Abstract
Synapses are the fundamental units of neuronal circuits. Synaptic plasticity can occur through changes in synaptic strength, as well as through the addition/removal of synapses. Two-photon microscopy, in combination with fluorescence labeling, offers a powerful tool to peek into the living brain and follow structural reorganization at individual synapses. Time-lapse imaging depicts a dynamic picture, in which experience-dependent plasticity of synaptic structures varies between different cortical regions and layers, as well as between neuronal subtypes. Recent studies have demonstrated that the formation and elimination of synaptic structures happens rapidly in a subpopulation of cortical neurons during various sensorimotor learning experiences, and that stabilized synaptic structures are associated with long-lasting memories for the task. Thus, circuit plasticity, mediated by structural remodeling, provides an underlying mechanism for learning and memory.
Introduction
Neuronal plasticity refers to structural and functional changes of neuronal circuits in response to experience. This concept was first proposed by William James in the nineteenth century to correlate structural changes of the brain with the habitual behavior of animals [1]. In the 1960’s and 70’s, Hubel and Wiesel examined the plasticity of cat and monkey visual systems and identified a ‘critical period’ during which deprivation of normal visual experience irreversibly altered neuronal connections and functions in the visual cortex [2–5]. Since then, similar findings have been made in other systems, and the ‘critical period’ is defined as a period of time during which neuronal connections are susceptible to experience-dependent modifications [6]. For a long time, it was believed that neuronal circuits lost plasticity after the end of the critical period, and remained fixed in adult life. However, this idea has been greatly challenged in recent decades. Cumulative data have shown that experience-dependent plasticity occurs in adulthood and it is now well accepted that neuronal circuits of the mammalian brain are capable of changing in response to new experience throughout life [7–11].
The mammalian neocortex participates in a variety of brain functions, such as sensory perception, movement control, and cognition. Various cortical functions build upon different neuronal circuits, which are made up of different types of neurons communicating at individual synapses. It is generally believed that a functional, mature neuronal circuit is formed from an initial pool of less precise synaptic connections. Experience modifies these connections by selectively stabilizing some synapses and removing others [8, 12, 13]. In the adult brain, synaptic plasticity continues through modifications of synaptic strength, as well as through formation and elimination of synapses [8, 10]. Until a decade ago, much of our knowledge about neuronal structural changes had been inferred from single time-point observations of fixed tissues. Given the complexity of neuronal circuits and the variability among animals, it is crucial to obtain longitudinal data from the same synapses over time to understand their dynamics [14, 15]. Recent developments in both imaging and molecular tools enable the visualization of synapses in living animals (Figure 1). In particular, two-photon laser scanning microscopy offers low phototoxicity and deep penetration through thick, scattering preparations, which makes it suitable for imaging in the intact brain [16]. Both turnover (formation and elimination) and morphological changes of synaptic structures have been examined in various cortical regions over periods ranging from minutes to years. In this review, we will summarize recent in vivo studies on the structural plasticity of the cortex. We will also discuss how these structural changes relate to changes in synaptic connectivity and outline open questions remaining to be addressed in the field.
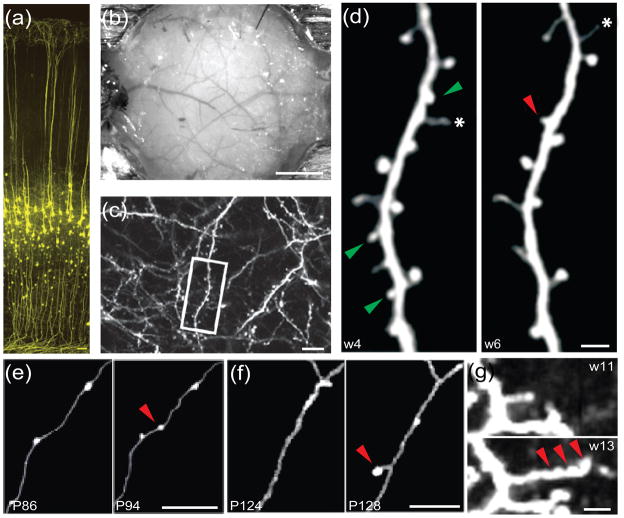
Figure 1.
