SUMMARY
Matrix metalloproteinase-2 (MMP-2) is a proteolytic enzyme degrading the extracellular matrix and over-expressed by many tumors. Here, we documented the presence of MMP-2-specific CD4+ T cells in tumor-infiltrating lymphocytes (TILs) from melanoma patients. Strikingly, MMP-2-specific CD4+ T cells displayed an inflammatory TH2 profile, i.e. mainly secreting TNFα, IL-4 and IL-13 and expressing GATA-3. Furthermore, MMP-2-conditioned dendritic cells (DCs) primed naïve CD4+ T cells to differentiate into an inflammatory TH2 phenotype through OX40L expression and inhibition of IL-12p70 production. MMP-2 degrades the type-I IFN receptor, thereby preventing STAT1 phosphorylation, which is necessary for IL-12p35 production. Active MMP-2, therefore, acts as an endogenous type-2 “conditioner” and may play a role in the observed prevalence of detrimental type-2 responses in melanoma.
SIGNIFICANCE
Several melanoma-associated antigens have been targeted in immunization strategies to treat melanoma patients. However, the therapeutic efficacy of these approaches remains limited, indicating an urgent need for improvement. Because MMP-2 activity is critical for melanoma progression, it represents an interesting target for vaccine therapy. We show that MMP-2 is an immunogenic tumor antigen. However, MMP-2-specific CD4+ T lymphocytes display a suboptimal inflammatory TH2 profile. MMP-2-conditoned DCs prime TH2 responses against several melanoma-associated antigen (MAA), suggesting that MMP-2 can create a TH2 skewing microenvironment in a bystander fashion. Elucidation of the underlying mechanisms opens the way to improving immune responses towards a more effective TH1 response, and highlights the potential of MMP2 as a target antigen in melanoma.
INTRODUCTION
A large array of human melanoma-associated antigens (MAA) has been identified and used in various immunization strategies to treat cancer patients. However, despite significant induction of tumor-specific T cells (Coulie and van der Bruggen, 2003; Rosenberg, 2004), the therapeutic efficacy of these approaches has been suboptimal indicating a need for improving current strategies. Possible explanations for failure (Loose and Van de Wiele, 2009) include malignant cells producing immunosuppressive cytokines (IL-10, TGFβ, IL-6 and M-CSF), prostaglandins and vascular endothelial growth factor thereby skewing the immune response towards type-2 or regulatory T cells and deleteriously modulating the differentiation, maturation and function of antigen presenting cells (APCs). Furthermore, malignant cells that chronically stimulate infiltrating T cells can actively exhaust and eliminate T cells through expression of molecules such as FasL, PDL-1 or RCAS1. Finally, due to immune pressure, immunoresistant tumor cell variants emerge through selection of mutants with reduced antigenicity. This can affect the expression/function of molecules implicated in antigen processing and presentation or the expression of tumor antigens themselves (Hirohashi et al., 2009; Yee et al., 2000).
A way to circumvent this latter limitation would be to vaccinate against immunogenic proteins whose expression is critical for tumor growth and/or invasiveness. The matrix metalloproteinase-2 (MMP-2), overexpressed in many tumors including melanoma, may be such an antigen. MMP-2 is a proteolytic enzyme that degrades numerous components of extracellular matrix such as collagens, laminin or fibronectin and contributes to cell migration by clearing the surrounding extracellular matrix and basement membrane barriers. MMP-2 over-expression has been associated with tumor progression. Indeed, MMP-2 modulates various oncogenic processes such as angiogenesis (Brooks et al., 1998; Itoh et al., 1998) and tumor dissemination (Kessenbrock et al., 2010; Liotta et al., 1980; Westermarck and Kahari, 1999).
We previously identified MMP-2 as a melanoma-associated antigen (MAA) recognized by HLA-A*0201-restricted CD8+ tumor infiltrating lymphocytes (TILs) (Godefroy et al., 2005). Because MMP-2 activity is critical for melanoma progression, MMP-2 is a promising tumor antigen to target in immunotherapy against malignant melanoma. Accordingly, several patients administered CD8+ T cells that recognize this epitope among others have remained tumor-free up to 15 years after treatment (Godefroy et al., 2005; Khammari et al., 2007).
As CD4 help is essential for generating effective anti-tumor immunity we evaluated whether MMP-2 could also be recognized by CD4+ T cells. Here we characterize these cells and identify mechanisms by which they are generated.
RESULTS
MMP-2-specific CD4+ T cell responses in melanoma patients
Whether CD4+ T cells recognize MMP-2-derived epitopes has not previously been established. We used a pool of 20 amino acid long, partially overlapping peptides spanning the entire sequence of MMP-2 (Fig.S1A) to evaluate specific responses in TILs derived from melanoma patients, in some of whom we had previously detected MMP-2-specific CD8+ T cells infiltrating their tumors and whose tumor cells produced MMP-2 protein (Godefroy et al., 2005). Strikingly, MMP-2-specific CD4+ T cells were found in 13 out of 31 unselected TIL populations (Fig.1A-B). Among these 13 responders, the percentage of cells secreting TNFα upon stimulation ranged from 0.04 to 2.12% (mean=0.78% ±0.53) and IL-4 from 1.17 to 4.91% (mean=2.44% ±1.21). Very few cells produced IFNγ and IL-2 (Fig.1A-B). Therefore, more than 40% of tested TILs contained MMP-2-specific CD4+ T cells preferentially secreting IL-4 and TNFα.
Figure 1. MMP-2-specific CD4+ T cell responses in melanoma patients.

TILs from representative melanoma patient M186 (A) or from 31 unselected patients (B) were stimulated with the MMP-2 peptide pool (2μM) for 6h and assessed for cytokine production by intracellular staining. Percentage of secreting cells upon peptide stimulation was considered specific/positive when it exceeded by more than twofold the background (cytokine-secreting cells in the absence of stimulation) and had more than 0.5% of responding cells (after background subtraction) for at least one cytokine. See also Fig.S1 for MMP-2-derived peptides and patient survival analysis.
To try and evaluate whether these TILs played any therapeutic role, we assessed the clinical outcome of patients who had CD4+ TILs targeting MMP-2. Survival status was available for 21 of these patients. Twelve patients displayed MMP-2-specific CD4+ T cell responses and demonstrated a trend towards a poorer clinical outcome compared to patients with no detectable MMP-2 responses (Fig.S1B). However, the observed difference in patient's survival was not statistically significant (p=0.121).
MMP-2-specific CD4+ T cell responses in healthy donors
To further study and characterize MMP-2-specific CD4+ T cells, we stimulated circulating isolated CD4+ cells from 16 healthy donors with the same pool of MMP-2 peptides. Twelve days later, cultures were assessed for MMP-2-specific CD4+ T cells by monitoring intracellular cytokine staining upon stimulation with the peptide pool. We derived 14 MMP-2-reactive polyclonal populations (Fig.2A-B). Among these 14 responders and upon peptide re-stimulation, the percentage of cells secreting TNFα ranged from 0 to 2.8% (mean= 0.46% ±0.69), IFNγ from 0 to 0.44% (mean= 0.07% ±0.11), IL-2 from 0 to 0.24% (mean= 0.06% ±0.06), IL-4 from 0.55 to 4.93% (mean= 1.69% ±1.3) and IL-13 from 0 to 1.21% (mean= 0.15% ±0.31) (Fig.2B). No MMP-2-specific CD4+ T cells secreted IL-10 and very few cells secreted type-1 cytokines (Fig.2A-B). To determine whether MMP-2 responses can be detected directly ex vivo, we stimulated PBMCs from 6 healthy donors with MMP-2 peptides and measured their cytokine responses. Responding gated CD4+ memory T cells (CD4+/CD45RO+/CD62L−) secreted IL-4 (mean=1.09% ±0.61 of CD4+/CD45RO+ cells), but none of the other cytokines (Fig.2C-D). The absence of detectable TNFα responses is likely to be due to the small percentages of MMP-2-specific CD4+ T cells, producing cytokine below the detection threshold. Gated naïve CD4+/CD45RA+/CD62L+ T cells, derived either from healthy donors or cord blood, were used as controls, and failed to respond to the MMP-2 peptides (Fig.2D). Therefore, MMP-2-specific CD4+ T cells can be frequently expanded from normal donors and secrete primarily TNFα and IL-4, but little type-1 cytokines and no IL-10, consistent with an inflammatory TH2 profile (Ito et al., 2005; Soumelis et al., 2002) acquired in vivo.
