Abstract
Glucose-stimulated insulin secretion [GSIS] involves interplay between small G-proteins and their regulatory factors. Herein, we tested the hypothesis that Arf nucleotide binding site opener [ARNO], a guanine nucleotide exchange factor [GEF] for the small G-protein Arf6, mediates the functional activation of Arf6, and that ARNO/Arf6 signaling axis, in turn, controls the activation of Cdc42 and Rac1, which have been implicated in GSIS. Molecular biological [i.e., expression of inactive mutants or siRNA] and pharmacological approaches were employed to assess the roles for ARNO/Arf6 signaling pathway in insulin secretion in normal rat islets and INS 832/13 cells. Degrees of activation of Arf6 and Cdc42/Rac1 were quantitated by GST-GGA3 and PAK-1 kinase pull-down assays, respectively. ARNO is expressed in INS 832/13 cells, rat islets and human islets. Expression of inactive mutants of Arf6 [Arf6-T27N] or ARNO [ARNO-E156K] or siRNA-ARNO markedly reduced GSIS in isolated β-cells. secinH3, a selective inhibitor of ARNO/Arf6 signaling axis, also inhibited GSIS in INS 832/13 cells and rat islets. Stimulatory concentrations of glucose promoted Arf6 activation, which was inhibited by secinH3 or siRNA-ARNO, suggesting that ARNO/Arf6 signaling cascade is necessary for GSIS. secinH3 or siRNA-ARNO also inhibited glucose-induced activation of Cdc42 and Rac1 suggesting that ARNO/Arf6 might be upstream to Cdc42 and Rac1 activation steps, which are necessary for GSIS. Lastly, co-immunoprecipitation and confocal microscopic studies suggested increased association between Arf6 and ARNO in glucose-stimulated β-cells. These findings provide the first evidence to implicate ARNO in the sequential activation of Arf6, Cdc42 and Rac1 culminating in GSIS.
Keywords: Insulin secretion, pancreatic islet, ARNO, Arf6, Rac1, secinH3
1. Introduction
It is widely accepted that small G-proteins regulate various cellular functions including proliferation, survival and demise. At least four major classes of small G-proteins have been identified in the pancreatic β-cell. These include the Ras, Rho, Rab and ADP-ribosylation factor [Arf] family of G-proteins [1], of which Arf family of G-proteins is less studied in the islet. Though originally identified as an ADP-ribosylator for cholera toxin, Arf has gained much importance as a critical modulator of membrane traffic in eukaryotic cells. Among the six members of the Arf family, Arf6 is a well documented protein for its positive modulatory roles in multiple cell types including regulation of various effector proteins [e.g., phospholipase-D; PLD] and trafficking of secretory granules to the plasma membrane for exocytosis [2]. Regazzi and coworkers first described localization and regulation of Arfs in insulin-secreting RINm5f cells [3, 4]. More recently, Lawrence and Birnbaum have demonstrated regulatory roles for Arf6 in insulin secretion mediated by glucose, GTPγS and membrane depolarization. They further demonstrated that Arf6 regulates insulin secretion by maintaining plasma membrane phosphatidylinositol 4, 5-biphosphate [PIP2; 5]. Existing evidence also supports a potential role for PLD in physiological insulin secretion [6, 7, 8].
Arf6 cycles between the GDP-bound [inactive] and GTP-bound [active] configurations; which are tightly regulated by two distinct classes of regulatory factors namely the GTPase activating proteins [GAP] and the GTP/GDP exchange factors [GEF; 9, 10]. GAPs inactivate Arf6 by promoting its conversion to the inactive GDP-bound form, while GEFs facilitate its activation. The GEF activity is a rate-defining step and involves coordination of multiple intracellular signals. In this context, many GEFs with distinct size and structure have been identified for Arf6 [11-16]. However, in the majority of the signaling events, only one member belonging to the cytohesin family has been closely linked to activate Arf6 [13, 14, 17, 18]. Recently, Hafner et al., reported a small molecule inhibitor, secinH3, which selectively blocks ARNO-mediated activation of Arf6 [19]. Previous studies have utilized secinH3 to determine the regulatory roles for ARNO/Arf6 signaling in cellular signal transduction [20, 21].
In an attempt to identify precise regulatory mechanisms involved in glucose-mediated activation of Rac1 and insulin secretion, we first proposed [22] and subsequently confirmed experimentally [23] that certain biologically-active lipid second messengers [e.g., PA, PIP2] promote dissociation of Rac1 from Rac1/GDI complex to facilitate activation of Rac1 in rodent islets and clonal β-cells. Therein, we also proposed that Arf6 could represent one of the upstream regulators of Rac1 activation by generating relevant lipid second messengers via phospholipase activation to dissociate the Rac1/GDI complex [22, 23]. Studies from Thurmond's laboratory have demonstrated the requirement of Cdc42, a Rho family GTPase in the Rac1 activation process for actin remodeling and insulin exocytosis [24, 25]. The current study is undertaken to test the hypothesis that ARNO mediates sequential activation of Arf6, Cdc42 and Rac1 leading to GSIS. Using molecular biological and pharmacological approaches we provide below the first evidence to in support of this hypothesis in normal rodent islets and insulin-secreting INS 832/13 cells.
2. Materials and Methods
2.1 Materials
SecinH3 was from Tocris Biosciences [Ellisville, MO]. siRNA-Arf6 consisting of pools of three to five target-specific 19-25 nt siRNAs were from Santa Cruz Biotechnology [Santa Cruz, CA]. siRNA-ARNO was from Dharmacon [Lafayette, IL]. The rat insulin ELISA kit was from American Laboratory Products [Windham, NH]. Antisera directed against Arf6, ARNO and Dbl were from Santa Cruz Biotechnology [Santa Cruz, CA]. Cdc42 and Rac1 antisera were from BD Biosciences [San Jose, CA]. Cdc42 and Rac1 activation kits were from Cytoskeleton Inc., [Denver, CO]. Arf6 activation assay kit and the Classic Co-IP kit were from PIERCE [Rockford, IL]. Alexa-fluor secondary antibody was from Invitrogen Molecular probes [Carlsbad, CA]. All other reagents used in these studies were from Sigma Aldrich Co. [St. Louis, MO] unless stated otherwise.
2.2 Insulin-secreting INS 832/13 cells, rat islets and human islets
INS 832/13 cells were kindly provided by Dr. Chris Newgard (Duke University Medical Center, Durham, NC). The cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 11 mM glucose, and 10 mM HEPES (pH 7.4). Islets were isolated from pancreas of male Sprague-Dawley rats (Harlan Laboratories, Oxford, MI), using collagenase digestion and a ficoll gradient as we described previously [22]. All experiments were reviewed and approved by the Wayne State University Institutional Animal Care and Use Committee. Human pancreatic islet lysates were kindly provided by Dr. Karl Olson [Michigan State University, Lansing, MI].
2.3 Hydrophilic and hydrophobic phase partitioning method using Triton X-114
Total hydrophobic and hydrophilic phases of lysates derived from INS 832/13 cells and pancreatic islets were separated using Triton X-114 according to method described earlier by us [22]. Briefly, about 400 μg of cell [INS 832/13 cell or islet] homogenate protein, prepared in 400 μl of buffer (20 mM Tris-HCl, pH 7.5, 0.5 mM EGTA, 2 mM MgCl2, 10 μg/ml leupeptin, and 2 μg/ml aprotinin), supplemented with 1 % (w/v) Triton X-114 was overlaid on 400 μl sucrose cushion 6 % (w/v) prepared in 20 mM Tris-HCl buffer (pH 7.4) containing 0.06 % (w/v) Triton X-114. Following brief incubation at 30 °C, samples were centrifuged at 300 × g for 3 min and the aqueous phase was mixed with 0.5 % (w/v) fresh Triton X-114 at 4 °C. Following dissolution, the mixture was again overlaid on the same sucrose cushion, incubated for 3 min at 30 °C and centrifuged at 300 × g for 3 min. The lower hydrophobic phase was diluted to a final volume of 400 μl with homogenization buffer, while the aqueous phase was transferred into a separate tube supplemented with 2 % fresh Triton X-114, incubated for 3 min at 30 °C, and centrifuged at 300 × g without sucrose cushion. The supernatant obtained thereof served as total hydrophilic phase. The relative abundance of ARNO in hydrophilic and hydrophobic phases were determined by Western blotting.
2.4 Transfection of Arf6 or ARNO mutants and siRNAs
INS 832/13 cells were subcultured at 50-60% confluency and transfected using Effectene [Qiagen, Valencia, CA], with 0.2 μg of plasmid DNA constructs against either dominant-negative of Arf6 [T27N] or ARNO [E156K] per well of a 24-well plate. Endogenous Arf6 or ARNO expression was depleted by transfecting cells using small interfering RNA [siRNA; 100 nM] using HiPerfect transfection reagent [Qiagen, Valencia, CA]. Efficiency of mutant expression or protein knockdown was determined by Western blotting.