Transcranial two-photon imaging of fluorescently labeled pyramidal cortex neurons in the living mouse brain. (a) This image illustrates a subset of YFP-expressing L5 pyramidal neurons that extend their apical dendrites to L1 in the barrel cortex of a one-month-old YFP-H transgenic mouse. Scale bar: 50 μm. Modified, with permission, from [36]. (b) A CCD camera image of the brain vasculature as observed in the mouse cortex using the thinned skull method of preparation. Scale bar: 1 mm. (c–d) Dendritic spine dynamics of L5 pyramidal neurons in the barrel cortex of a YFP-H line adolescent mouse. (c) A low-magnification 2D projection from a 3D stack of dendritic branches and axons. Scale bar: 10 μm. (d) A higher magnification view of the dendritic segment demarcated in the white box in (c). Two images of the same dendritic segment, taken at the ages of weeks (w) 4 and 6, respectively, reveal spine elimination (green arrowheads), spine formation (red arrowheads) and filopodia (asterisks). Scale bar: 2 μm. (c) and (d) are modified, with permission, from [27]. (e) En passant bouton dynamics of intracortical axons from L2/3 and L4 neurons in the barrel cortex of a GFP-M transgenic mouse. Two images of the same axon segment, taken at P86 and P94, respectively, reveal the addition of an en passant bouton (red arrowhead). Scale bar: 10 μm. (f) Terminaux bouton dynamics of thalmocortical axons in the barrel cortex of a GFP-M transgenic mouse. Two images of the same axon segment, taken on P124 and P128, respectively, reveal the addition of a terminaux bouton (red arrowhead). Scale bar: 5 μm. (e) and (f) are modified, with permission, from [59]. (g) Dendritic tip dynamics of layer 2/3 interneurons in the visual cortex of a GFP-S transgenic mouse. Two images of the same dendrite, taken at the age of weeks 11 and 13, respectively, reveal elongation of a dendritic tip (pointed by 3 red arrowheads). Scale bar: 5 μm. Modified, with permission, from [58].
Dendritic plasticity of excitatory pyramidal neurons
A majority of cortical synapses are axodendritic synapses. Dendrites are the sites where neurons receive and integrate information. The first in vivo imaging of dendrites was performed over a decade ago in the rat barrel cortex, using a Sindbis virus containing the gene for enhanced green fluorescent protein (EGFP) to label layer (L) 2/3 pyramidal neurons [17]. Most subsequent studies have taken advantage of thy1 transgenic mice [18]. These mice express fluorescent proteins (i.e., GFP or yellow fluorescent protein, YFP) under the control of the thy1 (thymocyte differentiation antigen 1) promoter, which results in fluorescent labeling of a subset of neurons. These mice have been extensively used by numerous research groups to study structural dynamics of pyramidal neurons during postnatal development and in adulthood.
In vivo imaging of cortical pyramidal neurons has shown that, except for small tip extensions and retractions, dendritic branches are mostly stable in adulthood [19, 20]. Such dendritic stability is tightly regulated. Recent in vivo studies show that mutant mice lacking the cell adhesion molecule, δ-catenin, undergo progressive retraction of dendrites and spine loss after 5 weeks of age [21]. In contrast, absence of the tumor suppressor protein, phosphatase and tensin homolog (PTEN), results in continuous growth of L2/3 pyramidal neuron apical dendrites and expansion of the mature cortex [22].
Along dendrites, many small protrusions emanate from dendritic shafts. Not only are these protrusions heterogeneous in shape [23–25], their density also changes during development and varies among different types of neurons [26–28]. In fact, a long-standing debate exists in the field regarding the classification of these protrusions. Some researchers believe that dendritic protrusions cover a continuous spectrum of morphologies and do not fall into recognizable structural subtypes. Hence, all dendritic protrusions are identified as spines in their studies [19, 28–32]. These researchers argue that even the thinnest spine could have synapses or contain synaptic proteins [19, 33–35]. In contrast, other researchers categorize protrusions into spines and filopodia, based on their distinct morphologies. Spines are indentified as protrusions with bulbous heads, while filopodia are long thin protrusions without clearly defined heads [17, 27, 36–40]. These researchers argue that filopodia are much more dynamic than spines [36, 39, 40], and even daily imaging could underestimate the dynamics of filopodia. Furthermore, they argue that filopodia act as precursors of spines [34, 36, 41–43] and do not necessarily have functional synapses [44]. In this review, results obtained for both filopodia and spines are summarized. While filopodia have mostly been described during development, when they are abundant, spines have generally been the focus of adult studies. Given the fact that filopodia are rare in adults, conclusions of adult spine dynamics are largely not affected by including or excluding filopodia from the analyses. The cumulative results from these in vivo studies reveal that development, experience, and various pathologies all affect spine and filopodium dynamics in significant ways, as discussed in more detail below (see also Tables 1 and 2, and Box 1).