Figure 2. MMP-2-specific polyclonal CD4+ T cell responses in healthy donors.
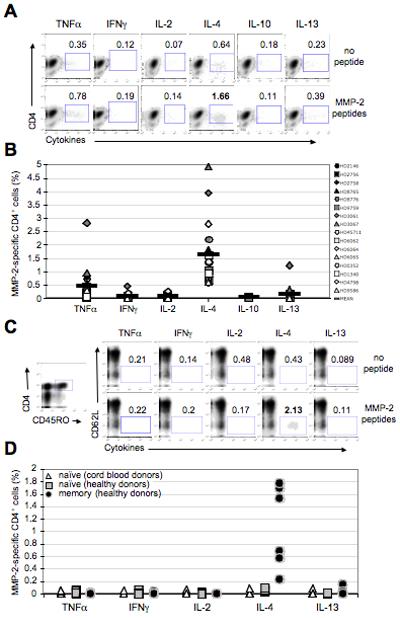
(A) Isolated CD4+/CD25− T cells from 16 healthy donors were individually stimulated with the MMP-2 peptide pool (2μM) pulsed on the irradiated (35grays) autologous CD4− fraction. 12 days later, cells were re-stimulated, in an auto-presentation manner (no APCs), with the same peptides (2μM) for 6h, and assessed for cytokine production by intracellular staining. Density plots represent cytokine production by representative donor HD9759. (B) Percentages of MMP-2-specific CD4+ T cells (% of producing CD4+ cells after peptide stimulation - % of producing CD4+ cells non stimulated (background)) are shown for all donors. Percentage of secreting cells upon peptide stimulation was considered specific/positive when it exceeded by more than twofold the background (cytokine-secreting cells in the absence of peptide stimulation) and had more than 0.5% of responding cells (after background subtraction) for at least one cytokine. (C) Ex vivo PBMCs from 6 healthy donors were stimulated by adding the MMP-2 peptide pool (2μM) onto the cells (no specific APCs were targeted). The gating strategy for visualizing memory CD4+ T cells is shown on the left plot. Density plots showing cytokine production of CD4+/CD45RO+/CD62L− memory cells are represented for representative donor HD7957. Percentages of producing cells among CD4+/CD45RO+ gated cells are indicated. (D) MMP-2-specific memory CD4+ T cell responses for all donors are represented. CD4+/CD45RA+/CD62L+ naïve cells derived from healthy (n=6) and cord blood (n=9) donors were used as controls. See also Fig.S2.
To more accurately characterize the in vitro MMP-2-specific CD4+ T cell differentiation state, T cell clones were generated from healthy donors (Fig.S2B). Nineteen CD4+ T cell clones responding to the MMP-2 peptide pool were subsequently characterized (as exemplified by clone HD9828/52) for epitope specificity (Fig.S3A), CD4/CD8, TCR Vβ expression (Fig.S3B) and HLA class-II isotype restriction (Fig.S3C). Results for all CD4+ T cell clones are shown in Table S1. We looked for production of TNFα, IL-4 and IFNγ upon stimulation with individual peptides separately to assess epitope specificities. Nineteen CD4+ T cell clones recognized eleven distinct MMP-2-derived peptides, within the following amino acid positions: 1-20, 11-30, 21-40, 41-60, 161-180, 361-380, 551-570, 571-590, 601-620, 621-640 and 631-650 (Fig.S3A and Table S1). Antibodies towards most TCR Vβ chains were used to confirm clonality and distinguished at least 13 distinct clones among the original 19 CD4+ T cell clones (Fig.S3B and Table S1), as >99% of each culture expressed only one Vβ chain (not shown). Blocking antibodies against HLA class II isotypes showed that most of the MMP-2-specific T cell clones were HLA-DR restricted (Fig.S3C and Table S1).
To assess the physiological relevance of MMP-2-derived epitope presentation, CD4+ T cells from healthy donors were stimulated with autologous DCs loaded with whole MMP-2 protein. Twelve days later, we tested T cell responses to the peptide pool. Donors HD6026 and HD6028 had 0.12% and 0.17% of cells secreting IFNγ as well as 0.73% and 1.99% secreting IL-4, respectively (Fig.S3D). These results showed, on the one hand, that the whole protein is naturally processed and presented by DCs to T cells and, on the other hand, that MMP-2-specific human CD4+ T cells preferentially secrete IL-4 upon DC stimulation.
MMP-2-specific CD4+ T cells display an inflammatory TH2 phenotype
Because the majority of MMP-2-specific CD4+ T cell clones, upon peptide stimulation, secreted more IL-4 and TNFα than IFNγ (Fig.S3A and not shown), we tested them for production of a broader panel of cytokines to further assess their cytokine profile. MMP-2-specific T cell clones, as shown by the representative clone HD5950/MC2/43 (Fig.3A) or the 19 clones (Fig.3B), secreted mainly TNFα (mean=38.2% of secreting cells, after background subtraction), IL-4 (mean=23.5%) and IL-13 (mean=19.7%), but fewer cells produced IFNγ (mean=6.2%) and IL-2 (mean=5.7%). This profile again corresponds to an inflammatory TH2 phenotype (Ito et al., 2005; Soumelis et al., 2002).
Figure 3. MMP-2-specific CD4+ T cells display an inflammatory TH2 phenotype.

(A, B) CD4+ T cell clones were stimulated with peptides (2μM) for 6h. Cytokine production was assessed by intracellular staining (clone HD5950/MC2/43 is shown as an example) (A). The results for the 19 MMP-2-specific CD4+ clones are also shown (B, black bars). Three Melan-A/MART-1- and 8 NY-ESO-1-specific CD4+ T cell clones were stimulated with peptides (2μM) for 6h before intracellular staining for cytokines (B, gray and white bars, respectively). Results are shown as mean ± SD. (C, D) T-bet and GATA-3 expression was measured by intracellular staining of MMP-2- (n=19), Melan-A/MART-1- (n=3) and NY-ESO-1-specific CD4+ T cell clones (n=8). Histogram plots show a representative example for one NY-ESO-1-specific CD4+ T cell clone (HD3014/1) and one MMP-2-specific CD4+ T cell clone (HD5950/33). (C) Isotype control is represented in tinted gray. (D) MFIs for all CD4+ T cell clones are represented. Cord blood-derived CD4+/CD45RA+/CD62L+ naïve cells (n=9) were used as a negative control.
For each cytokine or transcription factor, analyses of variance were used to compare T cells specific for MMP-2, Melan-A and NY-ESO-1, and t-tests were used for pairwise comparisons between groups. p values ≤ 0.025 (*) were considered statistically significant using a Bonferroni correction for 2 comparisons.
See also Fig. S3 and Table S1.
