors compared to healthy controls, with no differences observed for unpleasant odors. No effect was seen for sex. The findings from the current investigation suggest that odor identification accuracy in patients is influenced by odor valence. This pattern of results parallels a growing body of literature indicating that patients display aberrant pleasantness ratings for pleasant odors and highlights the need for additional research on the influence of odor valence on olfactory identification performance in individuals with schizophrenia.
Keywords: olfaction, olfactory, hedonics, anhedonia, pleasantness
1. Introduction
The relationship between olfactory and emotional processing is an area of increasing interest in schizophrenia research, due to the involvement of overlapping neural substrates in the orbitofrontal and temporo-limbic brain regions. Emotional disturbances such as flat affect and anhedonia are prominent and well-characterized clinical features of the illness. Structural and functional abnormalities in olfactory networks are also robust neurobiological findings (Turetsky et al., 2009). How individuals with schizophrenia process the hedonic characteristics of odors is therefore a relevant yet understudied probe of frontotemporal limbic dysfunction and the resulting emotional disturbances observed in schizophrenia.
While schizophrenia deficits in odor identification have been widely replicated (Moberg et al., 1999), considerably less attention has been given to odor hedonic processing. There are, though, an increasing number of studies which suggest that patients’ ability to appreciate the hedonic quality of odors is impaired. The precise nature of this abnormality, however, remains unclear. Schizophrenia patients have been variously reported to exhibit a nonspecific attenuation of their hedonic responses (Hudry et al., 2002) though a selective decrease in their ability to appreciate the pleasantness of odors (Crespo-Facorro et al., 2001; Plailly et al., 2006) as well as an exaggerated assessment of odor pleasantness has been shown (Rupp et al., 2005; Doop & Park, 2006). Consistent with these latter reports, previous work from our laboratory (Moberg et al., 2003) found that male patients failed to appreciate the increasing negative valence associated with higher concentrations of an odorant, even as they correctly perceived the increase in odor intensity. This finding, that schizophrenia patients mis-assign hedonic valence even as they correctly rate the intensity of odors, is one that has been observed repeatedly (Crespo-Facorro et al., 2001; Hudry et al., 2002; Moberg et al., 2003; Doop et al., 2006). Recent data suggest that the tendency to over-rate vs. under-rate the pleasantness of odors may reflect the extent of a patient’s negative symptomatology though here, too, contradictory results have been reported. In a study that was limited to 12 olfactory probe items, but which examined both deficit syndrome and nondeficit patients, Strauss et al. (2010) reported that both groups undervalued the unpleasantness of negative odors, but only deficit syndrome patients undervalued the pleasantness of pleasant odors. In contrast, Doop and Park (2006) found that patients with greater negative symptomatology were more likely to judge an odor as pleasant.
Differences in methodology and sample characteristics are likely to underlie some of the specific differences in these study results. Cumulatively, though, they suggest that schizophrenia patients not only fail to identify odors correctly, but also fail to appreciate their hedonic qualities appropriately. This raises the intriguing question of whether these two domains of olfactory impairment might interact with each other. In particular, since olfactory identification performance is also highly correlated with negative symptomatology (Ishizuka et al., 2010), it is plausible that differences in the hedonic qualities of different odors affect the ability of patients to correctly identify them. Only two studies have actually attempted to look at the interaction of odor valence and identification performance in schizophrenia. Doop and Park (2006) reported that both patients and controls rated correctly identified items as being more pleasant than incorrectly identified items, consistent with the idea that familiarity is associated with liking. More interestingly, Strauss et al. (2010) considered whether identification performance was different for positive and negative valence odors, based on an established normative assessment of each odor’s hedonic attributes (Doty et al., 1984). Although they found no difference in the ability to identify pleasant vs. unpleasant odors, this study was limited by the fact that only 12 items (7 pleasant, 5 unpleasant) were included. Also, because there were so few items, odors considered to be on the pleasant side of a neutral categorization were included in the pleasant group and, similarly, odors on the unpleasant side of neutral were considered unpleasant. This may have militated against the likelihood of finding an association between odor valence and identification performance.