2.5 Insulin release studies
Arf6 or ARNO mutant or siRNA-transfected or secinH3 inhibitor-treated cells were cultured overnight in low serum and low glucose containing medium and then stimulated either with high glucose, KCl or arginine in Krebs-Ringer bicarbonate buffer [KRB, pH 7.4] for different time intervals as indicated in the text. In studies involving KCl-induced insulin secretion, we noticed that INS 832/13 cells were not responsive to 40 mM KCl in releasing insulin. However, higher KCl concentrations [60 mM] were found to elicit robust insulin release. Therefore, in KCl-stimulated insulin secretion studies, cells were incubated with 60 mM KCl in an osmolarity-balanced KRB medium [5]. For arginine [L-Arg]-stimulated insulin release, a stock solution was prepared in Tris.HCl [pH 7.4], and diluted to desired concentration with KRB. The insulin released into the medium was quantitated by ELISA [22].
2.6 Quantitation of Arf6.GTP in pancreatic β-cells
Active Arf6 was quantitated by a pull-down assay. Briefly, the incubation medium was aspirated and cells were washed with ice-cold PBS. Cells were lysed with 500 μl lysis buffer and the lysate was clarified by centrifugation at 16,000 × g at 4 °C for 15 min and incubated ∼400 μg protein with 100 μl of glutathione resin and 100 μg of GST-GGA3-PBD beads at 4 °C for 1 hr with gentle rocking, following which the reaction mixture was spun at 6,000 × g for 30 sec. The GST-tagged beads were washed [3×] and proteins were separated by SDS-PAGE and activated Arf6 was identified by Western blotting.
2.7 Quantitation of Cdc42 and Rac1 activation
Relative degree of activated Cdc42 and Rac1 [i.e., GTP-bound form] were determined by p21-activated kinase-p21-binding domain pull-down assay as described in [22]. Briefly, INS 832/13 cells treated with either diluent or secinH3 [50 μM] or cells were either mock-transfected or transfected with ARNO-siRNA were cultured overnight in low serum-low glucose media. Cells were stimulated with either low [2.5 mM] or high [20 mM] glucose for 3 or 30 min at 37 °C in the continued presence of either diluent or secinH3 with respect to inhibitor studies. The GTP-bound forms of Cdc42 and Rac1 in the pull-down samples were quantitated by Western blotting and densitometry.
2.8 Co-immunoprecipitation studies
Immunoprecipitation studies were performed using the Classic Co-IP kit as suggested by the manufacturer [26]. Briefly, β-cell lysates [500 μg protein] were incubated with anti-ARNO for 2 hr at 4 °C followed by incubation with agarose resin for an additional 1 hr at 4 °C. Beads were washed; eluted using sample buffer and proteins were resolved by SDS page to quantify Arf6.
2.9 Immunofluorescence studies
INS 832/13 cells were plated onto coverslips and incubated with [2.5 or 20 mM] glucose for 30 min at 37 °C followed by washing in PBS and fixed with 4% paraformaldehyde solution for 15 min at room temperature. They were then permeabilized with 0.2% Triton X-100 for 15 min at room temperature. After blocking with 1% BSA for 1 hr, the cells were further incubated with primary antibodies Arf6 [1:150] and ARNO [1:150] in 0.1% BSA solution for 1 hr. After extensive washes, the cells were further incubated with secondary antibodies Alexa-fluor 488 anti-mouse [1:1000] and Alexa-fluor anti-goat 546 [1:1000] in 0.1% BSA solution for 1 hr at 37 °C. The coverslips were then mounted on glass slides containing mounting media [DAKO corporation, Carpinteria, CA] and visualized under a confocal LSM 510 microscope in the midplane using a 63× oil-immersion lens [26].
2.10 Statistical analyses
The statistical significance of the difference between the experimental conditions was determined by Student's t test unless mentioned otherwise. p values < 0.05 were considered significant.
3. Results
3.1 Distribution of ARNO in pancreatic β cells
Data shown in Figure 1A indicated that ARNO is expressed in normal rat islets, human islets and INS 832/13 cells. The relative abundance of ARNO in the total hydrophilic and hydrophobic compartments [isolated from cell lysates using Triton X-114 phase separation protocol; see Methods] indicated that ARNO remains associated with the hydrophilic compartment [Figure 1B].
Figure 1. Expression of ARNO in INS 832/13 cells, rat islets and human islets.
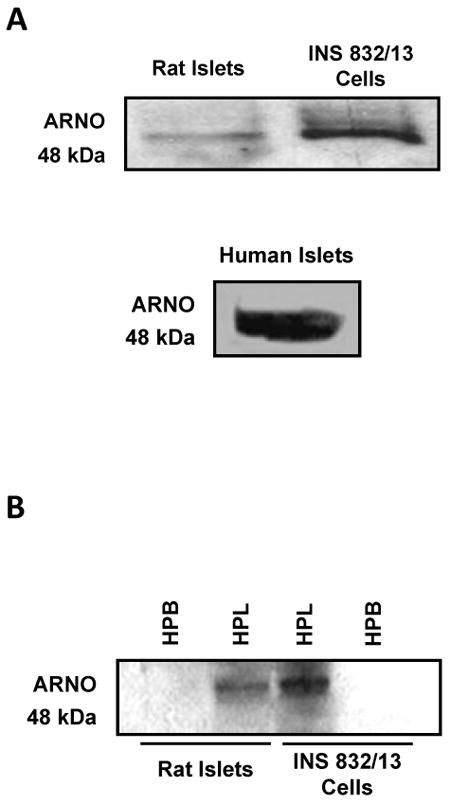
Panel A: Lysates from rat islets, human islets and INS 832/13 cells were separated by SDS-PAGE and probed for ARNO [48 kDa].
Panel B: Hydrophilic [HPL] and hydrophobic [HBP] phases of the homogenates from rat islets and INS 832/13 cells were isolated using Triton X-114 partition technique [see Methods for additional details]. Proteins were separated by SDS-PAGE and probed for ARNO. A representative blot from three independent experiments is shown here.
3.2 Molecular biological inhibition of Arf6 or ARNO attenuates insulin release by various insulin secretagogues in INS 832/13 cells
We first verified potential consequences of overexpression of dominant negative mutants of Arf6 or ARNO on GSIS from INS 832/13 cells. To examine this, INS 832/13 cells were either mock-transfected or transfected either with Arf6-T27N or ARNO-E156K [see Methods]. Transfection efficiency of mutants was verified by Western blotting [Figure 2A]. Insulin secretion was quantitated in these cells in the presence of 2.5 or 20 mM glucose. Data in Figure 2C showed a marked reduction in GSIS in cells expressing Arf6-T27N. Likewise, overexpression of ARNO-E156K, a mutant lacking the GEF function, significantly inhibited GSIS in these cells [Figure 2D]. Together, these data suggested key roles for Arf6 and ARNO in signaling events leading to GSIS. Next, we verified if knockdown of endogenous Arf6 and ARNO by using siRNA affects insulin secretion elicited by high glucose. We observed 50-60% reduction in the expression of Arf6 or ARNO by Western blotting analysis [Figure 2B]. Under these conditions, GSIS was significantly inhibited in Arf6- and ARNO-knocked-down cells [Figure 2E and 2F]. Together, data in Figure 2 [Panels C-F] implicated Arf6/ARNO signaling axis in GSIS.
Figure 2. Overexpression of inactive mutants or siRNA-Arf6 or siRNA-ARNO markedly inhibits glucose-induced insulin secretion in INS 832/13 cells.
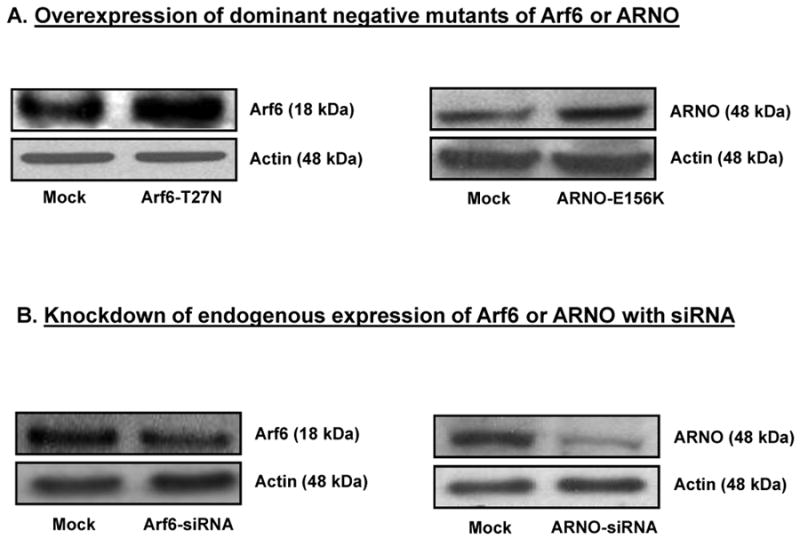
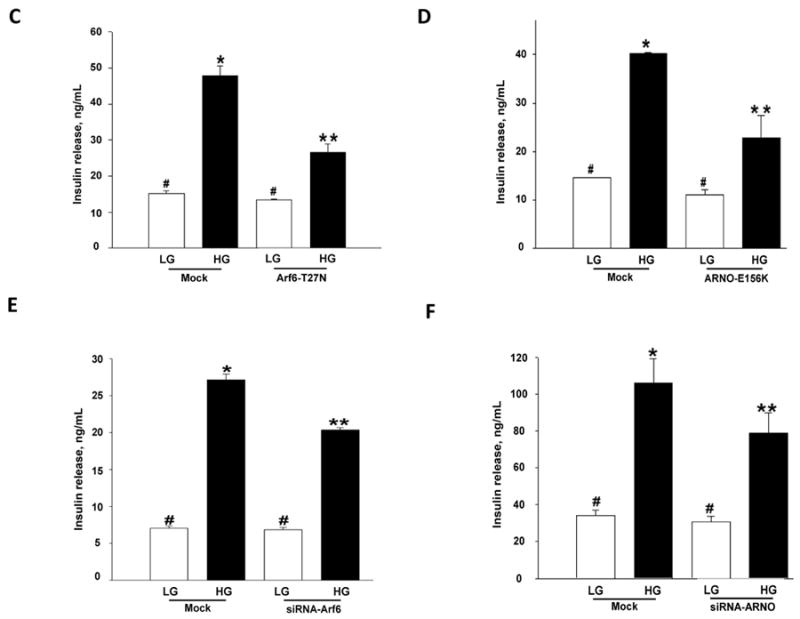
Panel A: INS 832/13 cells were either mock-transfected or transfected with dominant mutants of Arf6 [T27N] or ARNO (E156K; see Methods). Relative degrees of expression of the mutants was verified by Western blotting. A representative blot from three independent studies yielding similar results is provided here.