Because of the unique inflammatory TH2 profile of MMP-2-specific CD4+ T cells, we next examined CD4+ T cell responses to other MAA, under the same conditions. CD4+ T cells from healthy donors were stimulated with overlapping 20 amino acid long peptides spanning the length of the differentiation antigen Melan-A/MART-1 and the cancer testis antigen NY-ESO-1 sequences. Antigen-specific T cells were then enriched before generating antigen-specific CD4+ T cell clones. We obtained 3 CD4+ T cell clones specific for Melan-A/MART-1 (recognizing the epitopes P21-40, P51-70 and P61-80) and 8 for NY-ESO-1 (recognizing P61-80, P81-100, P119-143, P131-160, P139-160, and P161-180). Upon peptide stimulation, these clones secreted TNFα (mean=84.8% of secreting cells after background subtraction), IFNγ (mean=76.1%; overall ANOVA p≤0.0001, compared to MMP-2-specific T cell clones), IL-2 (mean=35.0%; overall ANOVA p≤0.0001 compared to MMP-2-specific T cell clones) and IL-13 (mean=58.5%), but low amounts of IL-4 (mean=14.1%) (Fig.3B). To further characterize and compare MMP-2-, Melan-A/MART-1- and NY-ESO-1-specific T helper profiles, clones were stained for type-1 and type-2-associated transcription factors, T-bet and GATA-3 respectively. MMP-2-specific T cell clones expressed GATA-3 (mean MFI (mean fluorescence intensity) =105) and insignificant levels of T-bet (mean MFI=5.5), while Melan-A/MART-1- and NY-ESO-1-specific T cell clones expressed T-bet (mean MFI=16.6) and lower amounts of GATA-3 (mean MFI=72.3), confirming that CD4+ T cells recognizing MMP-2 exhibited a TH2-like phenotype (Fig.3C-D). Cord blood-derived naïve CD4+ T cells, gated on CD4+/CD45RA+/CD62L+ cells, were used as a negative control (Fig.3D).
Active MMP-2 enzyme is required for differentiation of naïve MMP-2-specific CD4+ T cells into inflammatory TH2 cells
To determine whether the active full-length protein was required for MMP-2-specific CD4+ T cells to differentiate towards an inflammatory TH2 phenotype, we primed naïve CD4+ T cells (from 11 cord blood donors) in vitro using autologous CD4− cells loaded with different sources of MMP-2: overlapping peptides, or the protein in its active or inactive conformation. The CD4− fraction was used as a source of APCs to include any cell type that could potentially be involved in priming. Peptide-specific responses of primed T cells were measured after 15 days. The percentage of MMP-2-specific CD4+ T cells secreting IL-4 (after background subtraction) was significantly higher when cells were primed with the active MMP-2 (mean=2.38%), rather than with peptides (mean=0.71% ; mean of the individual differences=1.34, p≤0.013) or inactivated MMP-2, whether inactivation occurred by heating (mean=0.45% ; mean of differences=1.80, p≤0.0002) or using a specific inhibitor (mean=0.12% ; mean of differences=1.47, ns) (Fig.4A-B). Furthermore, active MMP-2-primed cells produced less IFNγ and IL-2 compared to inactive MMP-2 or peptides (Fig.4A-B). Thus the inflammatory TH2 lineage commitment of MMP-2-specific CD4+ T lymphocytes depends on the presence of active MMP-2 both as a source of antigen and as an endogenous TH2 conditioner.
Figure 4. Effect of MMP-2 enzyme on specific CD4+ T cell differentiation.

Cord blood-derived CD4+/CD25− cells from 11 donors were stimulated with irradiated autologous CD4− cells loaded either with active MMP-2, heat-inactivated (HI) MMP-2 or with MMP-2 pre-incubated with a specific inhibitor (+ I). After 15 days, CD4+ T cells were stimulated with the MMP-2 peptide pool (2μM) for 6h before intracellular staining of cytokines. (A) Density plots representing cytokine production by CD4+ T cells are shown for the representative donor CB35 primed to MMP-2 either in its active form or after heat-inactivation. Numbers indicate percentages of cytokine-producing cells upon MMP-2 peptide stimulation. (B) Cytokines secreted by MMP-2-specific CD4+ T cells primed to inactive protein or peptides for all donors are shown and compared to MMP-2-specific CD4+ T cells generated after priming with active MMP-2. MMP-2 enzymatic activity was controlled (Fig.S5C-D).
For each cytokine, 3 paired t-tests were used to compare active MMP-2 to heat inactivated MMP-2, to MMP-2+I, and to MMP-2 peptides. p values ≤ 0.0167 (*) were considered statistically significant using a Bonferroni correction for 3 comparisons.
Active MMP-2 also influences other tumor-specific T helper differentiation
To evaluate whether active MMP-2 also affected CD4+ T cell differentiation specific for other MAA in a bystander fashion, naïve CD4+ T cells from 10 cord blood donors were primed to Melan-A/MART-1 or NY-ESO-1 using autologous poly(I:C)-matured DCs pulsed with the corresponding overlapping peptides. After 15 days, primed CD4+ T cells secreted IFNγ (mean=0.23% of secreting cells after background subtraction) and IL-2 (mean=0.15%). CD4+ T cells specific for either tumor associated antigen, secreted little IL-4 and IL-13 (mean=0.75% and 0.02%, respectively) (Fig.5A-B). On the other hand, when DCs were pre-incubated with active MMP-2 before maturation with poly(I:C), both Melan-A/MART-1- (Fig.5A-B) and NY-ESO-1-specific (Fig.5A) primed CD4+ T cells significantly failed to produce type-1 cytokines (p≤0.0004 and p≤0.0073 for IFNγ and IL-2, respectively) upon peptide re-stimulation (Fig.5A-B). Primed CD4+ T cells instead significantly produced more IL-4 (mean=2.50% ; mean of differences=1.85, p≤0.0001) and IL-13 (mean=0.08% ; mean of differences=0.07, p≤0.0174) (Fig.5A-B). In addition, CD4+ T cells primed using DCs pre-incubated with heat-inactivated MMP-2 significantly secreted similar cytokines than CD4+ T cells primed in the absence of MMP-2. Accordingly, the former CD4+ T cells (primed with HI MMP-2) were significantly different relative to CD4+ T cells primed in the presence of active MMP-2 (mean of differences=0.21 with p≤0.0007 for IFNγ, mean of differences=0.11 with p≤0.015 for IL-2 and mean of differences=1.92 with p<0.0001 for IL-4) (Fig.5A-B). Therefore, pre-exposure to active MMP-2, but not to inactive MMP-2, confers DCs with the ability to skew the differentiation of naïve CD4+ T cells towards a TH2-like phenotype, regardless of their antigen-specificity. Hence, MMP-2 could participate in the detrimental TH2 skewing observed in certain tumors, including melanoma, thus impairing host anti-tumor immune responses (Botella-Estrada et al., 2005; Lauerova et al., 2002; McCarter et al., 2005; Minkis et al., 2008; Tatsumi et al., 2002).
Figure 5. MMP-2 influences Melan-A/MART-1- and NY-ESO-1-specific T helper differentiation.