The current study was designed to investigate, in greater detail, the effects of odor valence on odor identification deficits in schizophrenia patients, and to address the weaknesses noted above. We used a well-established 40-item measure of odor identification that included normative data to classify the odors as pleasant, unpleasant or neutral (Doty et al., 1984). Patients were characterized with multiple clinical assessment tools to examine the relationship between specific clinical measures and valence-modulated odor identification performance.
2. Method
2.1 Participants
Thirty-three patients (21 males and 12 females) diagnosed with schizophrenia using DSM-IV criteria (American Psychiatric Association, 2000) and thirty-one healthy volunteers (20 males and 11 females) were recruited by the Schizophrenia Research Center at the University of Pennsylvania Medical Center. All participants underwent a medical and psychiatric evaluation that included a physical examination and the administration of standardized clinical scales. Exclusion criteria included history of psychiatric disorder (other than schizophrenia), neurological disorder, head trauma, loss of consciousness, substance abuse history, or the presence of one of the following: a medical condition that could alter cerebral functioning, an upper respiratory infection, or a condition that could affect olfactory functioning (e.g., common cold). Normal participants were screened for a history Axis I psychiatric illness in themselves and in their first-degree relatives. After providing a complete description of the study to participants, written informed consent was obtained.
Of the patients with schizophrenia, nine were taking atypical antipsychotic medication, 10 were taking typical antipsychotic medication, and six were taking a combination of both typical and atypical antipsychotic medications at the time of testing. Patients and controls did not differ with regard to age (F[1,62] = 3.37, P = 0.11), sex composition (χ2 = 0.01, df = 1, P = 0.94), or smoking status (χ2 = 2.84, df = 2, P = 0.24). They did however differ with regard to ethnic background (χ2 = 7.80, df = 3, P = 0.05) and educational attainment (F[1,62] = 27.65, P < 0.001). Sample demographics are provided in Table 1.
TABLE 1.
Sample Characteristics for schizophrenia (SZ) and healthy comparison (HC) group
| Variable | SZ (N= 33) | HC (N= 31) |
|---|---|---|
| Mean (± SD) | Mean (± SD) | |
| Age (years) | 34.9 (10.2) | 30.7 (10.3) |
| Gender | ||
| Male | N = 28 | N = 25 |
| Female | N = 13 | N = 15 |
| Education (years) | 12.0 (2.0) | 14.7 (2.1)* |
| Ethnic Background+ | ||
| Caucasian | N = 15 | N = 23 |
| African-American | N = 17 | N = 7 |
| Asian-American | N = 0 | N = 1 |
| Other | N = 1 | N = 0 |
| UPSIT (Total Score) | 31.0 (4.1) | 35.8 (3.6)* |
| Pleasant Odors (n=16) | 12.5 (2.0) | 14.4 (1.7)* |
| Neutral Odors (n=15) | 11.1 (2.3) | 13.4 (1.7)* |
| Unpleasant Odors (n=9) | 7.5 (1.6) | 8.0 (1.1) |
| Illness Duration (years) | 11.1 (7.6) | – |
| Age of Onset (years) | 22.5 (7.7) | – |
| SANS Total score | 39.4 (21.9) | – |
| SAPS Total score | 23.2 (21.3) | – |
| BPRS Total score | 35.4 (12.4) | – |
| HAM-D Total score | 11.2 (7.7) | – |
| QOLS Total score | 29.9 (20.0) | – |
SANS and SAPS: Scales for the Assessment of Negative Symptoms (Andreasen, 1983) and Positive Symptoms (Andreasen, 1984); BPRS: Brief Psychiatric Rating Scale (Overall & Gorham, 1980); QOLS: Quality of Life Scale (Heinrichs et al., 1984); HAM-D: Hamilton Rating Scale for Depression (Hamilton, 1960).
patient – control difference, p<.00001
patient – control difference, p=.05
2.2 Clinical assessment
Clinical rating scales were completed on all patients prior to testing, by trained raters with an inter-rater reliability (ICC) greater than 0.85. Patients were administered the following instruments to characterize their clinical and emotional status: Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983), Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984), Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1980), Quality of Life Scale (QOLS; Heinrichs et al., 1984), and the Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1960). Clinical rating scale scores are presented in Table 1.