Panel B: INS 832/13 cells were either mock-transfected or transfected with siRNA-Arf6 or siRNA-ARNO as described in the Methods section. Relative degrees of knockdown of these proteins were verified by Western blotting. A representative blot from three independent studies yielding similar results is shown here.
Panel C: INS 832/13 cells were transfected with dominant negative Arf6 [T27N] at a final concentration of 0.2 μg of DNA and cultured for 48 hr. Following this, cells were stimulated with either low [2.5 mM] or high [20 mM] glucose in KRB for 30 min at 37°C. Insulin released into the media was quantitated and expressed as ng/mL. Data are mean ± SEM from three independent experiments. * represents p < 0.05 vs. mock low glucose; **p < 0.05 vs. mock transfected cells treated with high glucose, and data points with similar symbol did not differ significantly.
Panel D: INS 832/13 cells were transfected with dominant negative ARNO [E156K] at a final concentration of 0.2 μg of DNA and cultured for 48 hr following which cells were stimulated with either low [2.5 mM] or high [20 mM] glucose for 30 min at 37°C. Insulin released into the medium was quantitated and expressed as ng/mL. Data are mean ± SEM from three independent experiments. * represents p < 0.05 vs. mock transfected low glucose and **p < 0.05 vs. mock transfected high glucose, and data points with similar symbol do not differ significantly.
Panel E: INS 832/13 cells were either mock-transfected or transfected with Arf6-siRNA at a final concentration of 100 nM. After 48 hr culture in regular medium, cells was stimulated with low [2.5 mM] or high [20 mM] glucose for 30 min. Insulin released into the medium was quantitated and expressed as ng/mL. Data are mean ± SEM from three independent experiments. * represents p < 0.05 vs. mock low glucose; **p < 0.05 vs. mock transfected cells treated with high glucose, and data points with similar symbol did not differ significantly.
Panel F: INS 832/13 cells were either mock-transfected or transfected with ARNO-siRNA at a final concentration of 100 nM. After 48 hr culture in regular medium, cells were stimulated with low [2.5 mM] or high [20 mM] glucose for 30 min. Insulin released into the medium was quantitated and expressed as ng/mL. Data are mean ± SEM from five independent experiments. *represents p < 0.05 vs. mock transfected low glucose; **p < 0.05 vs. mock transfected high glucose. Insulin release values between mock low glucose or siRNA transfected low glucose did not differ significantly.
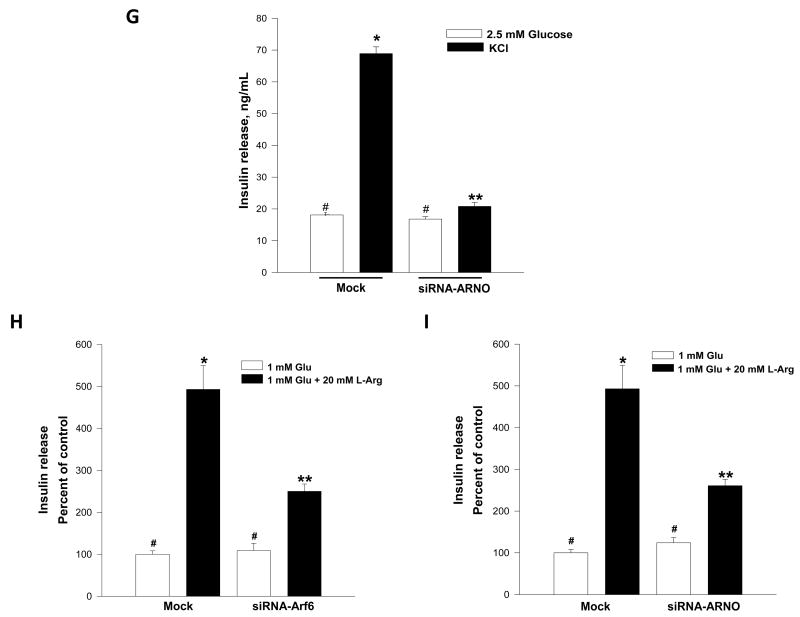
Panel G: INS 832/13 cells were either mock-transfected or transfected with ARNO-siRNA at a final concentration of 100 nM. After 48 hr culture in regular medium, cells were stimulated with low [2.5 mM] or KCl [60 mM; osmolarity adjusted] for 60 min. Insulin released into the medium was quantitated and expressed as ng/mL. Data are mean ± SEM from three independent experiments. *represents p < 0.05 vs. mock-transfected low glucose; **p < 0.05 vs. mock transfected K+. Insulin release values between mock low glucose or siRNA transfected low glucose did not differ significantly.
Panels H and I: INS 832/13 cells were mock-tranfected or transfected either with Arf6-siRNA/ARNO-siRNA at a final concentration of 100 nM. After 48 hr culture in regular medium, cells were stimulated with 1 mM glucose [Glu] or 1 mM glucose + 20 mM arginine [L-Arg] for 15 min. Insulin released into the medium was quantitated and expressed as percent of control. Data are mean ± SEM from replicates. * represents p < 0.05 vs. mock-transfected 1 mM glucose; **p < 0.05 vs. mock-transfected 1 mM Glu + 20 mM L-Arg. Insulin release values between mock or siRNA transfected under low glucose [1 mM] conditions did not differ significantly.
In the next series of studies, we determined potential roles of Arf6 or ARNO in insulin secretion elicited by a membrane-depolarizing concentration of KCl or arginine. To address this, INS 832/13 cells were transfected with either siRNA-Arf6 or siRNA-ARNO, and insulin secretion in the presence of KCl [60 mM; Figure 2G] or arginine [20 mM; Figure 2H and 2I] was quantitated. Data depicted in Figure 2 [panels G – I] demonstrated that insulin secretion elicited by KCl or arginine was inhibited in cells in which the endogenous Arf6 or ARNO was knocked-down. Together, data described in Figure 2 implicate regulatory roles for ARNO/Arf6 in insulin secretion elicited by a variety of secretagogues.
3.3 Glucose activates Arf6 in pancreatic β-cells
To further understand the role of Arf6 in stimulus-coupled secretion, we next examined whether insulinotropic concentrations of glucose activates Arf6 in the β-cell. This was accomplished by using a recently developed GST-GGA3 pull-down assay, which utilizes GGA proteins to capture activated forms of Arf6 by interacting with the Arf-binding domain [27]. Efficiency and specificity of the activation assay was confirmed by the ability of GTPγS to stimulate Arf6 activation in broken cell preparations [additional data not shown]. A time-course study for Arf6 activation by glucose [Figures 3A and 3B] suggested that glucose-induced activation was seen as early as 1 min and reached optimum at 3 min time point. Even though, we noticed a reduction in activated Arf6 at 5 min time point, Arf6 remained active [Arf6.GTP] above basal till 30 min [additional data not shown]. Together, these data suggest that Arf6 activation might represent one of the early signaling steps leading to GSIS [see below].
Figure 3. Time-dependent activation of Arf6 by glucose in pancreatic β-cells.