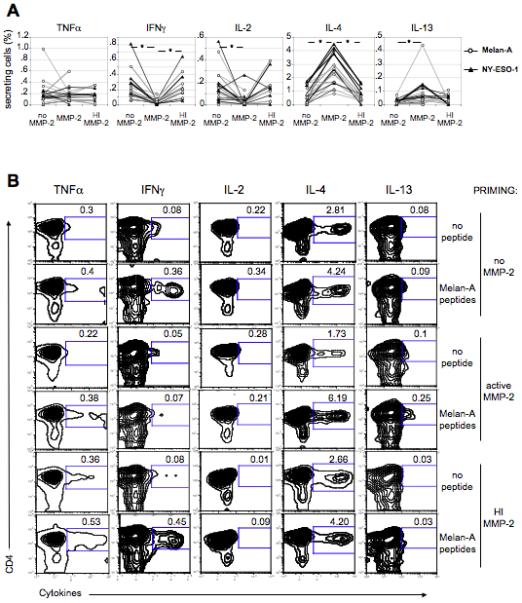
(A) Cord blood-derived CD4+/CD25− cells were stimulated with autologous poly(I:C)-matured DCs pulsed with overlapping peptides spanning either Melan-A/MART-1 or NY-ESO-1. Prior to poly(I:C) addition, DCs were incubated with or without MMP-2 (active or heat-inactivated) for 1h. MMP-2 enzymatic activity was controlled (Fig.S5C-D). After 15 days, CD4+ T cells were stimulated with the MelanA/MART-1 or NY-ESO-1 peptide pools (2μM) for 6h before intracellular staining of cytokines. Percentages of antigen-specific CD4+ T cells, secreting indicated cytokines, are represented for all donors. (B) Contour plots showing percentages of cytokine-producing CD4+ T cells are shown for representative donor CB45 primed to Melan-A/MART-1. Percentage of secreting cells upon peptide stimulation was considered specific/positive when it exceeded by more than twofold the background (cytokine-secreting cells in the absence of stimulation) and had more than 0.5% of responding cells (after background subtraction) for at least one cytokine.
For each cytokine, 2 paired t-tests were used to compare priming in the presence of active MMP-2 to either priming in the absence of active MMP-2 or with HI MMP-2. The Bonferroni adjustment was used for multiple comparisons. p values ≤ 0.025 (*) were considered statistically significant using a Bonferroni correction for 2 comparisons.
MMP-2 induces inflammatory TH2 cells in a basophil-independent but DC-dependent manner
We next studied the cellular requirements underlying the inflammatory TH2 polarization of MMP-2-specific cells. Basophils, recently described as APCs (Sokol et al., 2009), are thought to play an important role in TH2 polarization by secreting factors such as IL-4 or TSLP (Ito et al., 2005; Oh et al., 2007; Sokol et al., 2008). However, MMP-2 neither induced production of IL-4 nor thymic stromal lymphopoietin (TSLP) by human basophils (Fig.S4A-C). In addition, MMP-2 did not increase the expression of the basophil activation marker CD203c (Ocmant et al., 2007), further demonstrating that MMP-2 did not stimulate basophils (Fig.S4A).
We next evaluated whether DCs could be involved in the polarization of MMP-2-specific CD4+ T cells. Expression levels by DCs of maturation markers CD40, CD80, CD83, CD86, HLA-DR and CCR7 were not significantly affected by either active or inactive MMP-2 (not shown).
OX40L expressed on DCs can function as an inflammatory TH2-skewing molecule (Ito et al., 2005). Both active and inactivated MMP-2 significantly increased OX40L expression on DCs (n=9) irrespective of their maturation stage (Fig.6A-B). However, differentiation into inflammatory TH2 cells required pre-exposure of DCs to MMP-2 in its active conformation only (Fig.5), suggesting the involvement, in the polarization mechanism, of another molecule specifically sensitive to active MMP-2.
Figure 6. MMP-2 protein induces OX40L expression on DCs.

Immature and poly(I:C)-matured DCs were incubated with active or HI MMP-2 (0.5μg/mL or 5μg/mL) for 24h. MMP-2 enzymatic activity was controlled (Fig.S5C-D). OX40L expression was assessed by surface staining. (A) Histogram plots are shown for representative donor HD1228. Isotype control is represented in tinted gray. (B) Mean fluorescent intensities are represented for all 9 donors tested.
For immature and mature DCs, paired t-tests were used to compare low and high values of glycerol, active MMP-2 and inactive MMP-2. p values ≤ 0.0167 (*) were considered statistically significant using a Bonferroni correction for 3 comparisons for each DC type.
See also Fig.S4.
Inhibition of IL-12 production by MMP-2
Importantly, MMP-2-treated DCs produced significantly less TH1-associated IL-12p70 (47.8% mean inhibition with 5μg/mL MMP-2; mean of differences=41.7 with p≤0.001, n=11 donors), in a dose-dependent manner, when stimulated with the TLR3 agonist poly(I:C). The reduction in IL-12 was not observed using inactivated MMP-2, MMP-2 peptides, rhPEX (190 amino acid long C-terminal fragment of MMP-2 lacking protease activity) or controls including glycerol (vehicle control for MMP-2), DMSO (peptides diluted in DMSO) and a MMP-2-specific inhibitor alone (Fig.7A). To assess whether MMP-2 diminished IL-12p70 levels by inhibiting DC production/secretion or by directly degrading extracellular IL-12, we incubated rhIL-12 for 24h in the presence or absence of MMP-2. IL-12p70 levels remained the same in every condition (Fig.S5A). Single chains IL-12p35 and IL-12p40 as well as trypsin-exposed IL-12p70 were not recognized by the CBA kit (Fig.S5B), confirming its specificity for bioactive IL-12p70 and therefore strongly suggesting that IL-12 was not a substrate for MMP-2 and that instead MMP-2 directly inhibited DCs to produce IL-12. MMP-2 activity was controlled by measuring its capacity to degrade MCP-3 (Fig.S5C). Furthermore, supernatants of MMP-2-exposed DCs did not affect IL-12p70 production by fresh DCs, suggesting that no cleaved part of a MMP-2 substrate is directly responsible for this effect (Fig.S5E). Following poly(I:C) activation, 4.33% ±0.94 DCs expressed the IL-12p35 subunit, whereas only 1.5% ±0.22 expressed it when pre-incubated with active MMP-2 (Fig.7B). Thus pre-exposure to active MMP-2 reduces DC production of IL-12p35 (mean inhibition=65%), thereby preventing the formation of the bioactive IL-12p70. IL-12p40 production was not affected overall (Fig.7B). Optimal IL-12p35 transcription is thought to depend on STAT1 activation in human moDCs (Gautier et al., 2005). As expected, STAT1 phosphorylation was decreased when DCs were pre-incubated with active (p≤0.0047) but not inactive MMP-2 prior to poly(I:C) stimulation (Fig.7C,D), suggesting that active MMP-2 inhibits IL-12p35 production by preventing STAT1 phosphorylation.
Figure 7. MMP-2-dependent mechanism blocking IL-12 production by DCs.
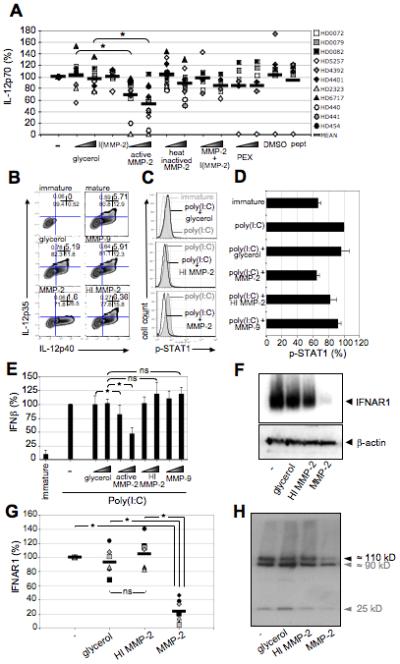
Immature DCs were incubated with MMP-2 (0.5μg/mL or 5μg/mL) and poly(I:C)-matured 1h later. IL-12 levels were measured 16h later. (A) Results are represented as a percentage of IL-12p70 levels, measured by CBA, produced when DCs were incubated with poly(I:C) only (n=11). (B) For intracellular staining of IL-12p35 and IL-12p40 chains, Brefeldin A was added 4h after poly(I:C) activation and cells were stained after an additional 12h incubation. Numbers in the upper right quadrants are the percentages of each population for a representative donor (n=3). (C,D) Immature DCs were incubated with MMP-2 for 1h (5μg/mL) before poly(I:C) maturation. Intracellular staining of phosphorylated (Y701) STAT1 (P-STAT1) was performed 3h later. A representative donor (C) and results for all 3 donors (D) are shown. Active MMP-2 only significantly inhibits STAT1 phosphorylation (p≤0.0047). Results are shown as mean ± SD. (E) Immature DCs were incubated with MMP-2 and poly(I:C)-matured 1h later. IFNβ levels were measured by ELISA 16h later. Results are represented as a percentage of IFNβ levels produced when DCs were incubated with poly(I:C) only (n=9). Results are shown as mean ± SD. (F,G) 106DCs/lane were treated with 0.1μg MMP-2 or controls. Poly(I:C) was added 1h later. IFNAR1 protein expression was detected the next day by Western blot analysis using a rabbit monoclonal antibody. A representative donor (F) and results for all 7 donors (G) are shown. (H) The rhIFNAR1 (5μg ; ≈110kD) was incubated overnight with 0.3μg MMP-2 before performing Western blot analysis for IFNAR1. The second band (around 90 kD) and the third band (25 kD) very likely correspond to either a degraded form of the protein still recognized by the Ab or a contaminant present in the commercial protein preparation and non-specifically recognized by the Ab.