2.3 Odor identification assessment
Odor identification ability was assessed using the University of Pennsylvania Smell Identification Test (UPSIT; Doty et al., 1984). The 40-item UPSIT is a standardized, four-alternative, forced-choice test of olfactory identification comprised of four booklets containing ten odorants apiece, one odorant per page. The stimuli are embedded in “scratch and sniff” microcapsules fixed and positioned on strips at the bottom of each page. A multiple-choice question with four response alternatives for each item is located above each odorant strip. The specific stimuli, basis for their selection, as well as the reliability and sensitivity of the test, have been described in detail elsewhere (Doty et al., 1984; Doty et al., 1989). The UPSIT was administered birhinally by a trained technician, who released the microencapsulated stimuli, placed the stimuli under the participant’s nares, and recorded the answer following the subject’s response.
To assess the effect of valence on identification performance, the 40 odorants were divided into pleasant, neutral, and unpleasant valence categories using published pleasantness ratings from the UPSIT manual (Doty et al., 1984). UPSIT items from Doty’s original study (1984) were rated on a Likert scale ranging from 1 to 9 with 5 designated as the neutral point. Mean item ratings were obtained and odors with mean item ratings between 4 and 6 were classified as neutral, greater than 6 were classified as pleasant, and less than 4 were classified as unpleasant. In total, 16 items were categorized as pleasant (bubble gum, cherry, banana, fruit punch, licorice, cinnamon, strawberry, chocolate, root beer, pineapple, lime, orange, wintergreen, watermelon, grape, and lemon), 15 items were categorized as neutral (menthol, mint, clove, coconut, cheddar cheese, cedar, ginger bread, lilac, peach, dill pickle, grass, pine, soap, rose, and peanut), and 9 items were categorized as unpleasant (pizza, motor oil, leather, onion, gasoline, turpentine, paint thinner, smoke, and natural gas).
2.4 Data analysis
Analysis of variance (ANOVA) was used to examine differences in the odor identification total score, with diagnosis and sex as grouping factors. To examine differences in the odor identification performance by valence category, the raw scores for pleasant, neutral, and unpleasant odors were rescaled to standard equivalents (Z-transformation) using the means and standard deviations of the healthy control group to control for differences in the number of items in each category. A multivariate repeated measures analysis of variance (MANOVA) was used to examine differences in the identification of pleasant, neutral, and unpleasant odors with diagnosis and sex as grouping factors and valence as the repeated measures factor. Within the patient group, relationships between olfactory performance and clinical measures (duration of illness, age of onset, negative symptoms, positive symptoms, general psychiatric symptoms, quality of life, and depression ratings) were assessed using Spearman rank order correlations, since several of the scales were not normally distributed.
3. Results
3.1 Odor identification
Consistent with the extant literature, patients demonstrated a significant deficit in total UPSIT performance relative to controls (F[1,60] = 24.33, P < 0.001). No main effect was seen for sex (F[1,60] = 0.09, P = 0.77) and no diagnosis by sex interaction (F[1,60] = 0.84, P = 0.36) was observed. Within the schizophrenia group, no differences in total UPSIT score were observed between medicated and unmedicated patients (F[1,28] = 0.76, P = 0.39). There were similarly no differences between patients treated with typical antipsychotics, those treated with atypical agents, or those taking a combination of both typical and atypical antipsychotic medications.