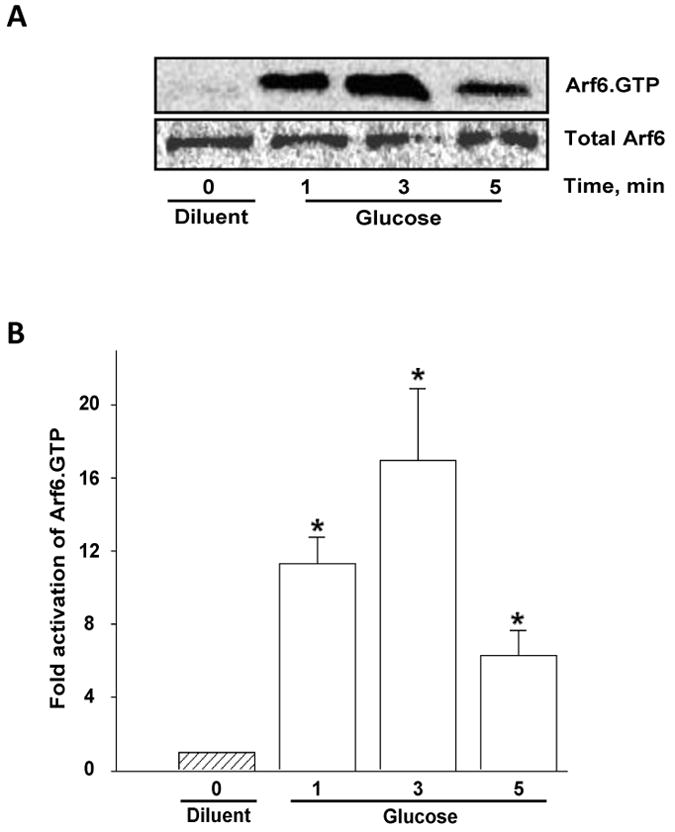
Panel A: INS 832/13 cells were incubated with KRB for 1 hr and either left unstimulated [diluent] or stimulated with high glucose [20 mM] for different time points as indicated. Cell lysates were used for detecting activated Arf6 [Arf6.GTP] by GST-GGA3-PBD pull down assay [see Methods]. Total Arf6 was used as the loading control and a representative blot from three independent experiments is shown.
Panel B: Densitometric quantitation of Arf6 activation depicted in Panel A is shown here. * represents p < 0.05 vs. diluent. Statistical analysis was performed using One-way ANOVA, All pairwise multiple comparison method (Dunnetts').
3.4 siRNA-mediated knockdown of ARNO or pharmacological inhibition of ARNO/Arf6 signaling results in attenuated glucose-induced activation of Arf6
The conversion of the GDP-bound inactive forms of G-proteins to their GTP-bound active state is mediated by GEFs. The above data prompted us to investigate if ARNO represents one of the GEFs for glucose-mediated activation of Arf6. This was verified by two complementary methods. In the first, glucose-induced activation of Arf6 was examined in cells in which ARNO expression was reduced by siRNA-ARNO. Data in Figure 4A and 4B indicated complete inhibition of glucose-induced activation of Arf6 under these conditions. This was further verified by a second approach involving pharmacological inhibition of ARNO/Arf6 signaling by secinH3 [19-21]. Data in Figure 4C and 4D suggested a complete inhibition of glucose-induced activation of Arf6 by secinH3. Taken together, these data indicate that glucose-induced activation of Arf6 requires the intermediacy of ARNO.
Figure 4. Molecular biological or pharmacological inhibition of ARNO attenuates glucose-induced activation of Arf6 in INS 832/13 pancreatic β-cells.
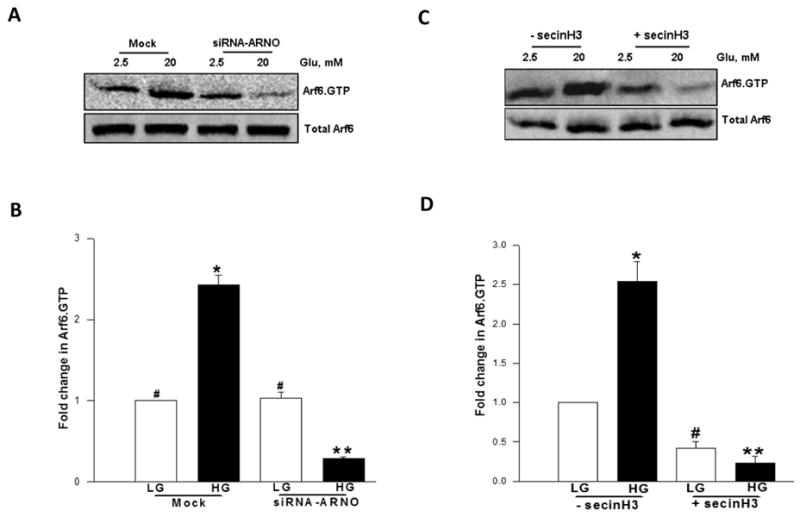
Panel A: INS 832/13 cells were either mock-transfected or transfected with siRNA-ARNO and cultured for 48 h following which cells were stimulated in the presence of either low glucose [LG, 2.5 mM] or high glucose [HG, 20 mM] for 30 min at 37°C. The relative amounts of activated Arf6 [i.e, Arf6.GTP] were determined by pull down assay. Total Arf6 from cell lysates was used as the loading control and a representative blot from three independent experiments is shown.
Panel B: Data shown in the panel A were analyzed densitometrically and expressed as fold change in Arf6.GTP over basal and are mean ± SEM of three independent experiments. * represents p < 0.05 vs. mock transfected low glucose; **p < 0.05 vs. mock transfected high glucose, and data points with similar symbol do not differ statistically.
Panel C: INS 832/13 cells were incubated overnight in the presence or absence of secinH3 [50 μM] and stimulated with either low glucose [LG, 2.5 mM] or high glucose [HG, 20 mM] in the continuous presence or absence of secinH3 [50 μM] for 30 min. Relative degrees of Arf6 activation was quantitated as described in Panel A. Total Arf6 from cell lysates was used as the loading control and a representative blot from three independent experiments is shown.
Panel D: Data shown in the Panel C are analyzed densitometrically and expressed as fold change in Arf6.GTP over basal. Data are mean ± SEM from three independent experiments. * and # represents p < 0.05 vs. low glucose without secinH3; and ** p < 0.05 vs. high glucose without secinH3.
3.5 SecinH3 markedly attenuates GSIS in INS 832/13 cells and rat islets
As a logical extension to the above studies, we next investigated potential impact of pharmacological inhibition of ARNO on GSIS in INS 832/13 cells and normal rat islets. Data in Figure 5 demonstrated complete inhibition of GSIS by secinH3 in INS 832/13 cells [Figure 5A] and normal rat islets [Figure 5B]. Under these conditions secinH3 failed to increase basal secretion in either INS 832/13 cells or rat islets [Figure 5A and 5B]. It should be noted that inhibitory effects of secinH3 on Arf6 activation [Figure 4] or GSIS [Figure 5] were not due to its cytotoxic effects since we noticed no significant effects of this inhibitor on the metabolic cell viability of β-cells under these conditions [not shown]. Together, these pharmacological data confirm the above molecular biological data to support our hypothesis that ARNO/Arf6 signaling cascade plays a positive modulatory role in GSIS.
Figure 5. secinH3, a selective inhibitor of ARNO attenuates GSIS in INS 832/13 cells and normal rat islets.
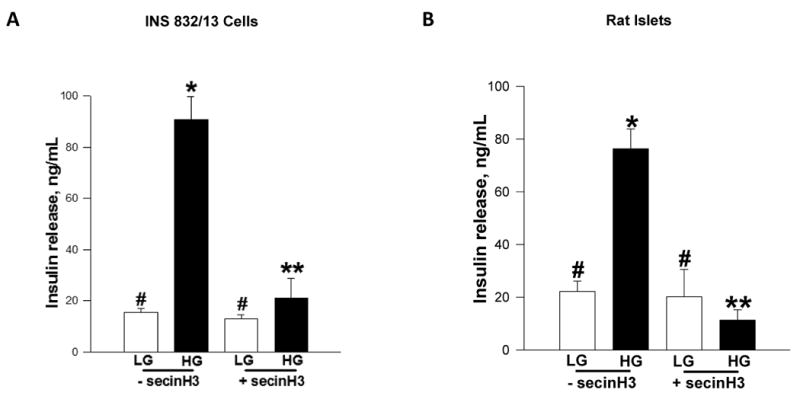
Panel A: INS 832/13 cells were incubated in low serum-low glucose overnight in the continuous presence of 50 μM secinH3 or diluent and stimulated with either low [LG, 2.5 mM] or high glucose [HG, 20 mM] for 30 min in KRB. Insulin released into the medium was quantitated by ELISA and expressed as ng/mL. Data are mean ± SEM from four independent experiments. * represents p < 0.05 vs. low glucose without secinH3; **p < 0.05 vs. high glucose without secinH3 and data points with similar symbol do not differ statistically.
Panel B: Normal rat islets were incubated in low serum-low glucose overnight in the continuous presence of either 50 μM secinH3 or diluent and stimulated with either low [LG, 2.5 mM] or high glucose [HG, 20 mM] for 30 min in KRB. Insulin released into the medium was quantitated by ELISA. Data are expressed as ng/mL insulin released and are mean ± SEM from four independent experiments. * represent p < 0.05 vs. low glucose with secinH3 and without secinH3; and **p < 0.05 vs. high glucose without secinH3. Data points with similar symbol do not differ significantly.