MMP-2 enzymatic activity was controlled (Fig. S5C-D).
Stastistical analysis: A: Two paired t-tests were used to compare low levels of glycerol with low levels of active MMP-2 and high levels of glycerol with high levels of active MMP2; E: For low and high levels, 3 comparisons were carried out (glycerol to active MMP-2, glycerol to HI MMP-2 and active MMP-2 to active MMP-9); G: 3 comparisons were carried out (glycerol to active MMP-2, glycerol to HI MMP-2 and active MMP-2 to HI MMP-2). For the 2 comparisons, p-values ≤ 0.025 (*) were considered statistically significant; for the 3 comparison analyses, p-values ≤ 0.0167 (*) were considered statistically significant
See also Fig.S5.
MMP-2 binds integrins such as αvβ3 (Brooks et al., 1996), αIIbβ3 (Choi et al., 2008), or other β2 integrins (Stefanidakis et al., 2003) and the scavenger receptor CD91 (Emonard et al., 2004), all of which are expressed on human DCs. We therefore tested whether MMP-2 signaled through such molecules to block IL-12 production and induce OX40L expression. PEX peptide, known to prevent MMP-2 binding to integrins such as αvβ3 (Brooks et al., 1998), and blocking mAbs specific for most of these receptors did not restore IL-12p70 production by MMP-2-exposed DCs (not shown), suggesting that other molecules may be involved.
STAT1 phosphorylation (Severa et al., 2006) and subsequent IL-12 production (Gautier et al., 2005) can be induced by type-I IFN receptor triggering. We confirmed that IFNβ induced IL-12p70 production, as blocking mAbs to IFNβ and its receptor significantly inhibited IL-12p70 production and with a similar magnitude as MMP-2 (Fig.S5F). We consequently tested involvement of this cytokine and its receptor in our system. IFNβ signaling through its receptor leads to STAT1 phosphorylation (Severa et al., 2006), which triggers IL-12p35 transcription and enhanced transcription of IFNβ itself through an amplification loop (Decker et al., 2005; Gautier et al., 2005). Accordingly, Poly (I:C)-matured DCs pre-exposed to active MMP-2 produced decreased levels of IFNβ (Fig.7E). Strikingly, we found that MMP-2-exposed DCs drastically lost expression of IFNAR1 chain of the receptor for type-I IFNs (n=7 donors, p≤0.001) (Fig.7F,G). Furthermore, recombinant rhIFNAR1 (≈110kD) incubated overnight with active MMP-2 was degraded by MMP-2 (Fig.7H). Therefore, MMP-2 cleaves IFNAR1 on DCs leading to loss of IL-12 production through reduction of STAT1 phosphorylation.
In summary, both active and inactive MMP-2 induce OX40L expression by DCs while active MMP-2 inhibits IL-12p35 production, and subsequent IL-12p70 formation by degrading IFNAR1.
MMP-2-specific CD4+ T cell differentiation depends on IL-12 and OX40L expression by DCs during priming
To test whether OX40L and/or IL-12 were directly responsible for inflammatory TH2 polarization of MMP-2-specific T cells, naïve CD4+ T cells from 19 cord blood donors were primed by autologous poly(I:C)-matured DCs pulsed with the active MMP-2 protein. As expected, primed MMP-2-specific CD4+ T cells secreted IL-4 and TNFα corresponding to the inflammatory TH2 phenotype observed so far. However, when exogenous rhIL-12 was added throughout priming, MMP-2-specific T cells partially reverted their phenotype towards a TH1-like profile, i.e. secreting less IL-4 (p≤0.0001; n=19) and more IFNγ (p=0.022; n=19). These results are consistent with the concept that lack of IL-12 is critical for inflammatory TH2 differentiation of MMP-2-specific CD4+ T cells (Fig.8A-B). When naïve T cells were primed in the presence of anti-OX40L, MMP-2-specific cells secreted less IL-4 (p≤0.003; n=10) and more IFNγ (p=0.023; n=10) than CD4+ T cells primed without additional IL-12 (Fig. 8A-B). Addition of both rhIL-12 and anti-OX40L during priming triggered MMP-2-specific CD4+ T cells secreting less IL-4 (p≤0.002; n=10) and more IFNγ (p≤0.006; n=10) than anti-OX40L alone (Fig.8B), indicating that both molecules are involved in MMP-2-specific CD4+ T cell polarization.
Figure 8. MMP-2-specific CD4+ T cell differentiation depends on IL-12 and OX40L expression by DCs.

(A) Cord blood-derived CD4+/CD25− cells from 19 donors were stimulated with autologous DCs previously loaded with active MMP-2 (MMP-2 enzymatic activity was controlled (Fig.S5C-D)) for 15 days in the absence or in the presence of exogenous rhIL-12 (10ng/mL) or blocking mAb for OX40L or IL-4 (10μg/mL). CD4+ T cells were then stimulated with the MMP-2 peptide pool (2μM) for 6h before intracellular staining of cytokines. Contour plots representing cytokine production by CD4+ T cells are shown for donor CB31 CD4+ T cells primed to MMP-2 alone, or together with rhIL-12, anti-OX40L mAb or anti-IL-4 mAb (not shown). (B) Percentages of MMP-2-specific CD4+ T cells, secreting indicated cytokines, are represented for all donors.
For each cytokine, 5 paired t-tests were used to compare “no conditioning” versus various conditions. p values ≤ 0.01 (*) were considered statistically significant using a Bonferroni correction for 5 comparisons.
IL-4 is critical for TH2 differentiation in many models (Sokol et al., 2008; Swain et al., 1990). We next primed naïve MMP-2-specific CD4+ T cells to active MMP-2 while blocking potential DC-derived IL-4 with a specific mAb. Primed cells maintained an identical inflammatory TH2 phenotype (n=8) (Fig.8B), confirming that, in this TH2 differentiation model, IL-4 is not a major determinant. Therefore, inflammatory TH2 polarization of MMP-2-specific T cells is dependent upon both IL-12 and OX40L, but not on IL-4.
In summary, our data show that MMP-2 induces priming of TH2 inflammatory CD4+ T cells, by diminishing IL-12 production (through degradation of type-1 IFN receptor) and inducing OX40L expression by DCs. Therefore, MMP-2 acts as an endogenous type-2 conditioner in humans.