3.2 Odor hedonics
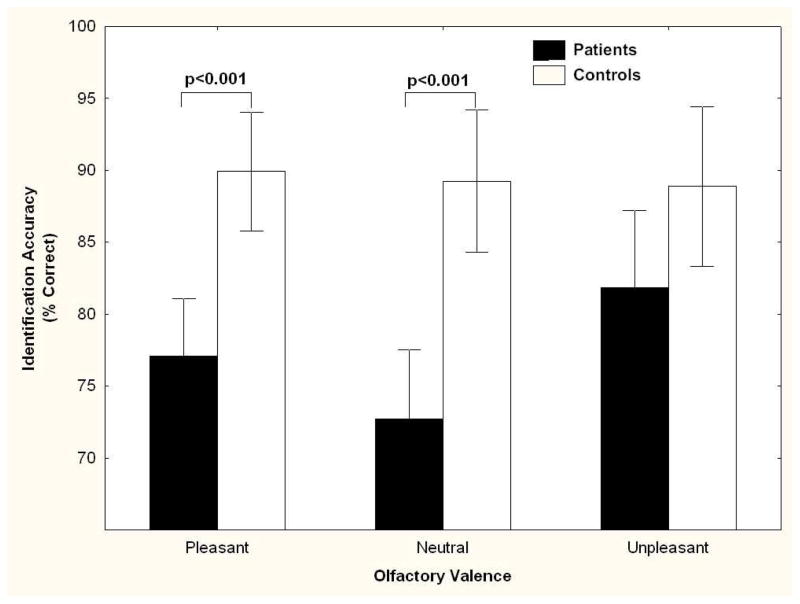
Analysis of identification z-scores for pleasant, neutral, and unpleasant odors revealed a significant diagnosis by valence interaction, F[2,120] = 3.56, P = 0.03 (See Figure 1). Post-hoc contrasts revealed that the schizophrenia participants made significantly more identification errors on pleasant (F[1,60] = 16.63, P < 0.001) and neutral odors (F[1,60] = 20.97, P < 0.001) compared to the healthy control group. In contrast, accuracy did not differ significantly between the schizophrenia and control groups for unpleasant odors (F[1,60] = 2.29, P = 0.14). No effect was seen for sex (F[1,60] = .10, P = 0.75). As shown in Figure 1, the lack of a patient-control difference for unpleasant odors reflected improved performance by patients, not poorer performance by controls. The healthy participants had approximately 90% accuracy in each of the three valence classes.
FIGURE 1. Odor Valence Identification in Schizophrenia Patients Relative to Control Group.
Odor identification accuracy (mean percent correct ± 95% confidence interval) across each odor valence for schizophrenia patients (N=33) relative to healthy controls (N=31).
Analysis of odor identification performance by valence category within the schizophrenia group revealed that significantly more identification errors were made in response to both pleasant (F[1,62] = 9.08, P = .003) and neutral odors (F[1,62] = 12.57, P < 0.001) relative to unpleasant odors. No differences between identification of pleasant and neutral odors was observed (F[1,62] = 0.61, P = 0.44). Inclusion of education and race as covariates in the analysis did not alter the pattern of results observed.