3.6 siRNA-mediated knockdown of ARNO or pharmacological inhibition of ARNO leads to marked reduction in glucose-induced activation of Rac1 and Cdc42
Several recent studies, including our own, have implicated Rho subfamily of small G-proteins [e.g., Cdc42 and Rac1] in cytoskeletal remodeling leading to the translocation of insulin-laden secretory granules to the plasma membrane for fusion and exocytotic secretion of insulin [28, 29]. In this context, recent evidence appears to suggest a significant cross-talk between ARNO/Arf6 and Rac1 in the regulation of cell function in multiple cell types [30-35]. Therefore, we asked if ARNO/Arf6 signaling axis regulates glucose-induced Rac1 activation in the pancreatic β-cell. We addressed this question by quantitating glucose-induced Rac1 activation in INS 832/13 cells in which ARNO function is compromised via pharmacological [e.g., secinH3] or molecular biological [e.g., siRNA-ARNO] approaches. As expected, we noticed a significant Rac1 activation in control cells exposed to glucose [Figure 6A]. Interestingly, siRNA-mediated knockdown of ARNO increased Rac1 activation under basal glucose conditions. However, glucose-induced activation of Rac1 was completely inhibited in ARNO-depleted β-cells [Figure 6A]. Likewise, pharmacological inhibition of ARNO/Arf6 signaling axis with secinH3 abolished glucose-induced activation in these cells [Figure 6B] suggesting that ARNO/Arf6 signaling step might be upstream to the Rac1 activation step in the cascade of events leading to GSIS. These data, which are compatible with our original proposal [22] also fit into the time-frame for glucose-induced activation of these proteins. We noticed in this study that Arf6 activation is seen as early as 1 min while glucose-induced activation of Rac1 is maximal at 15-20 min [28, 29].
Figure 6. Molecular biological or pharmacological inhibition of ARNO function attenuates glucose-induced Rac1 or Cdc42 activation in INS 832/13 cells.
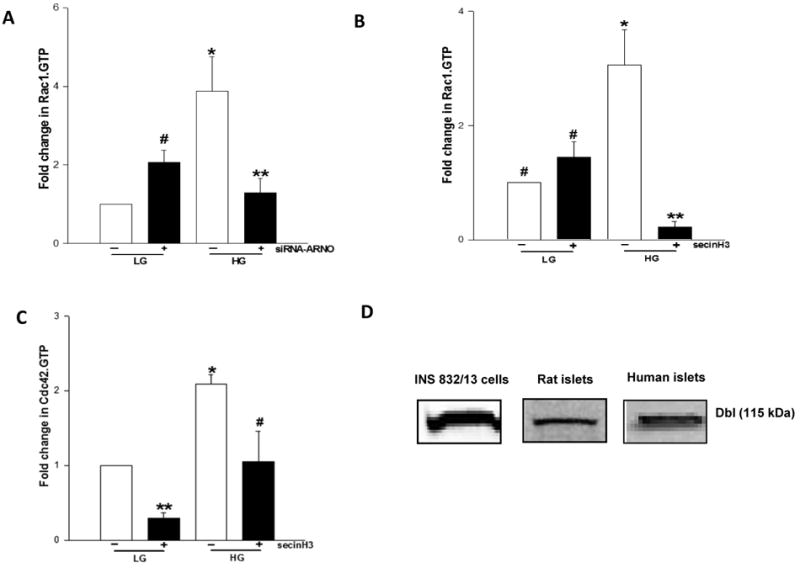
Panel A: INS 832/13 cells were either mock-transfected or transfected with siRNA-ARNO at a final concentration of 100 nM and after 48 hr culture, cells were stimulated with either low glucose [LG, 2.5 mM] or high glucose [HG, 20 mM] for 30 min at 37°C. The relative amounts of activated Rac1 [i.e, Rac1.GTP] were quantitated by PAK-PBD pull down [see Methods for additional details]. Total Rac1 from cell lysates was used as the loading control. Data were analyzed densitometrically and expressed as fold change in Rac1.GTP over basal. Data are mean ± SEM of five independent experiments. * and # represent p < 0.05 vs. low glucose without siRNA-ARNO; and **p < 0.05 vs. high glucose without siRNA-ARNO.
Panel B: INS 832/13 cells were cultured overnight in the presence or absence of secinH3 [50 μM] and further stimulated with low glucose [LG, 2.5 mM] and high glucose [HG, 20 mM] for 30 min in the continuous presence or absence of secinH3. The relative amounts of activated Rac1 [i.e, Rac1.GTP] were determined by PAK-PBD pull down assay as described in Panel A. Total Rac1 from cell lysates was used as the loading control. Data were analyzed densitometrically and expressed as fold change in Rac1.GTP over basal and are mean ± SEM of three independent experiments. * represents p <0.05 vs. low glucose without secinH3; ** p < 0.05 vs. high glucose without secinH3, and data points with similar symbol do not differ statistically.
Panel C: INS 832/13 cells were starved overnight in the presence or absence of secinH3 [50 μM] and were stimulated with low glucose [LG, 2.5 mM] and high glucose [HG, 20 mM] for 3 min in the continuous presence or absence of secinH3. The relative amounts of activated Cdc42 [i.e, Cdc42.GTP] was determined by PAK-PBD pull down assay. Total Cdc42 from cell lysates was used as the loading control. Data were densitometrically analyzed and is expressed as fold change in Cdc42.GTP over basal and are mean ± SEM of three independent experiments yielding similar results. * and ** represents p<0.05 vs. low glucose without secinH3 and #p < 0.05 vs. high glucose without secinH3.
Panel D: Lysates from rat islets, human islets and INS 832/13 cells were separated by SDS-PAGE and probed for Dbl by Western blotting.
Along these lines, earlier studies from our laboratory have suggested that the carboxylmethylation of Cdc42 is stimulated by glucose within 1 min of exposure [36]. More recent and comprehensive investigations by Thurmond's group [24, 25] have reported glucose-induced activation of Cdc42 within 3 min of exposure. They also demonstrated that Cdc42 activation is upstream to Rac1 activation in the cascade of events leading to GSIS [25]. Therefore, we examined if inhibition of ARNO/Arf6 signaling step affects Cdc42 activation. Our findings suggested ∼50% inhibition of glucose-induced activation of Cdc42 in INS 832/13 cells following inhibition of ARNO/Arf6 by secinH3 [Figure 6C]. Together, our findings are suggestive of sequential activation of Arf6, Cdc42 and Rac1 by ARNO in glucose-stimulated β-cell culminating in insulin secretion.
It should be noted that recent investigations from our laboratory have described the roles for Tiam 1, a GEF for Rac1 in GSIS. For example, we have shown that siRNA-mediated knockdown of Tiam1 or pharmacological inhibition of Tiam1/Rac1 signaling axis [e.g., NSC23766] markedly attenuated GSIS in INS 832/13 cells [37]. Along these lines, Western blot data indicated expression of Dbl, a known GEF for Cdc42 [38], in INS 832/13 cells, normal rat islets and human islets [Figure 6D]. Together, these data are suggestive expression of at least three GEFs [i.e., ARNO, Tiam1 and Dbl] in the islet β-cell. Potential cross talk between these GEFs, if any, in the context of GSIS, remains to be verified.
3.7 Glucose promotes association between Arf6 and ARNO in pancreatic β-cells
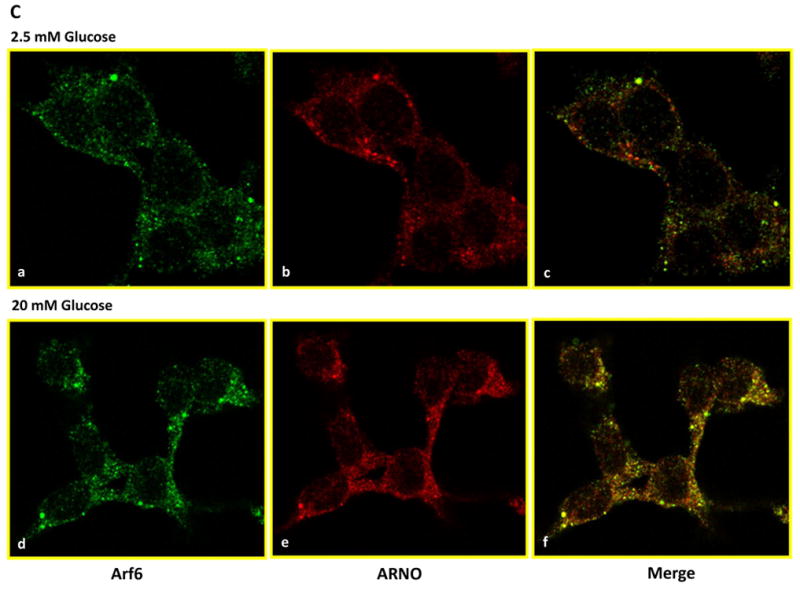
In the last series of studies we utilized co-immunoprecipitation and immunofluorescence approaches to further determine if exposure of isolated β-cells to an insulinotropic concentration of glucose promotes association between Arf6 and ARNO. Data shown in Figure 7A indicate detectable levels of Arf6 in ARNO immunoprecipitates suggesting that these two proteins stay complexed under basal conditions. Moreover, incubation of these cells with stimulatory glucose resulted in a significant increase [∼2-fold] in the amount of Arf6 in the ARNO immunoprecipitates [Figure 7B]. Together, these data suggest that glucose promotes physical association between ARNO and Arf6 in insulin-secreting cells. We verified these findings via a complementary immunofluorescence approach. Data in Figure 7C suggested that both Arf6 [green] and ARNO [red] remain diffused throughout the cell under basal conditions [2.5 mM glucose]. Merged images of subpanels a and b in Figure 7 [i.e., subpanel c] further suggested that the two proteins remain localized in the cytosolic compartment. However, exposure of these cells to a stimulatory concentration of glucose [20 mM] led to a significant association of these proteins as evidenced in the merged images of subpanels d and e of Figure 7 [i.e., sub-panel f]. Together, these findings [Figure 7] provide evidence for increased association of Arf6 and ARNO in the presence of glucose leading to the activation of ARNO/Arf6 signaling pathway followed by sequential activation of Cdc42/Rac1 culminating in insulin secretion.