Table 1.
Effect of development and experience on spine dynamics in different regions of rodent cortex in vivo.
| Species/cells labeled | Age | Main findings | Refs. |
|---|---|---|---|
| Visual cortex | |||
| Mouse (H-line), L5 neurons | 1–10m | Spines in the adult visual cortex were found to be very stable, with a half-life of >13 months. Over 95% of spines persisted over 1 month. | [39] |
| Mouse (H/M-line), L2/3/5 neurons | P28-61 | Spine motility varied in different cortices at 1 month of age, with the visual cortex being the most stable. Rewiring visual inputs into the auditory cortex did not change intrinsic spine motility in the auditory cortex. | [40] |
| Mouse (M-line), L5 neurons | >3m | Focal retinal lesions dramatically increased spine turnover in the lesion projection zone, leading to an almost complete replacement of original spines within 2 months. | [46] |
| Mouse (M-line), L2/3/5 neurons | >1.5m | MD increased spine formation in L5, but not L2/3 neurons, in the binocular cortex. Spines formed during MD persisted during binocular vision restoration, and were strengthened during the second MD. | [30] |
| Mouse (M-line), L5 neurons | ~P28 | Dark-rearing resulted in high motility and immature morphology of spines. While brief light exposure increased spine motility, prolonged light exposure stabilized spines over time. | [32] |
| Barrel cortex | |||
| Rat, virus-labeled L2/3 neurons | P8-16 | Spines and filopodia were highly motile during early postnatal development. Trimming all the whiskers unilaterally reduced spine and filopodium motility in the contralateral barrel cortex during a critical period between P11 and P13. | [17] |
| Mouse (M-line), L5 neurons | P34-74 | Spines displayed a spectrum of stability. Chessboard deprivation increased the pool of transient spines and decreased the pool of stable spines. | [19] |
| Mouse (M/H-line), L2/3/5 neurons | P14-511 | The fraction of persistent spines increased with an animal’s age. The visual cortex was observed to be more stable than the barrel cortex in 6m old mice. | [28] |
| Mouse (H-line), L5 neurons | P14-23m | Spines and filopodia displayed distinct dynamics. Spine dynamics decreased with age. In adults, a majority of spines were stable throughout the animal’s life. | [36] |
| Mouse (H-line), L5 neurons | 1–6m | Trimming all the whiskers unilaterally prevented spine elimination in the contralateral barrel cortex in both adolescent and adult mice. This activity-dependent spine elimination was NMDA receptor dependent. | [27] |
| Mouse (M-line), L5 neurons | 2–5m | Chessboard whisker trimming stabilized new spines and destabilized previously persistent spines, preferentially on L5 neurons with complex apical tufts. | [29] |
| Mouse (H-line), L5 neurons | 1–18.5m | Sensory enrichment increased spine formation and elimination. Most spines formed early during development and a small fraction of new spines induced by novel experience survived experience-dependent elimination and were preserved into adulthood. | [38] |
| Mouse (M-line), L5 neurons | 2–3m | Chessboard whisker trimming increased new spine stabilization at the border between spared and deprived barrel columns. CaMKII autophosphorylation-defective mutant mice lacked such experience-dependent spine stabilization. | [31] |
| Motor cortex | |||
| Mouse (H-line) L5 neurons | 1m, >4m | Forelimb-specific motor skill training induced rapid spine formation, followed by prolonged spine reorganization selectively in the forelimb region. Different motor skills affected different populations of spines. | [37] |
| Mouse (H-line) L5 neurons | 1m, >4m | Rotarod training increased spine turnover. | [38] |
Abbreviations: months (m), Ca2+/calmodulin-dependent protein kinase II (CAMKII).
Table 2.
