Abstract
A 60-year-old man presented with melena and hematemesis in 1984. Esophagogastroduodenoscopy (EGD) detected a small protruding lesion in the duodenal bulb, which was diagnosed as Brunner's adenoma. No significant change was detected in subsequent annual EGD and biopsies for 10 years, after which the patient was not observed for 7 years. The patient presented with melena again in 2001. The lesion had changed shape to become a 10 mm sessile tumor with a central depression, and following a biopsy was diagnosed as an adenocarcinoma. The patient underwent partial resection of the duodenum. Histopathological assessment showed acidophilic cells with swollen nuclei, and clear cells forming a tubular or papillary tubule in the mucosal lamina propria and submucosal layer. The tumor cells stained positive for lysozyme, indicating that they arose from Brunner's gland. The patient showed no sign of recurrence and was disease-free for more than 34 months after surgery. The patient died of pneumonia. This is an extremely rare case of primary duodenal carcinoma arising from Brunner's gland in a patient observed for 17 years.
Key Words: Brunner's gland, Adenocarcinoma, Duodenal cancer
Introduction
The increased use of endoscopies in recent years has led to an increase in the identification of duodenal lesions; however, primary duodenal carcinomas remain comparatively rare and account for only 0.3% of digestive organ carcinomas [1, 2, 3]. Furthermore, tumors arising from Brunner's gland are extremely rare [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23], and only two reports describe the preoperative observation period [11, 21]. Here we report on an extremely rare case of primary duodenal carcinoma arising from Brunner's gland in a patient who had been under observation for 17 years.
Case Report
A 60-year-old man presented with melena and hematemesis in 1984. Esophagogastroduodenoscopy (EGD) detected a small protruding lesion in the duodenal bulb, which was diagnosed as Brunner's adenoma. Subsequent annual EGD and biopsies showed no significant changes for 10 years, after which the patient was lost to follow-up for 7 years. He again presented with melena in 2001. The lesion had changed shape to become a 10 mm sessile tumor with a central depression according to EGD (fig. 1) and hypotonic duodenography (fig. 2), and following a biopsy was diagnosed as adenocarcinoma. Laboratory test results, including those from tumor marker (CEA and CA19-9) assays, were within normal limits. The patient underwent partial resection of the duodenum.
Fig. 1.

Esophagogastroduodenoscopy showing a 10 mm sessile tumor with a central depression in the duodenal bulb.
Fig. 2.
Hypotonic duodenography showing a contrast media-positive elevated lesion in the center of the duodenal bulb.
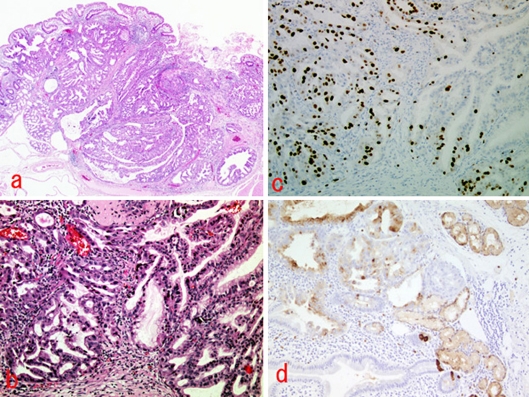
Histopathology assessment found acidophilic cells with swollen nuclei, and clear cells forming a tubular or papillary tubule in the mucosal lamina propria and submucosal layer (fig. 3a, b). Immunohistological staining showed the tumor to be negative for chromogranin-A, p-53, lipase and amylase. Immunochemical staining showed the tumor cells to be positive for MIB-1 (fig. 3c) and lysozyme (fig. 3d), indicating that they arose from Brunner's gland.
Fig. 3.
Microscopic findings of the tumor. a Gross appearance following hematoxylin and eosin staining (×2). b Adenocarcinoma in an adenoma (hematoxylin and eosin stain, ×10). c MIB-1-positive neoplastic cells (×10). d Lysozyme-positive neoplastic cells (×10).
The patient showed no sign of recurrence in abdominal computed tomography and tumor markers and was disease-free for more than 34 months after surgery. He subsequently died of pneumonia.
Discussion
Duodenal carcinoma may arise in different types of cells of the duodenal mucosa. It has been proposed that they arise as either de novo lesions, or from adenomas or aberrations of the pancreas or gastric mucosa [24, 25]. Carcinomas arising from Brunner's gland are very rare, and only 21 such cases have been reported in the literature, the first of which was by Shorrock et al. in 1986 [4]. Immunohistochemical examination is essential for determining the origin of carcinomas arising from Brunner's gland. In the present case, a Brunner's gland adenocarcinoma was indicated by positive MIB-1 and lysozyme staining, the absence of a surrounding normal Brunner's gland, and negative staining for chromogranin A, p53, lipase and amylase.