Figure 7. Glucose promotes association between Arf6 and ARNO in INS 832/13 cells.
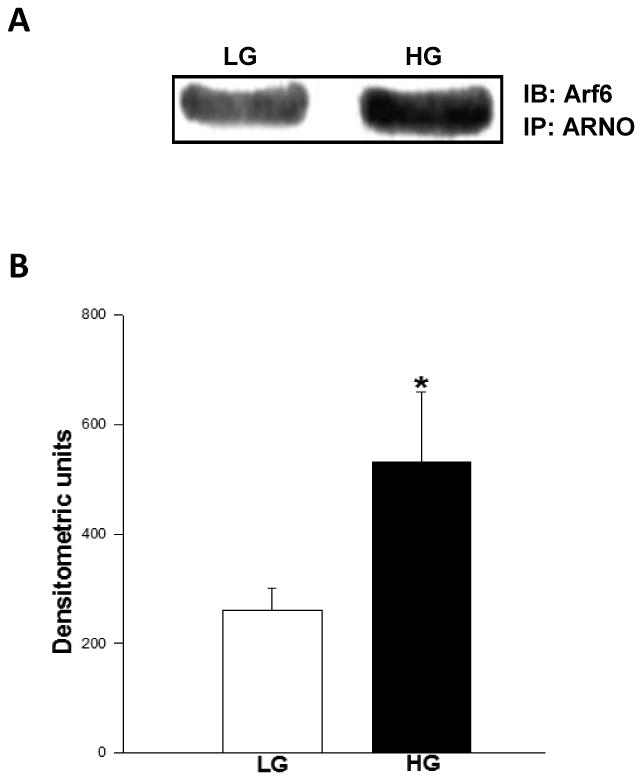
Panel A: Coimmunoprecipitation studies: Herein, INS 832/13 cells were incubated in the presence of low glucose [LG, 2.5 mM] or high glucose [HG, 20 mM] for 30 min at 37°C. ARNO was immunoprecipitated in the lysates using a specific antibody as described in Methods. The immunoprecipitates were separated by SDS-PAGE and probed for Arf6. A representative blot from three studies is shown.
Panel B: Data from multiple studies shown in Panel A are analyzed densitometrically and expressed as densitometric units and are mean ± SEM. * represents p <0.05 vs. low glucose.
Panel C: Immunofluorescence studies using confocal microscopy: INS 832/13 cells were cultured on coverslips and cultured overnight prior to the incubation with either 2.5 mM or 20 mM glucose for 30 min at 37°C. The cells were fixed in 4% paraformaldehyde solution in PBS for 15 min and permeabilized using 0.2% triton X-100 for 15 min. Fixed cells were examined for Arf6 [stained in green] and ARNO [stained in red] as described under Methods.
4. Discussion
It is widely accepted that the Arf family of G-proteins play a key regulatory role in membrane trafficking [9]. Among these, Arf6 is well studied and has been shown to regulate several cellular events including cell motility, vesicular transport, and cortical actin rearrangements [17, 32, 39]. In addition, Arf6 has been shown to activate several G-proteins and enzymes of lipid metabolism including PLD to generate fusogenic lipids such as PA and PIP2 [40, 41]. Such experimental evidence led to the postulation that Arf6 might represent one of the G-proteins whose activation may be necessary for the trafficking of secretory vesicles to the plasma membrane and fusion to lease their contents into the circulation.
The functions of Arf6 are regulated by factors such as GEFs. Despite compelling evidence in many cell types, little is known with regard to regulatory factors for Arf6 in β-cells. Therefore, in the current study, we tested the hypothesis that ARNO mediates the functional activation of Arf6, and that the ARNO/Arf6 signaling cascade, in turn, controls the activation of downstream regulatory proteins including Cdc42 and Rac1 leading to GSIS. In the present study we demonstrated that ARNO might subserve the function of a GEF for Arf6 in the islet β-cell. Salient features of our study are: [i] ARNO is expressed in clonal β-cells, rodent islets and human islets; [ii] overexpression of inactive mutants of ARNO or Arf6 or siRNAs of Arf6 or ARNO reduces insulin secretion elicited by glucose, arginine and KCl in insulin-secreting cells; [iii] secinH3, a selective inhibitor of ARNO/Arf6 signaling pathway, also inhibits GSIS in INS 832/13 cells and rodent islets; [iv] insulinotropic concentration of glucose stimulates Arf6 activation; [v] glucose-induced Arf6 activation is inhibited by secinH3 or siRNA-ARNO, suggesting a critical involvement of ARNO/Arf6 in insulin secretion; [vi] pharmacological or molecular biological inhibition of ARNO/Arf6 inhibits glucose-induced activation of Cdc42 and Rac1; and [vii] glucose promotes association between ARNO and Arf6 as evidenced by co-immunoprecipitation and confocal microscopic studies. These findings provide the first evidence to implicate novel roles for ARNO in insulin secretion.
Regazzi et al first reported [3,4] that incubation of permeabilized RINm5F cells with non-hydrolyzable analogs of GTP resulted in a significant redistribution of otherwise cytosolic Arfs to the Golgi and plasma membrane compartments. Based on these and insulin secretion data, they concluded that Arf is subjected to cycling between the membrane and soluble compartments and this cycling is governed by regulatory factors to mediate insulin secretion in clonal β-cells. And more recently Lawrence and Birnbaum demonstrated regulatory roles for Arf6 in insulin secretion from MIN6 cells [5]. Using adenoviral expression protocols involving the wildtype, dominant negative and constitutively active Arf6 mutants, these investigators provided compelling evidence to implicate regulatory roles for Arf6 in glucose-, KCl- and GTPγS-induced insulin secretion. Along these lines, at least two other recent studies have verified roles for Arf6 in islet function and insulin secretion. Grodnitzky et al have demonstrated a role for EFA6A, another GEF for Arf6 in somatostatin-mediated activation of PLD in clonal β [HIT-T15] cells. Interestingly, however, this pathway appears to be distinct from glucose-mediated effects in INS 832/13 cells and normal rat islets [current study] since overexpression of inactive ARNO mutant [E156K] failed to affect somatostatin-induced PLD activation [42]. More recent findings by Ma and coworkers further implicated Arf6 in glucose-induced PLD activation in MIN6N8 cells, which suggested binding of Arf6 to PLD. Furthermore, brefeldin A, a known inhibitor of Arf6, decreased glucose-induced PLD activity and insulin secretion [43].
What then is the potential connection between glucose-induced ARNO/Arf6 signaling cascade and Rac1 activation that we have demonstrated in the current study? We proposed earlier [22] that Rac1 activation might be downstream to Arf6 in the signaling events leading to GSIS. More recently we have provided further evidence to suggest that biologically-active, fusogenic lipids regulate Rac1 signaling in the β-cell [23]. For example, using lysates derived from normal rat islets and INS 832/13 cells we have demonstrated that these lipids promote the dissociation of Rac1/GDI complex, which is necessary for the membrane translocation and activation of Rac1 [23]. Earlier observations also demonstrated potential involvement of PLD activation in the signaling mechanisms leading to GSIS [6, 7, 44- 46]. Therefore, it is reasonable to speculate that glucose-induced Arf6-mediated activation of PLD1 [43] results in biologically active, fusogenic lipids, which, in turn, facilitate the dissociation of Rac1/GDI complex and leading to the translocation, membrane association and activation of Rac1 culminating in the cytoskeletal remodeling and fusion of granules with the plasma membrane. This remains to be verified experimentally.
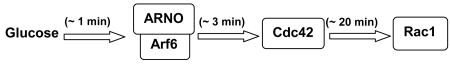
Our current findings from the time course studies suggested that Cdc42 activation [∼3 min] step as an intermediate between Arf6 [∼1 min] and Rac1 [∼20 min] activation. In support of sequential activation of Arf6, Cdc42 and Rac1, our current data demonstrated that inhibition of ARNO/Arf6 signaling leads to inhibition of Cdc42 and Rac1. Findings from Thurmond's laboratory have clearly demonstrated that Cdc42 plays an upstream regulatory role to Rac1 in GSIS [25]. Based on these findings, we propose that GSIS involves sequential activation of ARNO-mediated stimulation of Arf6, Cdc42 and Rac1 as shown below.