Alterations in dendritic structure and dynamics in the living mouse brain after various neurological insults/injuries or disease progression.
| Mice line or cell labeling | Main findings | Refs. |
|---|---|---|
| Stroke | ||
| H/M-line | >90% reduction of blood flow caused rapid loss of spines and dendrite structures and reperfusion within an hour was capable of restoring a majority of damaged structures. | [67–69] |
| H-line | Turnover of apical dendritic spines increased in the peri-infarct area within weeks after stroke, with balanced arbor extension and retraction. | [70, 71] |
| Alzheimer’s disease | ||
| PSAPP transgenic crossed with H line | Dendrites near amyloid plaques underwent dramatic spine loss and shaft atrophy. | [72] |
| Tg2576, Virus | Damage of dendrites and loss of spines was observed within ~20μm of Aβ plaques. | [73] |
| APP KO crossed with H line | Spine density was 2x higher in adult APP KO mice compared to wildtype mice. Spine dynamics in APP KO mice were insensitive to γ-secretase inhibition. | [74] |
| Fragile X syndrome | ||
| Fmr1 KO, in utero electroporation | Fmr1 KO mice were observed to have an increase in spine turnover and a delay in transition from immature to mature spines during early postnatal development. | [75] |
| Fmr1 KO crossed with H line | Fmr1 KO mice showed increased spine turnover and lack of sensory manipulation-induced changes in spine dynamics. | [76] |
| Prion disease | ||
| H-line | Prion infected mice exhibited a loss in persistent spines (lifetime >8d), but no change in transient spines (lifetime ≤4d), as compared to non-infected control mice. | [77] |
Abbreviations: presenilin-1 and amyloid precursor protein (PSAPP), amyloid precursor protein (APP).
Box 1. Alterations of dendrites and spines in neuropathology.
While experience-dependent spine remodeling provides a structural basis for learning and memory, altered spine morphology and dynamics are hallmarks of injuries and neurological diseases (Table 2). Ischemic stroke causes a rapid (within minutes) loss of spines and distortion of dendrites, which can be mostly recovered if reperfusion happens within one hour [67–69]. Following stroke, dendritic branch remodeling and spine formation are selectively elevated in the peri-infarct area, leading to a gradual recovery of spine density between 1 and 6 weeks [70, 71]. Since post-stroke behavioral recovery is thought to be dependent on adjacent, surviving tissues taking over the function of damaged tissues, these studies provide a structural basis for functional reorganization in the peri-infarct region. Similar dendritic deterioration and remodeling have also been observed in neurodegenerative diseases. For example, dramatic spine loss and neurite dystrophy have been observed in the vicinity of β-amyloid plaques in the cortex of mouse models of Alzheimer’s disease [72, 73]. Such structural changes likely contribute to altered neuronal circuits and functions.
In contrast to the spine loss in injuries and neurodegenerative diseases, elevated spine density is a hallmark for Fragile X Syndrome (FXS), the most frequent form of inherited mental retardation. Two recent studies have investigated spine dynamics in Fragile X mental retardation-1knockout mice (Fmr1 KO, a mouse model of FXS) during postnatal development [75, 76]. Both studies reveal a significant increase in spine turnover in the barrel cortex of Fmr1 KO mice. They also show a delayed maturation of spines [75] and a decreased sensitivity of spine plasticity to sensory manipulation [76], suggesting developmental defects of these mice.
Plasticity during development
Filopodia are predominant during early development. In vivo imaging shows that the proportion of dendritic filopodia gradually decreases as animals mature, from over 50% at 2 weeks of age to <10% at one month of age, and to 2–3% in adulthood in the mouse barrel cortex [36]. At one month of age, a small percentage (~15%) of filopodia has been observed to form bulbous heads within 4 hours in vivo. However, less than 20% of these newly formed spines persist for more than 2 days, suggesting that spine stabilization is a highly selective process [36].
In early postnatal development (i.e., around 2 weeks of age), both dendritic spines and filopodia are very dynamic, appearing, disappearing or changing shape over tens of minutes [17]. By the age of one month, spines and filopodia display markedly different dynamics. While filopodia turn over on a daily basis, spines are much more stable [36, 39]. Between 1 to 4 months of age, more spines are lost than formed in all cortical regions examined, resulting in a ~30% pruning of total spines [27, 36]. In adults, more than 70% of spines are stable over 18 months, with comparable rates of spine formation and elimination [36, 39]. In summary, despite the debate on the baseline of spine dynamics (Box 2), it is now believed that dendritic spines change their morphology and turn over rapidly in developing animals but become much more stable in adults.