In 2002, Akino et al. summarized 16 cases of carcinoma arising from Brunner's gland [3, 4, 9, 11, 14, 19, 26, 27], and five further cases were later reported [20, 21, 22, 23]. Our analysis of these 20 cases shows they involved 15 men and 5 women, with a mean age of 67.4 years (range 39–85 years) [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23] (table 1). Eight tumors were located in the first portion of the duodenum and twelve in the second portion. In terms of shape, five tumors mimicked submucosal tumors, seven were sessile, four were type 2 carcinomas, and four were polypoid lesions. The mean tumor diameter was 25.6 mm (range 7–70 mm). Four tumors were limited to the mucosal layer, nine showed submucosal invasion, and six were advanced carcinomas. The variety in the tumor forms reflects that Brunner's gland exists in the deep lamina propria of the mucosa or in a submucosal layer [28]. All tumors were diagnosed as highly differentiated adenocarcinomas. Ten cases underwent limited resection, comprising six partial resections of the duodenum, two endoscopic mucosal resections, and two polypectomies. Those ten resection cases showed relatively good outcomes over a mean observation period of 21.6 months (range 3–45 months).
Table 1.
Published cases of duodenal cancer arising from Brunner's gland (1986-2007)
| No. | Author | Year | Age | Gender | Location | Macroscopic appearance | Maximum size (mm) | Depth of invasion | Histological type | Preoperative observation time (years) | Operation | Outcome (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shorrock [4] | 1986 | 51 | M | 2nd | polypoid | unknown | sm | adenocarcinoma | - | PD | A (18) |
| 2 | Ohmachi [5] | 1989 | 64 | M | 1st | sessile | 7 | sm | pap | - | DG | A (9) |
| 3 | Kubota [6] | 1990 | 70 | M | 1st | type 2 | 70 | ss | pap – tub | - | PD, TG, RHC | unknown |
| 4 | Fukuda [7] | 1990 | 72 | M | 1st | sessile | 15 | sm | adenocarcinoma | - | PR | unknown |
| 5 | Sasaki [8] | 1991 | 66 | F | 1st | type 2 | 50 | si (pane) | muc | - | PD | A (9) |
| 6 | Miyamoto [9] | 1991 | 45 | F | 1st | sessile | 19 | m | tub1 | - | PR | unknown |
| 7 | Sakai [10] | 1992 | 39 | F | 2nd | type 2 | 40 | ss | tub1 | - | PD | A (18) |
| 8 | Itsuno [11] | 1993 | 75 | M | 1st | sessile | 14 | unknown | pap | 3.3 | - | D (3) |
| 9 | Komatsu [13] | 1994 | 71 | M | 2nd | SMT | 33 | al | tub1 | - | PD | D (45) |
| 10 | Kawamoto [14] | 1994 | 69 | M | 1st | SMT | 20 | sm | pap | - | PR | A (36) |
| 11 | Suzuki [15] | 1995 | 77 | M | 2nd | polypoid | 15 | m | tub1 | - | polypectomy | unknown |
| 12 | Arai [16] | 1998 | 55 | F | 2nd | SMT | 17 | sm | tub1 | - | PR | A (21) |
| 13 | Shinohara [17] | 2000 | 71 | M | 2nd | polypoid | 24 | sm | tub1 | - | PR | A (6) |
| 14 | Kobayashi [18] | 2001 | 75 | M | 2nd | SMT | 12 | sm | adenocarcinoma | - | PR | unknown |
| 15 | Akino [19] | 2002 | 82 | M | 2nd | SMT | 10 | m | adenocarcinoma | - | EMR | A (36) |
| 16 | Kushima [20] | 2002 | 85 | F | 2nd | polypoid | 20 | m | adenocarcinoma | - | polypectomy | A (28) |
| 17 | Miyamoto [21] | 2003 | 57 | M | 2nd | type 2 | 15 | mp | sig | 5 | PD | A (24) |
| 18 | Sakurai [22] | 2005 | 73 | M | 2nd | sessile | 13 | sm | adenocarcinoma | - | unknown | A (20) |
| 19 | Sakurai [22] | 2005 | 73 | M | 1st | sessile | 78 | ss | adenocarcinoma | - | unknown | D (27) |
| 20 | Kimura [23] | 2007 | 77 | M | 2nd | sessile | 15 | sm | adenocarcinoma | - | EMR | A (unknown) |
| 21 | Our case | 2007 | 77 | M | 1st | sessile | 10 | sm | adenocarcinoma | 17 | PR | D (34) |
Macroscopic appearance: SMT = submucosal tumor-like. Depth of invasion: m = mucosa; sm = submucosa; mp = muscularis propria; ss = subserosa; si = invade adjacent structure; pane = pancreas. Histological type: pap = papillary adenocarcinoma; tub = tubular adenocarcinoma; tubl = tubular adenocarcinoma well differntiated type; muc = mucinous adenocarcinoma; sig = signet-ring cell carcinoma. Operation: PD = pancreatoduodenectomy; DG = distal gastrectomy; TG = total gastrectomy; RHC = right hemicolectomy; PR = partial resection; EMR = endoscopic mucosal resection. Outcome: A = alive; D = dead.