However, it remains to be verified if Cdc42 activation mechanism by ARNO/Arf6 also involves the dissociation of Cdc42/GDI complexes by biologically-active lipids as in the case of Rac1 [23]. Further, it is likely that ARNO/Arf6-mediated Cdc42 activation in the β-cell might involve a mechanism similar to the one described in MCF7 cells by Dubois et al [47]. These investigators have demonstrated that active Arf proteins bind to Cdc42. GTPase activating protein thereby preventing its GAP function and retain Cdc42 in the GTP-bound active conformation. Such a possibility remains to be verified for Arf6 in the islet.
Lastly, our current studies identify ARNO as one of the GEFs for glucose-induced activation of Arf6. The list of G-protein regulatory factors involved in GSIS continues to grow. Along these lines, we have identified Tiam1 as one of the GEFs for Rac1 [37]. Rho-GDI appears to subserve the roles of GDI for Cdc42 and Rac1 [29]. The above described Western blot data are suggestive of localization of Dbl, a known GEF for Cdc42, in INS 832/13 cells, normal rat islets and human islets. Studies are underway to determine potential cross-talk between various GEFs and their corresponding G-proteins in the cascade of events leading to insulin secretion.
In conclusion, based on the existing experimental data including those described herein, we propose a mechanism [Figure 8] involved in GSIS in pancreatic β-cells involving ARNO/Arf6 signaling steps. We propose that GSIS involves ARNO-mediated conversion of GDP-Arf6 [inactive] to GTP-Arf6 [active]. We verified this by over-expression of dominant negative mutants of ARNO and Arf6 as well as siRNA-mediated knockdown of these proteins in pancreatic β-cells. This postulation was further supported by our findings of pharmacological inhibition of glucose-induced ARNO-mediated activation of Arf6 by secinH3. Based on the data described herein and our previously published data [22, 23], it is likely that glucose metabolic events lead to the activation of endogenous phospholipases, including PLD [44, 45] and the subsequent generation of biologically-active lipid second messengers [e.g., PA and PIP2], which, in turn, facilitate the dissociation of Rac1/GDI complex for attaining the GTP-bound active conformation [23]. Potential involvement of ARNO/Arf6-mediated activation of Cdc42 and Rac1 via PLD remains to be verified. Finally, as we demonstrated previously [48], it is also likely that biologically-active lipids could exert direct effects on the functional activation of specific GTPases by increasing their GTP binding function and decreasing their GTPase activities thereby retaining putative G-proteins in their GTP-bound active conformation [29].
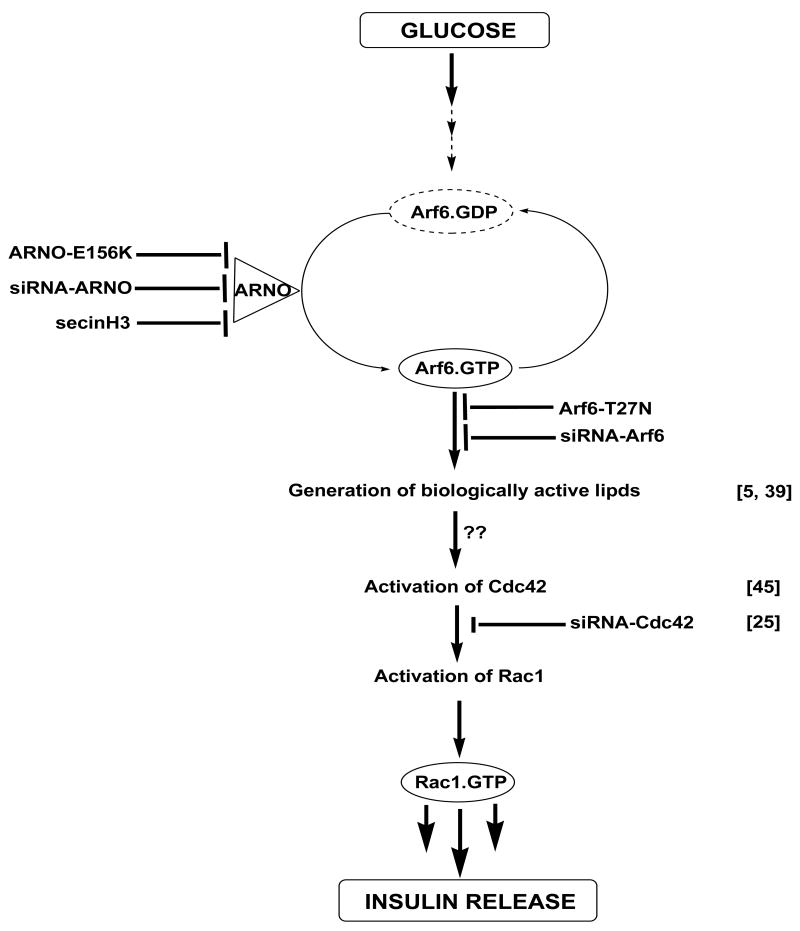
Figure 8. A proposed mechanism for ARNO signaling axis in GSIS via sequential activation of Arf6, Cdc42 and Rac1 in pancreatic β-cells.
Based on the data described herein and our previously published data [22, 23], we propose a model for potential involvement of ARNO as a GEF for Arf6 in promoting GSIS in pancreatic β-cells. We propose that glucose activates islet endogenous ARNO to facilitate the conversition of inactive GDP-bound Arf6 to its GTP-bound biologically active conformation. Essential nature for ARNO in this signaling cascade was demonstrated via the use of molecular biological [E156 mutant and siRNA-ARNO] and pharmacological approaches [e.g., secinH3]. A role for Arf6 in GSIS was confirmed via molecular biological [i.e., Arf6-T27N mutant and siRNA-Arf6] approaches. These data confirm the original observations of Lawrence and Birnbaum [5]. We propose that the activation of ARNO/Arf6 signaling pathway leads to the activation of PLD [43] leading to the generation of fusigenic lipids [e.g., PA], which in turn, promote dissociation of Rac1/GDI complexes leading to the activation and membrane association of Rac1 [22, 23]. Our time course studies also suggest that ARNO-mediated activation of Cdc42 [within 1 min] is unstream to Rac1 activation [15-20 min] together suggesting that ARNO facilitates sequential activation of Arf6, Cdc42 and Rac1 to promote insulin secretion. Potential mechanisms underlying Arf6 mediated activation of Cdc42 remain to be determined. It might include dissociation of Cdc42/GDI complex by biologically-active lipids as in the case of Rac1 [21, 22] or inhibition of GAP activity specific for Cdc42 by ARNO/Arf6 signaling pathway [see Discussion]. These aspects are being investigated in our laboratory currently.
Lastly, it may be germane to pint out that data described above implicated regulatory roles for ARNO/Arf6 signaling axis in insulin secretion elicited by KCl. Our current observations are compatible with studies of Lawrence and Birnbaum implicating a role for Arf6 in KCl-induced insulin secretion [5]. However, it remains to be seen if ARNO/Arf6 signaling cascade regulates additional pathways which are independent of Cdc42/Rac1 activation in insulin-secreting cells. Such an examination is necessary since recent studies have clearly implicated non-regulatory roles for Cdc42/Rac1 signaling pathways in KCl-induced insulin secretion [28, 29 and references therein]. To the best of our knowledge, very little is known with regard to potential involvement of small G-proteins [e.g., Rac1] in insulin secretion facilitated by amino acids, such as arginine. These aspects, which are not included in the current model [Figure 8], are being investigated in our laboratory currently.
Acknowledgments
This research was supported by a Merit Review Award from the Department of Veterans Affairs [to AK] and the National Institutes of Health [DK 74921 to AK and DK 55267 to CJR]. AK is also the recipient of the Senior Research Career Scientist Award from the Department of Veterans Affairs. We thank Dr. Chris Newgard for INS 832/13 cells, Dr. Karl Olson for human islets, Dr. Michel Franco for Arf6 [T27N] and Dr. Nicolas Vitale for ARNO [E156K]. We also thank Carmel Harkins and Mary Olive [Wayne State University Confocal Facility] for their help with these studies.