3.3 Olfactory performance and clinical status
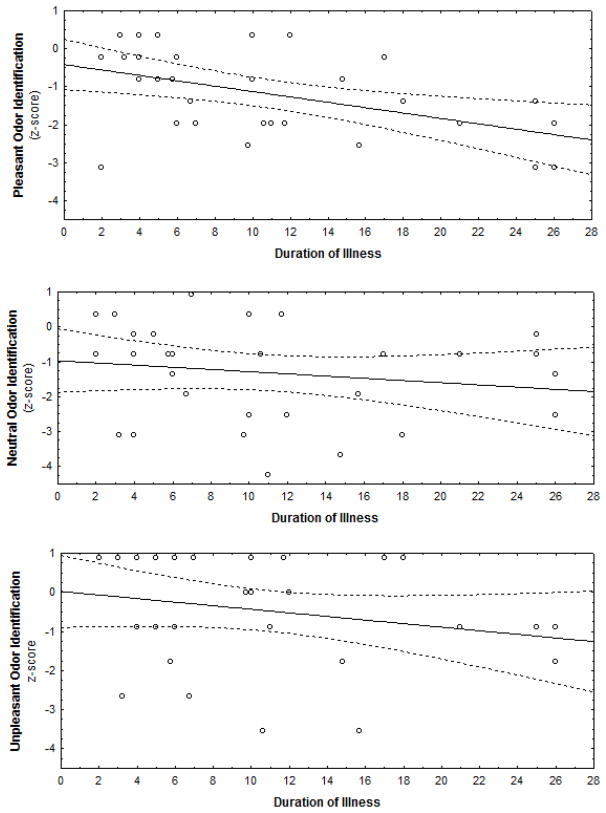
In patients, an inverse relationship between duration of illness and overall UPSIT performance was observed (rs = −.44, P = 0.01) such that longer duration of illness was associated with poorer odor identification abilities. As illustrated in Figure 2, decomposition of this effect by valence category revealed a significant negative relationship between identification of pleasant odors and duration of illness (rs = −.45, P = 0.01) (i.e., longer duration of illness associated with poorer identification of pleasant odors). Notably, the relationship between duration of illness and identification of neutral or unpleasant odors was not statistically significant (all P’s > 0.09). No relationship between age of onset and UPSIT identification indices was observed.
FIGURE 2. Relationship between Illness Duration and Valence Odor Identification Accuracy.
Correlations between odor identification accuracy across each odor valence for schizophrenia patients (N=33). Longer duration of illness was associated with poorer pleasant odor identification (rs = −.45, P = 0.01). Relationships between duration of illness and identification of neutral or unpleasant odors were not statistically significant (all P’s > 0.09)
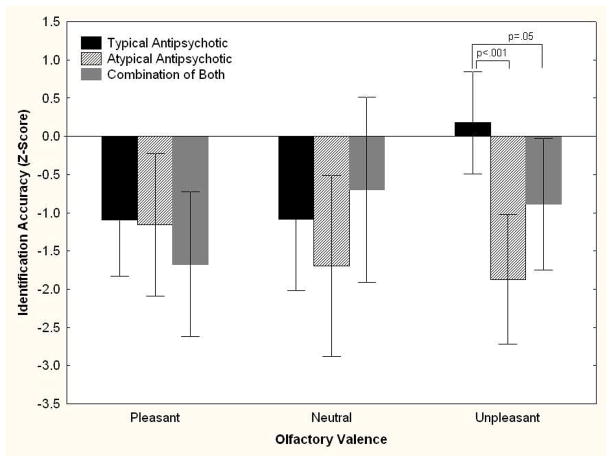
When patients were separated into those taking typical vs. atypical vs. a combination of both types of antipsychotic medications, a significant medication by valence interaction emerged, F[4,38] = 2.71, P = 0.04. Post-hoc comparisons revealed a group difference for unpleasant odors (F[2,19] = 8.10, P = 0.002), but not for pleasant (P = 0.58) or neutral (P = 0.47) odors. As illustrated in Figure 3, patients taking only 1st generation typical antipsychotics performed at the same level as control subjects, while those taking atypical agents performed nearly 2 standard deviations below the controls. Subjects taking both typical and atypical agents were intermediate. Medication dosages were converted to chlorpromazine equivalents using published reference tables (Kroken et al., 2009). The relationships between dosage and identification accuracy for pleasant (rs = −0.06, P = 0.76), neutral (rs = −0.002, P = 0.99), and unpleasant odors (rs = −0.26, P = 0.16) were not statistically significant.
FIGURE 3. Odor Identification in Schizophrenia Patients as a Function of Odor Valence and Antipsychotic Medication Type.
Odor identification accuracy (± 95% confidence interval) across each odor valence for schizophrenia patients prescribed typical antipsychotics (N=10), atypical antipsychotics (N=9), and a combination of both (N=6) relative to healthy controls (N=31), whose performance is set to zero with standard deviation = 1. There was a significant medication X valence interaction among patients (F[4,38] = 2.71, P = 0.04). As indicated, patients taking atypical agents performed better when identifying unpleasant odors.
