Abstract
We report the case of a 63-year-old woman who presented with the rare finding of a distal choledochocele in a pancreas divisum with recurrent abdominal pain and episodes of pancreatitis. She underwent successful resection with choledochectomy, papillectomy and reconstruction with a hepatico-jejunostomy and reinsertion of the uncinate pancreatic duct into the same jejunal loop. Comparable literature findings are discussed with regard to the presented case.
Key Words: Choledochal cyst, Pancreas divisum, Surgical management
Case Report
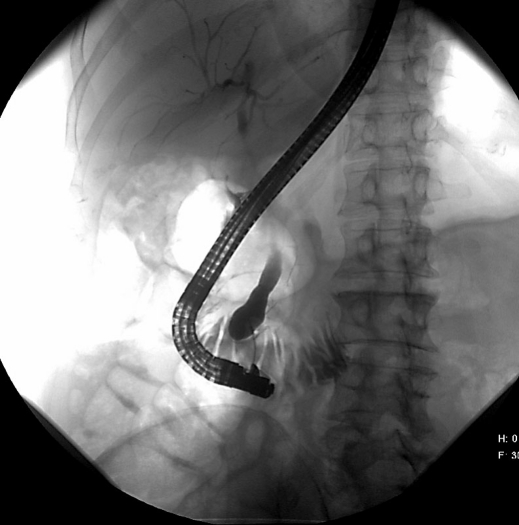
A 63-year-old woman presented to our department with recurrent episodes of postprandial right upper quadrant abdominal pain, nausea and vomiting with recurrent episodes of cholangitis and mild acute pancreatitis. She had undergone open cholecystectomy for similar symptoms 20 years earlier but had suffered from unchanged episodes of pain and nausea after the operation which had accentuated recently. Diagnostics included endoscopy with ERCP, MRI scan and standard laboratory analyses. In duodenoscopy, the minor papilla was regular. At the major papilla there was a spheric impression into the duodenal lumen. This correlated with the ERCP finding, which demonstrated a massive dilation of the distal choledochal duct, morphologically Todani type III [1], with a short pancreatic duct of about 1.5 cm in the uncinate process (fig. 1). Similar findings were described in the MRI without revealing any tumorous or other morphologic reason for the pathologies. Laboratory findings showed elevated AP (339 U/l), GGT (546 U/l) and LDH (281 U/l) with normal serum bilirubin, pancreatic enzymes and tumor markers (CEA, CA 19-9).
Fig. 1.
Endoscopic retrograde cholangiography showing cystic dilation of the distal bile duct.
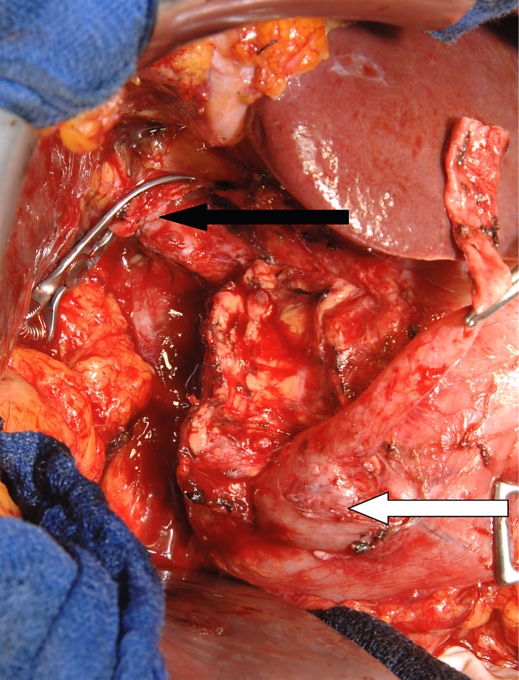
The patient underwent surgical exploration. Intraoperatively, the preoperative findings were confirmed with a massive dilation of the bile duct and soft pancreatic tissue without inflammatory alterations. There were no signs of a malignant tumor neither of the bile duct nor the pancreas. Consequently, a bile duct resection with dissection of the duct from the pancreatic head and excision of the papilla of Vater with a small duodenal wall segment was performed (fig. 2). The pancreatic duct draining the uncinate process was transected during bile duct preparation, the minor pancreatic papilla was not touched. Reconstruction included an end-to-side hepatico-jejunostomy and a pancreatic duct-jejunostomy end to side into the same jejunal loop with 7-0 PDS sutures as well as a Roux-en-Y jejunal reconstruction.