DISCUSSION
The first two subsets described of effector CD4+ T cells were the TH1 and TH2 subpopulations (Mosmann and Coffman, 1989). Both cells were shown to mediate anti-cancer functions (Nishimura et al., 1999), but IFNγ-secreting TH1 cells appeared to be superior in inducing memory cytotoxic responses and strong cellular immunity (Nishimura et al., 2000). TH1 cells are characterized by their production of IFNγ and, to a lesser extent, IL-2. IL-12 produced by APCs, particularly activated DCs, as well as IFNγ produced by NK cells and T cells, polarize CD4+ T cells toward the TH1 cell differentiation program through STAT1 and T-bet (Szabo et al., 2000). In contrast, TH2 cells mediate host defense against extracellular parasites including helminths and are important in the induction and persistence of allergic diseases. Conventional TH2 cells are classically defined as producers of IL-4, IL-5, IL-10 and IL-13 but the mechanism(s) underlying induction of TH2 differentiation has remained unclear.
IL-4, by inducing GATA-3 expression, is usually described as essential for TH2 differentiation (Sokol et al., 2008; Swain et al., 1990). Cellular sources of IL-4 for TH2 differentiation have not been clearly established. Granulocytes such as basophils readily produce IL-4 after crosslinking of their FcεRI receptors and have been proposed to be responsible for TH2 differentiation in several models (Falcone et al., 1996; Sokol et al., 2008). MMP-2 did not activate basophils (assessed by IL-4 and TSLP production as well as CD203c expression (Fig.S4A-C)), suggesting that these cells were not involved in this model. Moreover, MMP-2-induced type-2 differentiation does not appear to depend on IL-4 as naïve MMP-2-specific CD4+ T cells primed in the presence or in the absence of a blocking antibody specific for IL-4 gave rise to type-2 CD4+ T cells with a similar profile (Fig.8A-B). This observation is in accordance with accumulating in vivo studies indicating that, in some settings, IL-4 is not essential for TH2 differentiation (Everts et al., 2009; Jankovic et al., 2000; Steinfelder et al., 2009). A default mechanism could also explain TH2 differentiation, where the lack of TH1-polarizing signal, namely IL-12, would be sufficient and/or necessary (Minkis et al., 2008; Moser and Murphy, 2000). We found that active MMP-2 inhibited IL-12 production by DCs (Fig.7A-B), which was in part responsible for the MMP-2-induced type-2 polarization. Indeed, supplementing the priming cultures of MMP-2-specific CD4+ T cells with rhIL12, even at doses as low as 1ng/mL (not shown), induced lower percentages of IL4-secreting T cells and more IFNγ-secreting cells (Fig.8A-B). TSLP leads to TH2 cell recruitment and allergic inflammation (Liu et al., 2007). Basophils (Sokol et al., 2008), epithelial cells (Soumelis and Liu, 2004) or DCs (Ito et al., 2005; Liu, 2006; Watanabe et al., 2004) can be sources of TSLP. TSLP contributes to TH2 immunity either by inhibiting TH1 differentiation, by acting directly on T cells to promote TH2 differentiation or by activating DCs. Stimulation of DCs with TSLP, in the absence of IL-12, induces up-regulation of the costimulatory molecule OX40L and can promote differentiation of naïve T cells into cells secreting IL-4, IL-5, IL-13 and TNFα, named inflammatory TH2 (Ito et al., 2005; Soumelis et al., 2002). MMP-2-specific CD4+ T cells secreted IL-4, IL-13 and TNFα, but no IL-10, consistent with such an inflammatory TH2 profile. Interestingly, MMP-2 did not activate basophils to secrete TSLP (nor IL-4) (Fig.S4). On the other hand, MMP-2 induced DCs to express OX40L (Fig.6A-B), in a TSLP-independent manner (not shown). We showed that inflammatory TH2 differentiation of MMP-2-specific cells also relied on OX40L expression (Fig.8A-B). OX40 signaling in CD4+ T cells can directly induce type-2 lineage commitment by inducing NFATc1, which triggers IL-4 production and subsequent GATA-3 expression (So et al., 2006; Yu et al., 2009). MMP-2-induced type-2 differentiation likely works differently as we showed that IL-4 was not a major determinant in this model. Of note, addition of both rhIL-12 and anti-OX40L blocking mAb during priming induced MMP-2-specific CD4+ T cells secreting significantly less IL-4 and more IFNγ than anti-OX40L alone (Fig.8B), indicating that both molecules were involved in MMP-2-induced type-2 differentiation by triggering additive effects.
We found that MMP-2 degrades IFNAR1 (Fig.7F-H), constituting one chain of the heterodimeric type-I IFN receptor. The IFNAR1 chain, described here as a substrate for MMP-2, is essential for signaling (Decker et al., 2005). When cleaved, the type-I IFN amplification loop is abrogated, explaining the reduction of IFNβ secreted by MMP-2-exposed DCs (Fig.7E). Type-I IFN receptor signaling triggers STAT1 phosphorylation (Severa et al., 2006), which in turn also induces IL-12p35 transcription (Gautier et al., 2005). As a result, bioactive IL-12p70 formation is inhibited and cannot exert its function in TH1 differentiation. Through this mechanism, MMP-2 acts as an endogenous type-2 “conditioner”.
TNF+/IL10− TH2 cells are thought to represent the pathogenic TH2 cells that cause allergic inflammation, in contrast to the conventional IL-10-producing cells. Many potent allergens have intrinsic protease activity (Grobe et al., 1999; Kheradmand et al., 2002) and secreted proteases are essential for the infectious and reproductive cycles of helminthes (McKerrow et al., 2006). Recent studies showed that the T2 ribonuclease derived from soluble egg antigens of the parasitic helminth Schistosoma triggers potent TH2 responses by conditioning mouse DCs (Everts et al., 2009; Steinfelder et al., 2009). One can speculate that the innate immune system might have evolved a mechanism to detect abnormal proteases associated with helminth infection that could then be triggered by other proteases, such as allergens or in this case MMP-2. Our results clearly showed that an enzyme-induced TH2-conditioning directly resides in the intrinsic enzymatic activity rather than solely in the active conformation of MMP-2.
MMP-2 also acts as a type-2 conditioner for CD4+ T cells recognizing other MAA (Fig.5A-B), indicating that MMP-2 plays a dominant role in biasing the response against otherwise TH1 inducing tumor antigens. Our in vitro results suggest that increased MMP-2 secretion may locally influence the induction of immune responses and skew them towards type-2 responses. Accordingly, MMP-2-specific CD4+ T cell responses tended to be found in TILs from patients exhibiting a poorer clinical outcome (Fig.S1B). MMP-2 might therefore partially explain the observed prevalence, at least in some studies, of detrimental type-2 responses in various cancers including melanoma.
Our findings collectively support the idea that the pro-tumoral MMP-2 protein represents a good candidate to target in immunotherapy to treat melanoma patients, since it can induce both broad CD4+ and CD8+ T cell responses. Furthermore, elucidation of mechanisms underlying TH2 polarization, including the one identified in this study, opens the way to designing immune strategies for inducing effective anti-tumor TH1-like responses to treat cancer patients.
EXPERIMENTAL PROCEDURES
Human tissue specimens
Tumor infiltrating lymphocytes (TILs) were provided by Pr. F. Jotereau and B. Dréno. Following informed consent, TILs were obtained from tumor-invaded lymph nodes of melanoma patients (stage IIIb). These patients subsequently received autologous TILs and IL-2 infusions in a clinical trial (Labarriere et al., 2002). This protocol was approved by the Institutional Ethics Committee and registered with the regulatory state authority in France (Nantes). Moreover, TIL were expended in a specific genetic and cellular therapy unit (UTCG; CHRU, Nantes, France) under good manufacturing practice (GMP) conditions. Blood from healthy donors was purchased from the New York Blood Center. Of note, all samples, from donors and patients, have been de-identified prior to the analysis.