Nonstandard abbreviations used
- Arf6
ADP-ribosylation factor 6
- ARNO
Arf nucleotide binding site opener
- GAP
GTPase activating protein
- GDI
GDP-dissociation inhibitor
- GEF
Guanine nucleotide-exchange factor
- GGA3
Golgi-localized c-ear homology domain Arf-binding protein-3
- GSIS
Glucose-stimulated insulin secretion
- PA
Phosphatidic acid
- PIP2
Phosphatidyl inositol-4,5-bisphosphate
- PLD
Phospholipase-D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takai YT, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Galas MC, Helms JB, Vitale N, Thierse D, Aunis D, Bader MF. Regulated exocytosis in chromaffin cells. A potential role for a secretory granule-associated ARF6 protein. J Biol Chem. 1997;272(5):2788–93. doi: 10.1074/jbc.272.5.2788. [DOI] [PubMed] [Google Scholar]
- 3.Regazzi R, Ullrich S, Kahn RA, Wollheim CB. Redistribution of ADP-ribosylation factor during stimulation of permeabilized cells with GTP analogues. Biochem J. 1991;275(3):639–44. doi: 10.1042/bj2750639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regazzi R, Kikuchi A, Takai Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267(25):17512–9. [PubMed] [Google Scholar]
- 5.Lawrence JT, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2003;100(23):13320–5. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlop M, Metz SA. A phospholipase D-like mechanism in pancreatic islet cells: stimulation by calcium ionophore, phorbol ester and sodium fluoride. Biochem Biophys Res Commun. 1989;163(2):922–8. doi: 10.1016/0006-291x(89)92310-3. [DOI] [PubMed] [Google Scholar]
- 7.Metz SA. The pancreatic islet as Rubik's Cube. Is phospholipid hydrolysis a piece of the puzzle? Diabetes. 1991;40(12):1565–73. doi: 10.2337/diab.40.12.1565. [DOI] [PubMed] [Google Scholar]
- 8.Poitout V. Phospholipid hydrolysis and insulin secretion: a step toward solving the Rubik's cube. Am J Physiol Endocrinol Metab. 2008;294(2):214–6. doi: 10.1152/ajpendo.00638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 10.Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8(11):1476–85. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 11.Macia E, Chabre M, Franco M. Specificities for the small G proteins ARF1 and ARF6 of the guanine nucleotide exchange factors ARNO and EFA6. J Biol Chem. 2001;276(27):24925–30. doi: 10.1074/jbc.M103284200. [DOI] [PubMed] [Google Scholar]
- 12.Cox R, Mason-Gamer RJ, Jackson CL, Segev N. Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers. Mol Biol Cell. 2004;15(4):1487–505. doi: 10.1091/mbc.E03-06-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chardin P, Paris S, Antonny B, Robineau S, Béraud-Dufour S, Jackson CL, et al. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–4. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 14.Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell. 2007;18:2244–53. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Someya A, Sata M, Takeda K, Pacheco-Rodriguez G, Ferrans VJ, Moss J, et al. ARF-GEP(100), a guanine nucleotide-exchange protein for ADP-ribosylation factor 6. Proc Natl Acad Sci. 2001;98:2413–8. doi: 10.1073/pnas.051634798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunphy JL, Moravec R, Ly K, Lasell TK, Melancon P, Casanova JE. The Arf6 GEF GEP100/BRAG2 regulates cell adhesion by controlling endocytosis of beta1 integrins. Curr Biol. 2006;16:315–20. doi: 10.1016/j.cub.2005.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caumont AS, Vitale N, Gensse M, Galas MC, Casanova JE, Bader MF. Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in the regulated exocytotic pathway. J Biol Chem. 2000;275:15637–44. doi: 10.1074/jbc.M908347199. [DOI] [PubMed] [Google Scholar]
- 18.Frank S, Upender S, Hansen SH, Casanova JE. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem. 1998;273:23–7. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- 19.Hafner M, Schmitz A, Grune I, Srivatsan SG, Paul B, Kolanus W, et al. Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature. 2006;444:941–4. doi: 10.1038/nature05415. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi J, Miyamoto Y, Torii T, Mizutani R, Nakamura K, Sanbe A, et al. Valproic acid-inducible Arl4D and cytohesin-2/ARNO, acting through the downstream Arf6, regulate neurite outgrowth in N1E-115 cells. Exp Cell Res. 2009;315:2043–52. doi: 10.1016/j.yexcr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Torii T, Miyamoto Y, Sanbe A, Nishimura K, Yamauchi J, Tanoue A. Cytohesin-2/ARNO, through its interaction with focal adhesion adaptor protein paxillin, regulates preadipocyte migration via the downstream activation of Arf6. J Biol Chem. 2010;285:24270–81. doi: 10.1074/jbc.M110.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowluru A, Veluthakal R. Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes. 2005;54:3523–29. doi: 10.2337/diabetes.54.12.3523. [DOI] [PubMed] [Google Scholar]
- 23.McDonald P, Veluthakal R, Kaur H, Kowluru A. Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS 832/13 cells. Am J Physiol Cell Physiol. 2007;292:C1216–20. doi: 10.1152/ajpcell.00467.2006. [DOI] [PubMed] [Google Scholar]
- 24.Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol. 2003;285:698–710. doi: 10.1152/ajpcell.00093.2003. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–46. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath V, Kyathanahalli CN, Jayaram B, Syed I, Olson LK, Ludwig K, et al. Regulation of glucose- and mitochondrial fuel-induced insulin secretion by a cytosolic protein histidine phosphatase in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2010;29:E276–86. doi: 10.1152/ajpendo.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takatsu H, Yoshino K, Toda K, Nakayama K. GGA proteins associate with Golgi membranes through interaction between their GGAH domains and ADP-ribosylation factors. Biochem J. 2002;365(Pt 2):369–78. doi: 10.1042/BJ20020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santy LC, Ravichandran KS, Casanova JE. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr Biol. 2005;15:1749–54. doi: 10.1016/j.cub.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Cotton M, Boulay PL, Houndolo T, Vitale N, Pitcher JA, Claing A. Endogenous ARF6 interacts with Rac1 upon angiotensin II stimulation to regulate membrane ruffling and cell migration. Mol Biol Cell. 2007;18:501–11. doi: 10.1091/mbc.E06-06-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tushir JS, D'Souza-Schorey C. ARF6-dependent activation of ERK and Rac1 modulates epithelial tubule development. Embo J. 2007;26:1806–19. doi: 10.1038/sj.emboj.7601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu B, Shi B, Jarzynka MJ, Yiin JJ, D'Souza-Schorey C, Cheng SY. ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res. 2009;69:794–801. doi: 10.1158/0008-5472.CAN-08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muralidharan-Chari V, Hoover H, Clancy J, Schweitzer J, Suckow MA, Schroeder V, et al. ADP-ribosylation factor 6 regulates tumorigenic and invasive properties in vivo. Cancer Res. 2009;69:2201–09. doi: 10.1158/0008-5472.CAN-08-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, et al. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98:540–55. doi: 10.1172/JCI118822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A. Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol. 2009;77(1):101–13. doi: 10.1016/j.bcp.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami M, Meneses PI, Knight JS, Lan K, Kaul R, Verma SC, et al. Nm23-H1 modulates the activity of the guanine exchange factor Dbl-1. Int J Cancer. 2008;123(3):500–10. doi: 10.1002/ijc.23568. [DOI] [PubMed] [Google Scholar]
- 39.D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 40.Bader MF, Vitale N. Phospholipase D in calcium-regulated exocytosis: lessons from chromaffin cells. Biochim Biophys Acta. 2009;1791:936–41. doi: 10.1016/j.bbalip.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Begle A, Tryoen-Toth P, de Barry J, Bader MF, Vitale N. ARF6 regulates the synthesis of fusogenic lipids for calcium-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2009;284:4836–45. doi: 10.1074/jbc.M806894200. [DOI] [PubMed] [Google Scholar]
- 42.Grodnitzky JA, Syed N, Kimber MJ, Day TA, Donaldson JG, Hsu WH. Somatostatin receptors signal through EFA6A-ARF6 to activate phospholipase D in clonal beta-cells. J Biol Chem. 2007;282:13410–8. doi: 10.1074/jbc.M701940200. [DOI] [PubMed] [Google Scholar]
- 43.Ma WN, Park SY, Han JS. Role of phospholipase D1 in glucose-induced insulin secretion in pancreatic Beta cells. Exp Mol Med. 2010;42:456–64. doi: 10.3858/emm.2010.42.6.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metz SA, Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J. 1990;270:427–35. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metz S, Dunlop M. Phospholipase D in the pancreatic islet: evidence suggesting the involvement of phosphatidic acid in signal transduction. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:287–90. [PubMed] [Google Scholar]
- 46.Hughes WE, Elgundi Z, Huang P, Frohman MA, Biden TJ. Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic beta-cells. J Biol Chem. 2004;279:27534–41. doi: 10.1074/jbc.M403012200. [DOI] [PubMed] [Google Scholar]
- 47.Dubois T, Olivia Paléotti O, Mironov AA, Fraisier V, Stradal TEB, De Matteis Antonietta, et al. Golgi-localized GAP for Cdc42 functions downstream of ARF1to control Arp2/3 complex and F-actin dynamics. Nat Cell Biol. 2005;7:353–64. doi: 10.1038/ncb1244. [DOI] [PubMed] [Google Scholar]
- 48.Kowluru A, Metz SA. Regulation of guanine-nucleotide binding proteins in islet subcellular fractions by phospholipase-derived lipid mediators of insulin secretion. Biochim Biophys Acta. 1994;1222:360–8. doi: 10.1016/0167-4889(94)90041-8. [DOI] [PubMed] [Google Scholar]