Fig. 2.
Intraoperative aspect. Pancreatic head seen from the dorsal side, the hepatic duct is transected and proximally clamped (black arrow). The distal bile duct is carefully dissected from the pancreatic head, the distal choledochocele is still adherent to the duodenal wall (white arrow).
Histological examination of the specimen showed morphological signs of a bile duct dilation correlating to a choledochocele with chronic inflammation and beginning fibro-sclerotic alterations of the excised distal papilla. No malignant features were found in the specimen. The postoperative course was uneventful, the patient recovered quickly, abdominal drains were removed on the 4th postoperative day. At that time, pancreatic enzymes in the secretion fluid were identical to the serum equivalents within the normal range. Postoperative endocrine as well as exocrine pancreatic functions were normal. An asymptomatic elevation of AP and GGT (peak AP 240 U/l and GGT 340 U/l) as a sign of a slight cholangitis was treated with levofloxacin orally. The patient was discharged 10 days postoperatively.
Discussion
Bile duct anomalies with dilative or cystic character are mainly reported in children and are commonly observed as congenital anomalies [1, 2, 3]. Usually, these cystic malformations are classified according to the system of Todani, established in 1977 [1], although clinical practicability and prognostic relevance of this classification system is discussed controversely [4]. In adults, several cases of cystic malformations have been reported, most of them presenting as type I and type IV [4, 5]. Type III cysts presenting as distal choledochoceles are very rare and to our knowledge there are only 4 published cases of cystic malformations of the bile duct combined with a pancreas divisum. All of these cases were treated endoscopically [6]. Cystic bile duct anomalies require treatment due to two major aspects: first the clinical symptoms such as upper abdominal pain or recurrent episodes of cholangitis and pancreatitis that lead to the diagnosis in most of the cases; second due to the malignant potency with a high risk of developing cystic adenocarcinoma of the bile duct [4, 5]. In the reported case, the clinical symptoms were present for more than two decades before they consecutively led to the diagnosis after initial cholecystectomy had been performed for suspected cholecystolithiasis without long-lasting beneficial effects.
This underlines the importance of an accurate and detailed diagnostic workup of these patients who present with rather unspecific abdominal symptoms. Routine abdominal ultrasound may fail to reveal cystic malformations, especially in type III cysts located close to the duodenal wall due to interposition of air or poor examination conditions. Endoscopy, endoscopic ultrasound, ERCP (fig. 1) and CT or MRI scan should be applied as additional diagnostic tools to evaluate the surrounding tissue and detect other possible anomalies (e.g. pancreas divisum) [7, 8].
Therapy of choledochal cysts should be surgical by the time of detection [4, 5, 9]. Lipsett and Locke report that an early resection of the pathologic duct structures correlates with a good outcome and a very low incidence of late malignancy [9]. Therefore, it seems inadequate to simply observe cystic bile duct anomalies after diagnosis even in asymptomatic patients. The type of surgical resection ranges from cyst excision under conservation of the bile duct up to complete bile duct resection; even a Whipple procedure may be indicated depending on the type and extent of the cystic transformation [4, 5, 9, 10]. For understanding the anatomy and pathophysiology of distal pancreato-biliary duct anomalies, the evolution and variability of the ductal structures close to the papilla of Vater is essential [11]. As cystic variations of the pancreatic ducts can be a symptom of chronic pancreatitis as well as IPMN with completely different prognostic value, these anomalies must be treated with highly differentiated surgical concepts and individual patient-related therapies [12].
We performed a combination of bile duct resection, papillectomy, hepatico-jejunostomy and jejunal reinsertion of the uncinate pancreatic duct (fig. 2). With this surgical procedure all pathophysiological and anatomical particularities of our individual patient were respected. Resection of the pre-cancerous bile duct lesion was radical, the residual major pancreatic duct was reinserted to ensure drainage of the uncinate process and prevent chronic segmental pancreatitis. Besides, physiologic pancreatic drainage of the major part of the pancreas via the Santorini duct was preserved as well as physiologic transduodenal food passage. A Whipple procedure would have been an alternative surgical option. However, by the time of operation there was no evidence of a malignant transformation of the cyst, which was confirmed later by histological examination. Therefore, a major pancreatic resection, with the risk of a subsequent loss of exocrine or endocrine function as well as alteration of food passage, was not justified.