When patients taking typical antipsychotics were removed from the analysis, the overall interaction was no longer statistically significant (F(2,104) = 1.88, P =.16) as the remaining patients showed impaired identification accuracy across all valence categories. However, the magnitude of this impairment was still substantially greater for pleasant (F(1,52) = 14.78, P < .001, partial ε2 = 0.22) and neutral items (F(1,52) = 24.27, P < .00001; partial ε2 = 0.32) compared to unpleasant items (F(1,52) = 4.82, P =.03; partial ε2 = 0.08). Also, unmedicated patients showed a similar pattern of performance as the entire patient sample. Unmedicated patients were impaired for identification accuracy of pleasant (F[1,34] = 7.77, P = 0.01) and neutral odors (F[1,34] = 15.64, P < 0.001), but showed intact identification accuracy for unpleasant odors (F[1,34] = 1.65, P = 0.21).
Examination of the relationship between olfactory valence identification performance and SAPS and SANS total and subscale scores revealed a significant relationship between identification of unpleasant odors and the SANS anhedonia subscale (rs = 0.44, P = 0.01). Surprisingly, the patients with higher levels of anhedonia were better able to identify unpleasant odors. We observed a similar relationship between HAM-D scores and unpleasant odor identification scores, such that higher depression ratings were associated with improved accuracy for the unpleasant odors (rs = 0.44, P = 0.02). Not surprisingly, these two measures of anhedonia and depression seemed to be indices of the same underlying construct, as they were highly correlated (rs = 0.56, P = 0.004). HAM-D ratings were also significantly related to medication status (F[2,16] = 5.34, P = 0.02). Patients being treated with only typical antipsychotics had significantly higher depression rating scores than patients taking atypical agents, either alone (P = 0.009) or in combination with typicals (P = 0.04). There were no other associations with either SANS or SAPS indices or the BPRS measure of overall psychiatric symptomatology.
QOLS scores were also significantly associated with identification of both pleasant (rs = 0.48, P = 0.01) and unpleasant (rs = 0.39, P = 0.04) odors. Patients with higher quality of life ratings were better at identifying odors at both ends of the hedonic spectrum. No relationship was observed with the identification of neutral odors (rs = 0.21, P = 0.28). Quality of life was not related to the other significant moderating variables, i.e., depression, anhedonia, or medication.
4. Discussion
These findings suggest that the valence of the odor being identified differentially influences odor identification accuracy in schizophrenia patients, with no influence of sex, education, or race. Specifically, we observed that both males and females with schizophrenia displayed significantly reduced identification accuracy for normatively-defined pleasant and neutral odors while showing intact identification for unpleasant odors. These results coincide with a prior finding that patients with schizophrenia display aberrant hedonic ratings for pleasant but not unpleasant odors (Crespo-Facorro et al., 2001). The results of the current investigation extend a prior investigation by Strauss et al. (2010) in which the influence of valence on odor identification performance was examined in deficit and non-deficit patients with schizophrenia. These authors did not observe a differential pattern of identification accuracy for pleasant and unpleasant odors. Rather, patients had comparable levels of impairment for both pleasant and unpleasant odors. Consistent with the reported association between negative symptoms and olfactory impairments, deficit syndrome patients performed worse than non-deficit patients, but this was independent of odor valence. The discrepancy between our results and those of Strauss et al. (2010) may reflect a number of methodological differences, including sample size, sample characteristics (predominantly male schizophrenia sample with longer illness duration), number of olfactory test items (40-item UPSIT vs. 12-item B-SIT), and the strategy for dealing with relatively neutral odors. Strauss et al. (2010) classified all odors as either pleasant or unpleasant, which may have diluted the effects of strong negative hedonic valence. In particular, since odor identification performance deteriorates markedly with longer illness duration (Moberg et al., 1997), the fact that their sample was ill for approximately 10 years more than our sample may have eliminated the valence effect that we observed.