Reagents
Purified human MMP-2 (mixture of the proenzyme (50%) and the active form (50%)) was purchased from Biomol. The MMP-2 enzyme was inactivated either by heating to 56°C for 45min or by addition of the MMP-2 inhibitor III at 100nM (Calbiochem) for 20min. rhPEX was purchased from Genway. rhMMP-9 (Calbiochem) was used as a mixture of the proenzyme (50%) and the active protein (50%). Overlapping peptides (20 amino acid long overlapping of 10) spanning proMMP-2 sequence were from Proimmune (>80% pure). Lyophilized peptides were reconstituted in DMSO and were used either individually (2μM) or as a pool (2μM each). rhGM-CSF was purchased from Immunex. rhIL-12, rhIL-4, rhIL-7, rhIL-2 were from R&D Systems. The kits used for basophil isolation and IFNγ-secreting cell enrichment (Miltenyi Biotec) were used according to the manufacturer's instructions.
Antibodies
Allophycocyanin-conjugated antibody to CCR-7 (150503) was purchased from R&D Systems. Unconjugated (10μg/mL for blocking experiments) or phycoerythrin-conjugated antibody to OX40L (11C3.1) was from Biolegend. Blocking antibody to IL-4 (8F12; 10μg/mL) was from Acris. Antibodies to human Vβ chains covering 70% of the repertoire (IOTest Beta Mark; Beckman Coulter) were used according to the manufacturer's intructions. Fluorescein isothiocyanate-conjugated antibody to IL-12p35/IL-12p70 (B-T21) and IFNAR1 (EP899Y) were from Abcam. Phycoerythrine-conjugated antibodies to IL-12p40/IL-12p70 (C8.6), IL-10 (B-T10), CD203c (FR3-16A11) were from Miltenyi Biotec. Blocking antibody to HLA-DQ (SPVL3; 20μg/mL) was from NeoMarkers. Blocking antibodies to HLA-DP (B7/21; 20μg/mL) or HLA-DR (L243; 20μg/mL), phycoerythrine-conjugated antibodies to phosphorylated (Y701) STAT1 (4a), GATA-3 (L50-823), IL-2 (MQ1-17H12), IL-4 (8D4-8), IL-5 (JES1-39D10), IL-13 (JES10-5A2), TNFα (MAb11), IFNγ (25723.11), perforin (μδG9), GranzymeB (GB11), CD40 (5C3), CD80 (L307.4), CD83 (HB15e), CD86 (IT2.2), HLA-DR (TU36), fluorescein isothiocyanate-conjugated antibodies to CD45RA (HI100) and CD45RO (UCHL1), and antibody to CD4 (RPA-T4) were purchased from BD Biosciences Pharmingen. Alexa fluor 488-conjugated antibody to IL-17 (eBio64DEC17), phycoerythrine-conjugated antibody to T-bet (4B10) and allophycocyanin-conjugated antibody to CD62L (DREG-56) were from eBioscience. Antiboby to β-actin (C-2) was from Santa Cruz Biotechnology.
T cell culture, stimulation and priming
Peripheral blood mononuclear cells (PBMCs) were purified from healthy donor- (HD) or cord blood donor- (CB) derived buffy coats (New York Blood Center) by Ficoll-Paque Plus (GE Healthcare) centrifugation. CD4+/CD25− cells were enriched (>90%) by magnetic cell sorting (Miltenyi Biotec) and primed/stimulated for 12-15 days either with irradiated (35Gy) autologous CD4− cells or with autologous mature DCs in IMDM (GIBCO) supplemented with 1mM HEPES (Life Technologies), 2mM L-glutamine (Sigma), streptomycin (100UI/mL)/penicillin (100μg/mL) (Sigma) and 5% heat inactivated pooled human serum (PHS; Valley Biomedical) in the presence of rhIL-2 (10UI/mL) and IL7 (5ng/mL) (R&D Systems). Antigen presenting cells (CD4− cells or DCs) were loaded either with peptides (2μM) or with the MMP-2 protein (10μg/mL) for 2 and 5h, respectively. DCs were matured using the TLR3 agonist poly(I:C) at 5μg/mL/106 DCs (Amersham). To generate T cell clones, we originally relied on our published methodology (Godefroy et al., 2006; Godefroy et al., 2007), involving enrichment of IFNγ-secreting cells upon short-term culture and peptide stimulation to generate MMP-2 responsive clones. Although MMP-2-specific cells could be isolated, it was realized that IFNγ secretion was marginal compared to their secretion of IL-4 and TNFα. IFNγ-secreting cells in response to MMP-2 peptide pool were enriched by cytokine-guided magnetic cell sorting (Miltenyi Biotec) and cloned the following day by limiting dilution in the presence of irradiated allogeneic PBMCs, 1μg/mL phytohemagglutinin-L (Sigma) and 150UI/mL rhIL-2. Tumor infiltrating lymphocytes (TILs) were provided by Pr. F. Jotereau and B. Dréno. They were obtained from tumor-invaded lymph nodes of melanoma patients (stage IIIb) and expanded ex vivo. These patients received autologous TILs and IL-2 infusions in a clinical trial (Labarriere et al., 2002). This protocol was approved by the Institutional Ethics Committee and registered with regulatory state authority in France (Nantes).
Dendritic cell preparation and activation
PBMCs were purified from healthy- (HD) or cord blood- (CB) donors and plated at 40.106 cells/10mL/dish in complete IMDM with 5% PHS. Cells were allowed to adhere for 2h at 37°C. Non-adherent cells were removed. The monocyte-enriched fraction was supplemented with 100UI/mL rhGMCSF and 300UI/mL rhIL-4 (R&D Systems) on days 0, 2 and 4. Immature DCs were harvested on day 5 and matured using poly(I:C) at 5μg/mL/106 DCs (Amersham). Secretion of IL-12p70, TNFα, IL-1β, IL-6, IL-8 and IL-10 was assessed on both immature and mature DCs using the Human Inflammatory Cytokine Cytometric Bead Array (BD Pharmingen).
Enzyme-linked immunosorbent assay
Activation of T cell clones (10,000 cells/100μL/well), polyclonal T cell populations (100,000 cells/100μL/well), DCs (50,000 cells/100μL/well) and basophils (10,000 cells/100μL/well) was determined by ELISA. IFNγ (BioSource), TNFα (BioSource), IL-4 (BioSource), TSLP (Quantikine; R&D Systems), IFNβ (VeriKine; PBL interferon source) and MCP-3 (DuoSet; R&D Systems) contents in supernatants were measured according to the manufacturer's instructions.
Intracellular staining
T-bet and GATA-3 expression was measured on resting T cells. Cells were fixed (4% paraformaldehyde for 10min at RT), permeabilized with 0.1% saponin, and stained for intracellular transcription factors. Cytokine production by T cells was also assessed by intracellular staining. T cells were stimulated with 2μM overlapping peptides. After 1h, 10μg/mL brefeldin A was added to the cells. Five hours later, T cells were stained for surface markers, fixed, permeabilized, and stained for intracellular cytokines (TNFα, IFNγ, IL-2, perforin, granzymeB, IL-4, IL-5, IL-10, IL-13 and IL-17). Antigen-specificity was defined by the percentage of cells secreting cytokine as long as it exceeded background (cytokine-secreting cells in the absence of peptide stimulation) by more than twofold and consisted of more than 0.5% of responding cells following subtraction of background for at least one cytokine. It was not uncommon to find relatively high background levels of IL-4 and TNFα producing T cells, likely due to the fact that these highly sensitive cells continued to produce cytokine up to 2-3 weeks after stimulation. For assessment of IL-4 production by basophils, brefeldin A was added simultaneously to stimulation. For intracellular staining of IL-12p35, IL-12p40 and phosphorylated STAT1, immature DCs were incubated with or without MMP-2 as described in figure legends. After 1h, 5μg/mL poly(I:C) was added to activate immature DCs. For monitoring IL-12 subunits, brefeldin A was added 4h after poly(I:C) addition. DCs were then cultured for 12h, fixed, permeabilized and stained for intracellular IL-12. For detection of STAT1 phosphorylation on Y701, DC were fixed 3h after poly(I:C) addition, permeabilized and stained. Data were acquired with FACScalibur cytometer (BD Biosciences) and analyzed using FlowJo software.