Lipsett et al. [9, 10], who have published the largest series of patients with cystic bile duct lesions, recommend a life-long regular follow-up examination due to the persistent possibility of a malignant transformation of the bile duct disease after resection. Due to the low incidence of cystic bile duct disease there are no standardized guidelines for follow-up procedures, besides clinical examination, transabdominal ultrasound and laboratory parameters including AP, GGT, bilirubin and CA 19-9 seem sufficient for standard postoperative follow-up.
In conclusion, cystic bile duct lesions are rare anatomic varieties causing abdominal symptoms similar to other, especially gallbladder, pathologies. They should be taken into account in patients with these complaints and require detailed and accurate diagnostic measures. Therapy should usually be a surgical resection under consideration of all particular individual anatomical features, as these bile duct anomalies represent pre-cancerous lesions and surgical therapy can be performed with low morbidity and mortality in specialized centers.
References
- 1.Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 2.Saing H, Tam PK, Lee JM, Pe-Nyun Surgical management of choledochal cysts: A review of 60 cases. J Pediatr Surg. 1985;20:443–448. doi: 10.1016/s0022-3468(85)80238-4. [DOI] [PubMed] [Google Scholar]
- 3.Kaneyama K, Yamataka A, Kobayashi H, Lane GJ, Miyano T. Mixed type I and II choledochal cyst: a new clinical subtype? Pediatr Surg Int. 2005;21:911–913. doi: 10.1007/s00383-005-1510-x. [DOI] [PubMed] [Google Scholar]
- 4.Soreide K, Korner H, Havnen J, Soreide JA. Bile duct cysts in adults. Br J Surg. 2004;91:1538–1548. doi: 10.1002/bjs.4815. [DOI] [PubMed] [Google Scholar]
- 5.Visser BC, Suh I, Way LW, Kang SM. Congenital choledochal cysts in adults. Arch Surg. 2004;139:855–660. doi: 10.1001/archsurg.139.8.855. discussion 860-862. [DOI] [PubMed] [Google Scholar]
- 6.Dalvi AN, Pramesh CS, Prasanna GS, Rege SA, Khare R, Ravikiran CS. Incomplete pancreas divisum with anomalous choledochopancreatic duct junction with choledochal cyst. Arch Surg. 1999;134:1150–1152. doi: 10.1001/archsurg.134.10.1150. [DOI] [PubMed] [Google Scholar]
- 7.Prasad P, Wittmann J, Pereira SP. Endoscopic ultrasound of the upper gastrointestinal tract and mediastinum: diagnosis and therapy. Cardiovasc Intervent Radiol. 2006;29:947–957. doi: 10.1007/s00270-005-0184-z. [DOI] [PubMed] [Google Scholar]
- 8.Mazziotti S, Costa C, Ascenti G, Gaeta M, Pandolfo A, Blandino A. MR cholangiopancreatography diagnosis of juxtapapillary duodenal diverticulum simulating a cystic lesion of the pancreas: usefulness of an oral negative contrast agent. AJR Am J Roentgenol. 2005;185:432–435. doi: 10.2214/ajr.185.2.01850432. [DOI] [PubMed] [Google Scholar]
- 9.Lipsett PA, Locke JE. Biliary cystic disease. Curr Treat Options Gastroenterol. 2006;9:107–112. doi: 10.1007/s11938-006-0029-0. [DOI] [PubMed] [Google Scholar]
- 10.Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003;10:352–359. doi: 10.1007/s00534-002-0797-4. [DOI] [PubMed] [Google Scholar]
- 11.Flati G, Andrén-Sandberg A. Wirsung and Santorini: the men behind the ducts. Pancreatology. 2002;2:4–11. doi: 10.1159/000049441. [DOI] [PubMed] [Google Scholar]
- 12.Talbot ML, Foulis AK, Imrie CW. Total dorsal pancreatectomy for intraductal papillary mucinous neoplasm in a patient with pancreas divisum. Pancreatology. 2005;5:285–288. doi: 10.1159/000085284. [DOI] [PubMed] [Google Scholar]