Why might identification accuracy for unpleasant odors be preserved in patients, and why might this be associated, paradoxically, with greater levels of anhedonia and depression? We note that this finding is consistent with data indicating that schizophrenia patients have impaired visual recognition memory for positive valenced pictures, but preserved recognition memory for negative valenced pictures, especially those considered to be highly arousing (Herbener et al., 2007). It has been proposed that individuals with schizophrenia have a bias for preferential processing of negative, potentially threat-related stimuli (Pause et al., 2008) or, alternatively, that they fail to be motivated by potentially pleasant experiences (Herbener et al., 2007). Imaging studies of olfaction suggest that individuals with schizophrenia rely more on prefrontal cortical regions and less on subcortical limbic structures to perform the task of evaluating negative odors (Crespo-Facorro et al., 2001; Schneider et al., 2007). This was interpreted as a compensatory response, in the context of an underlying limbic disturbance, when confronted with a potentially environmentally important negative or threatening stimulus. Greater reliance on prefrontal cortical processing may facilitate, almost as a byproduct, enhanced cognitive processing of these negative stimuli, resulting in improvement in the identification of negative valence odors. If this model is correct, then it would not be surprising that patients with greater levels of affective symptomatology – i.e., those with the greatest limbic dysfunction – would also be those with the greatest ability to identify negative odors.
The finding that hedonic odor identification is modulated by patients’ antipsychotic medication regimen may be either causative or merely coincidental. We observed a strong correlation between treatment with typical antipsychotic agents and levels of depression. It is possible that the side effects associated with these medications increased affective symptom ratings. Alternatively, this may be a serendipitous association in our sample. The current data set does not allow us to entirely differentiate these two possibilities. This would require patient subsamples with different treatment regimens but with comparable levels of affective symptomatology. It is notable, though, that our small subset of unmedicated patients exhibited the same performance profile as the entire patient sample. That is, they differed significantly from controls in their ability to identify pleasant and neutral odors, but not unpleasant odors. These results indicate that the findings from the current study cannot be attributed entirely to medication effects.
Consistent with previous work (Moberg et al., 1997; Ugur et al., 2005) overall odor identification accuracy was strongly associated with longer duration of illness. In addition, an association between duration of illness and identification accuracy for pleasant odors was observed, suggesting that degradation of olfactory abilities differentially affects pleasant odor identification over the course of the illness. Alternatively, it suggests that the processes underlying the preservation of unpleasant odor identification are resistant to the olfactory decline associated with greater chronicity.
Several limitations should be noted. First, our available data on smoking status did not include pack-years, which is considered a more reliable assessment of lifetime smoking behavior, but which may or may not accurately reflect the current impact of smoking. Though extant literature in both schizophrenia (for reviews, see: Martzke et al., 1997; Moberg et al., 1999) and healthy people (Frye et al., 1990; Amoore, 1991) has indicated a negligible impact of smoking on olfactory function, less is known about how smoking history influences odor valence perception. Second, subjective participant ratings of odor pleasantness were not ascertained. So, although patients’ identification performance varied with respect to normative differences in hedonic quality, we do not know how this relates to subjects’ own perceptions of hedonic valence. The current study was also limited by the specific odors used for assessment. Odors are typically described across a variety of dimensions including pleasantness, edibility, familiarity, and intensity. Although the UPSIT was constructed to balance these factors and, in particular, to select odors of comparable intensity (Doty et al., 1984), subtle differences between odor groups on one or more of these dimensions may have influenced odor identification ability in our sample. Furthermore, there were a fewer number of unpleasant odorants assessed compared to pleasant and neutral odorants. Though standard deviations did not differ between the odor valence types, a balanced number of odorants for each valence would have been more helpful in comparing accuracy across subtypes. Lastly, our findings on relationships between mood states, medication status, and valence identification accuracy were preliminary given the methodological limitations of the current study. Future studies controlling for these limitations would be an important step in furthering our understanding of odor hedonic processing in schizophrenia and the influence of variables such as medication status and affective symptomatology.