Western blots
IFNAR1 and β-actin were detected by Western blot analysis using rabbit and mouse mAbs, respectively. HRP-linked anti-rabbit (Cell Signaling) or anti-mouse (Amersham Biosciences) IgG were used as secondary Abs before chemiluminescent detection (ECL Plus, Amersham Biosciences).
Statistical analysis
Separate analyses were performed for each experiment individually. Analyses take into account paired observations within donors when appropriate (e.g., MMP-2 vs no MMP-2, active vs inactive, active vs peptides). For three-group comparisons (i.e., MMP-2, Melan-A, NY-ESO-1), analyses of variance were performed for an overall comparison among independent groups, and t-tests were then used for specific pairwise comparisons between groups. Within each analysis, p-values were adjusted for multiple comparisons using a Bonferroni correction. For analyses in which each of two groups were compared to a third group (i.e., two comparisons, with no overall test of the three groups), two t-tests were performed, using the Bonferroni adjustment for the two analyses. Two-sided statistical tests were performed at an overall alpha-level of 0.05, with adjustments for multiple comparisons, as described above. Details for each analysis are provided in the figure legends.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dusan Bogunovic (New York University) and Davor Frleta (New York University) and Anne Gallois (New York University) for critical reading of this manuscript. This work was supported by the Cancer Research Institute, the Ludwig Institute for Cancer Research, the Alliance for Lupus Research, the Emerald Foundation, the Bill and Melinda Gates Foundation and the following National Institute of Health awards, R01 AI071078 and CCSG 5 P30 CA16087.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Botella-Estrada R, Escudero M, O'Connor JE, Nagore E, Fenollosa B, Sanmartin O, Requena C, Guillen C. Cytokine production by peripheral lymphocytes in melanoma. Eur Cytokine Netw. 2005;16:47–55. [PubMed] [Google Scholar]
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Choi WS, Jeon OH, Kim HH, Kim DS. MMP-2 regulates human platelet activation by interacting with integrin alphaIIbbeta3. J Thromb Haemost. 2008;6:517–523. doi: 10.1111/j.1538-7836.2007.02871.x. [DOI] [PubMed] [Google Scholar]
- Coulie PG, van der Bruggen P. T-cell responses of vaccinated cancer patients. Curr Opin Immunol. 2003;15:131–137. doi: 10.1016/s0952-7915(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N, et al. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonard H, Bellon G, Troeberg L, Berton A, Robinet A, Henriet P, Marbaix E, Kirkegaard K, Patthy L, Eeckhout Y, et al. Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2. TIMP-2 complex through a thrombospondin-independent mechanism. J Biol Chem. 2004;279:54944–54951. doi: 10.1074/jbc.M406792200. [DOI] [PubMed] [Google Scholar]
- Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone FH, Dahinden CA, Gibbs BF, Noll T, Amon U, Hebestreit H, Abrahamsen O, Klaucke J, Schlaak M, Haas H. Human basophils release interleukin-4 after stimulation with Schistosoma mansoni egg antigen. Eur J Immunol. 1996;26:1147–1155. doi: 10.1002/eji.1830260528. [DOI] [PubMed] [Google Scholar]
- Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrineparacrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy E, Moreau-Aubry A, Diez E, Dreno B, Jotereau F, Guilloux Y. alpha v beta3-dependent cross-presentation of matrix metalloproteinase-2 by melanoma cells gives rise to a new tumor antigen. J Exp Med. 2005;202:61–72. doi: 10.1084/jem.20042138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy E, Scotto L, Souleimanian NE, Ritter G, Old LJ, Jotereau F, Valmori D, Ayyoub M. Identification of two Melan-A CD4+ T cell epitopes presented by frequently expressed MHC class II alleles. Clin Immunol. 2006;121:54–62. doi: 10.1016/j.clim.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Godefroy E, Wang Y, Souleimanian NE, Scotto L, Stevanovic S, Chen YT, Valmori D, Ayyoub M. Assessment of CD4+ T cells specific for the tumor antigen SSX-1 in cancer-free individuals. Cancer Immunol Immunother. 2007;56:1183–1192. doi: 10.1007/s00262-006-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe K, Becker WM, Schlaak M, Petersen A. Grass group I allergens (beta-expansins) are novel, papain-related proteinases. Eur J Biochem. 1999;263:33–40. doi: 10.1046/j.1432-1327.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- Hirohashi Y, Torigoe T, Inoda S, Kobayasi J, Nakatsugawa M, Mori T, Hara I, Sato N. The functioning antigens: beyond just as the immunological targets. Cancer Sci. 2009;100:798–806. doi: 10.1111/j.1349-7006.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khammari A, Nguyen JM, Pandolfino MC, Quereux G, Brocard A, Bercegeay S, Cassidanius A, Lemarre P, Volteau C, Labarriere N, et al. Long-term follow-up of patients treated by adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2007;56:1853–1860. doi: 10.1007/s00262-007-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B, Jotereau F. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother. 2002;51:532–538. doi: 10.1007/s00262-002-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauerova L, Dusek L, Simickova M, Kocak I, Vagundova M, Zaloudik J, Kovarik J. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49:159–166. [PubMed] [Google Scholar]
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- Loose D, Van de Wiele C. The immune system and cancer. Cancer Biother Radiopharm. 2009;24:369–376. doi: 10.1089/cbr.2008.0593. [DOI] [PubMed] [Google Scholar]
- McCarter M, Clarke J, Richter D, Wilson C. Melanoma skews dendritic cells to facilitate a T helper 2 profile. Surgery. 2005;138:321–328. doi: 10.1016/j.surg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol. 2006;1:497–536. doi: 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- Minkis K, Kavanagh DG, Alter G, Bogunovic D, O'Neill D, Adams S, Pavlick A, Walker BD, Brockman MA, Gandhi RT, et al. Type 2 Bias of T cells expanded from the blood of melanoma patients switched to type 1 by IL-12p70 mRNA-transfected dendritic cells. Cancer Res. 2008;68:9441–9450. doi: 10.1158/0008-5472.CAN-08-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, Koda T, Nishimura S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandene L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320:40–48. doi: 10.1016/j.jim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–2927. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severa M, Remoli ME, Giacomini E, Ragimbeau J, Lande R, Uze G, Pellegrini S, Coccia EM. Differential responsiveness to IFN-alpha and IFN-beta of human mature DC through modulation of IFNAR expression. J Leukoc Biol. 2006;79:1286–1294. doi: 10.1189/jlb.1205742. [DOI] [PubMed] [Google Scholar]
- So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci U S A. 2006;103:3740–3745. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V, Liu YJ. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol. 2004;25:325–333. doi: 10.1007/s00281-003-0152-0. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Stefanidakis M, Bjorklund M, Ihanus E, Gahmberg CG, Koivunen E. Identification of a negatively charged peptide motif within the catalytic domain of progelatinases that mediates binding to leukocyte beta 2 integrins. J Biol Chem. 2003;278:34674–34684. doi: 10.1074/jbc.M302288200. [DOI] [PubMed] [Google Scholar]
- Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, de Waal Malefyt R, Liu YJ. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781–792. [PubMed] [Google Scholar]
- Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