The present case was unique in that it involved an adenocarcinoma developing after 17 years of observation. Other cases have been reported after 5 years and 3.3 years of observation, confirming the slow growth of this tumor type [11, 21]. De novo malignant degeneration of Brunner's gland has rarely been described [27, 29]. Given the nature of Brunner's gland adenocarcinoma, it should be removed if its shape or size changes significantly.
Conclusions
We report on an extremely rare case of a primary duodenal carcinoma arising from Brunner's gland in a patient under observation for 17 years. It is concluded that Brunner's gland adenomas should be followed up sequentially, and be removed if their shape or size changes significantly.
References
- 1.Moss WM, McCart PM, Juler G, Miller DR. Primary adenocarcinoma of the duodenum. Arch Surg. 1974;108:805–807. doi: 10.1001/archsurg.1974.01350300047013. [DOI] [PubMed] [Google Scholar]
- 2.Spira IA, Ghazi A, Wolff WI. Primary adenocarcinoma of the duodenum. Cancer. 1977;39:1721–1726. doi: 10.1002/1097-0142(197704)39:4<1721::aid-cncr2820390450>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Dixon CF, Lichtman AL, Weber HM, Mcdonald JR. Malignant lesions of the duodenum. Surg Gynecol Obstet. 1946;83:83–93. [PubMed] [Google Scholar]
- 4.Shorrock K, Haldane JS, Kersham MJ, Leach RD. Obstructive jaundice secondary to carcinoma arising in Brunner's glands. J R Soc Med. 1986;79:173–174. doi: 10.1177/014107688607900314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohmachi H, Nagao K, Matsuda M, Baba K, Nishimura R, Ueno Y, Morinaga H, Fukuda M, Narita K, Miyayama H. A case of primary early carcinoma of duodenum. Jpn J Gastroenterol Surg. 1989;22:2841–2844. [Google Scholar]
- 6.Kubota K, Yagawa H, Umeda H, Matsumoto N, Kumazawa K, Naritaka Y, Otani Y, Kikuchi T, Haga S, Ogawa K, Kajiwara T. A case of primary duodenal carcinoma presenting as a gastric submucosal tumor. J Jpn Surg Assoc. 1990;51:1763–1767. [Google Scholar]
- 7.Fukuda S, Morimatsu M. Brunner's gland carcinoma – A case report. J Kurume Med Assoc. 1990;53:210–220. [Google Scholar]
- 8.Sasaki M, Oshibuchi T, Hamasaki H, Fujimoto M. A case of mucinous adenocarcinoma of the duodenal bulb. Jpn J Gastroenterol Surg. 1991;24:2419–2423. [Google Scholar]
- 9.Miyamoto T, Matsuba S, Yokoyama Y, Ito M, Takeuchi T, Sugimura M, Nakamura T, Tatematsu M. Early duodenal cancer supposedly arising from the Brunner's gland, report of a case. Stomach Intestine. 1991;26:1395–1399. [Google Scholar]
- 10.Sakai H, Nakamura Y, Ohike Y, Sodeyama K, Iguchi K, Kumeda S, Aoki T, Akamatsu T, Matsuzawa K, Fujimori Y, Ota H, Katsuyama T. Adenocarcinoma of the duodenum arising from Brunner's gland – a case report. Endosc Forum Dig Dis. 1992;8:144–149. [Google Scholar]
- 11.Itsuno M, Mikiyama K, Omagari K, Tanaka T, Hara K, Tsuda N, Ajioka Y, Watanabe H. Carcinoma of duodenal bulb arising from the Brunner's gland. Gastroenterol Jpn. 1993;28:118–125. doi: 10.1007/BF02775012. [DOI] [PubMed] [Google Scholar]
- 12.Ajioka Y, Watanabe H, Narusawa R, Iwabuchi M, Kobayasi M, Maeo S, Yoshida M. Primary duodenal tumors and tumor-like lesions, focused on their incidence and Brunner's gland adenoma. Stomach Intestine. 1993;28:627–638. [Google Scholar]
- 13.Komatsu M, Kato H, Motohara T, Kaji M, Miyake T, Miyazaki N, Saito K, Kawamura M. A case of duodenal carcinoma of Brunner's gland origin. Jpn J Gastroenterol Surg. 1994;27:1815–1819. [Google Scholar]