Collectively, the results of the current study suggest that psychophysical probes of the olfactory network are relevant to difficulties with hedonic capacity observed in schizophrenia. While more research is needed to elucidate hedonic processing of odors in schizophrenia and its relationship both to structural and functional measures of brain function and to cognition, the findings of the current study suggest a preservation of processing for unpleasant odors. These findings highlight the need for additional research that examines the functional and structural deficits that underlie differences in odor valence identification in schizophrenia.
Acknowledgments
This study was funded in part by National Institutes of Health Grants MH-63381 to Paul Moberg, MH-59852 to Bruce Turetsky, and an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Paul Moberg. The authors thank the Hofmann Trust for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Author; Washington, DC: 2000. text revision. [Google Scholar]
- Amoore J. Specific anosmias. In: Getchell T, Doty R, editors. Smell and Taste in Health and Disease. Raven Press; New York: 1991. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. Journal of the American Medical Association. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Doop ML, Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophrenia Research. 2006;81:317–319. doi: 10.1016/j.schres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiology & Behavior. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Perception & Psychophysics. 1989;45:381–384. doi: 10.3758/bf03210709. [DOI] [PubMed] [Google Scholar]
- Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. Journal of the American Medical Association. 1990;263:1233–1236. [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT., Jr The Quality of Life Scale: An instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. Journal of Abnormal Psychology. 2007;116:43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- Hudry J, Saoud M, D’Amato T, Dalery J, Royet JP. Ratings of different olfactory judgements in schizophrenia. Chemical Senses. 2002;27:407–416. doi: 10.1093/chemse/27.5.407. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Tajinda K, Colantuoni C, Morita M, Winicki J, Le C, Lin S, Schretlen D, Sawa A, Cascella NG. Negative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification test. Neuroscience Research. 2010;66:106–110. doi: 10.1016/j.neures.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroken RA, Johnsen E, Ruud T, Wentzel-Larsen T, Jorgensen HA. Treatment of schizophrenia with antipsychotics in Norwegian emergency wards, a cross-sectional national study. BMC Psychiatry. 2009;9:24. doi: 10.1186/1471-244X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzke JS, Kopala LC, Good KP. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biological Psychiatry. 1997;42:721–732. doi: 10.1016/s0006-3223(96)00442-8. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Doty RL, Turetsky BI, Arnold SE, Mahr RN, Gur RC, Bilker W, Gur RE. Olfactory identification deficits in schizophrenia: correlation with duration of illness. American Journal of Psychiatry. 1997;154:1016–1018. doi: 10.1176/ajp.154.7.1016. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Kohler C, Kanes S, Seigel S, Gur RE, Turetsky BI. Impairment of odor hedonics in men with schizophrenia. American Journal of Psychiatry. 2003;160:1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- Overall M, Gorham DR. The brief psychiatric rating scale. Journal of Operational Psychiatry. 1980:48–64. [Google Scholar]
- Pause BM, Hellmann G, Goder R, Aldenhoff JB, Ferstl R. Increased processing speed for emotionally negative odors in schizophrenia. International Journal of Psychophysiology. 2008;70:16–22. doi: 10.1016/j.ijpsycho.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Plailly J, d’Amato T, Saoud M, Royet JP. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage. 2006;29:302–313. doi: 10.1016/j.neuroimage.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, Oberbauer H, Scholtz AW, Wanko C, Hinterhuber H. Various bilateral olfactory deficits in male patients with schizophrenia. Schizophrenia Bulletin. 2005;31:155–165. doi: 10.1093/schbul/sbi018. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Research. 2007;155:103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophrenia Bulletin. 2010;36:860–868. doi: 10.1093/schbul/sbn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophrenia Bulletin. 2009;35:1117–1131. doi: 10.1093/schbul/sbp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur T, Weisbrod M, Franzek E, Pfuller U, Sauer H. Olfactory impairment in monozygotic twins discordant for schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2005;255:94–98. doi: 10.1007/s00406-004-0536-8. [DOI] [PubMed] [Google Scholar]