- 14.Kawamoto K, Motooka M, Hirata N, Masuda K, Ueyama T, Yasukouchi A, Iwashita A, Matsuzawa K, Katsuyama T. Early primary carcinoma of the duodenal bulb arising from Brunner's glands. Gastrointest Endosc. 1994;40:233–236. doi: 10.1016/s0016-5107(94)70176-8. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Ito A, Takayasu H, Suzuki H, Yamashita T, Wada T, Asano T, Koda K, Yano K, Miwa T, Tada N, Tsutsumi Y. A case of duodenal Brunner's gland hyperplasia associated with in situ carcinoma. J Jpn Soc Gastroenterol. 1995;92:1189–1193. [PubMed] [Google Scholar]
- 16.Arai M, Ushimaru H, Imai Y, Furuta K, Terashima M, Furukawa K, Kumazawa S, Ishizaka M, Nakamura M, Nakafuji H, Katsuyama T. A case of early duodenal carcinoma arising from Brunner's glands. Gastroenterol Endosc. 1998;40:1872–1878. [Google Scholar]
- 17.Shinohara T, Terasaki M, Kuno T, Okamoto Y, Sakamoto E, Kamiya S, Kobayashi S, Asaba Y, Hoshi S. A case of cancer in adenoma arising in Brunner's glands. Jpn J Gastroenterol Surg. 2000;33:338–341. [Google Scholar]
- 18.Kobayashi T, Kamikawa Y, Ueyama S, Satomoto K, Fujii Y, Ogino T. Early duodenal cancer supposedly arising from Brunner's gland: A case report. Rinshogeka. 2001;56:973–977. [Google Scholar]
- 19.Akino K, Kondo Y, Ueno A, Yamazaki K, Hosokawa M, Shimoji H, Adachi T, Honda S, Ichiyanagi S, Akahonai Y, Fujisawa Y, Takahashi H, Arimura Y, Endo T, Imai K. Carcinoma of duodenum arising from Brunner's gland. J Gastroenterol. 2002;37:293–296. doi: 10.1007/s005350200038. [DOI] [PubMed] [Google Scholar]
- 20.Kushima R, Stolte M, Dirks K, Vieth M, Okabe H, Borchard F, Hattori T. Gastric-type adenocarcinoma of the duodenal second portion histogenetically associated with hyperplasia and gastric-foveolar metaplasia of Brunner's glands. Virchows Arch. 2002;440:655–659. doi: 10.1007/s00428-002-0615-z. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto T, Oshima I, Ozaki M, Ariga T, Kinoshita H, Yoshimura S, Otsuki K, Shoko T, Kawamura S, Kakuta Y. A case of duodenal signet-ring cell carcinoma suspected to originate in Brunner's gland. Jpn J Gastroenterol Surg. 2003;36:260–265. [Google Scholar]
- 22.Sakurai T, Sakashita H, Honjo G, Kasyu I, Manabe T. Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia. Am J Surg Pathol. 2005;29:1442–1448. doi: 10.1097/01.pas.0000180449.15827.88. [DOI] [PubMed] [Google Scholar]
- 23.Kimura Y, Sogabe M, Iwaki H, Okita Y, Hibino S, Wada H, Nakamoto J, Morimoto Y, Sano T. A case of primary duodenal cancer suspected of being derived from Brunner's glands from histopathological findings. Gastroenterol Endosc. 2007;49:1265–1272. [Google Scholar]
- 24.Oiko Y, Nishimura K, Murata T, Yoneshima M. A case of early duodenal cancer resected by endoscopic polypectomy. Gastroenterol Endosc. 1989;31:2704–2708. [Google Scholar]
- 25.Morozumi A, Fujino M. Duodenal tumour and tumour-like lesion, a review of literature. Stomach Intestine. 1993;28:621–626. [Google Scholar]
- 26.Stewart HL, Lieber MM. Carcinoma of the suprapapillary portion of the duodenum. Arch Surg. 1937;35:99–129. [Google Scholar]
- 27.Christie AC. Duodenal carcinoma with neoplastic transformation of the underlying Brunner's glands. Br J Cancer. 1952;7:65–67. doi: 10.1038/bjc.1953.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasugai K, Miyata M, Nagase F, Wada Y, Hibino K, Muramatu Y, Hayashi K, Aihara M, Yosida T, Nida M, Ohwa Y, Yokoi T, Mizuno H. Duodenal Brunner's gland hyperplasia detected from tarry stool, report of a case. Stomach Intestine. 2000;35:1091–1096. [Google Scholar]
- 29.Fujimaki E, Nakamura S, Sugai T, Takeda Y. Brunner's gland adenoma with a focus of p53-positive atypical glands. J Gastroenterol. 2000;35:155–158. doi: 10.1007/s005350050029. [DOI] [PubMed] [Google Scholar]