Box 2. Factors causing discrepancy of spine dynamics in different studies.
Despite the general consensus on the developmental pruning and experience-dependent plasticity that occurs throughout an animal’s life, the exact degree of synapse structural dynamics is still under debate. Several factors are likely to contribute to discrepancies that currently exist between studies:
Animal lines and labeling methods: Except a few virus labeling studies [17, 48], the majority of in vivo imaging of dendritic spines has been performed using two thy1 transgenic lines (GFP-M-line and YFP-H-line) [18]. Given the cell-type dependent dendrite morphology and dynamics [28–30], the inconsistency in baseline dynamics of dendritic spines could be due to variation in neuronal subtypes labeled in these two different mouse lines. Indeed, it has been shown that the YFP-H line labels a subpopulation of L5 pyramidal neurons with distinct local circuits [78].
Age of animals: Various ages are used in different studies. Since the dynamics of dendritic spines [28, 36] decrease with age, it could therefore be hard to compare between different baselines.
Sample preparation: Two popular methods have been used to prepare mice for in vivo imaging studies: (i) the cranial window [79] and (ii) the thin skull preparation [80]. While the contribution of imaging protocols to the data discrepancy is likely to be small, different methods are suitable for different experimental designs. For instance, the cranial window method may be more suited for studies that require many reimaging sessions, because no additional surgery is needed once the cranial window is implanted. In contrast, thin skull is more suitable for long imaging intervals, because bone regrowth under the window is not an issue. Recently, efforts have been made to combine both methods [81].
Data analysis: Analysis methods for structural dynamics vary among different research groups. For example, some investigators analyze spine formation and elimination [27, 30, 36–39, 48], while others analyze the percentage of consistent and new spines [19, 28, 29, 31]. Without the knowledge of the original data, it is hard to directly compare results from the different analyses. In addition, different studies categorize spines differently, while some count all dendritic protrusions as spines, others exclude filopodia from their analysis. Although filopodia are rare in adults, they account for ~10% of the total protrusions in adolescent mice at 1 month of age [36]. Furthermore, it has been shown that filopodia almost completely turnover on a daily basis [36], thus including or excluding filopodia in the analysis may greatly contribute to the discrepancy between results. Along the same line, various imaging intervals have been used in different studies. Since new spines are more vulnerable for elimination and only a small percentage of them are stabilized over time [37, 38], the degree of spine dynamics does not necessarily increase linearly with increased imaging intervals. Therefore, while shorter time intervals are biased towards the changes of transient structures, longer time intervals are likely to underestimate the structural plasticity.
Experience-dependent plasticity
Our understanding of experience-dependent spine plasticity has come mostly from sensory manipulation studies. Manipulations of visual inputs have been shown to affect spine morphology and dynamics. Mice raised in darkness since birth exhibit high spine motility and immature spine morphology in the visual cortex, compared with age-matched controls [32]. A few days of light exposure during the critical period (i.e., postnatal days (P) 21–28 in mice) decreases spine motility and restores the mature spine morphology [32]. The effect of light exposure can be partially mimicked by increasing inhibitory signaling, suggesting that a balance between excitatory and inhibitory circuits plays an important role in spine maturation during development [32]. This in line with previous findings that found that excitatory-inhibitory balance is crucial for correct development of visual function during the critical period [45], thus suggesting that spine maturation and plasticity is an important underlying component of the functional changes that occur in the visual pathway.
Manipulating visual input in the adult mouse, via a focused retinal lesion, leads to an almost complete spine turnover in the deafferented region of the visual cortex within 2 months from the time of lesion [46]. In addition, monocular deprivation (MD) increases spine formation and leads to a higher spine density selectively in the apical dendrites of L5 neurons in the mouse binocular region [30]. When binocular vision is restored, spine formation returns to the baseline. However, spine density remains elevated and many of the new spines formed during MD persist [30]. Interestingly, subsequent MD fails to increase spine formation, but selectively enlarges the spines formed during earlier MD, suggesting that new spines formed during the initial MD bear functional synapses and are reactivated during the second MD [30] (Figure 2d–e).
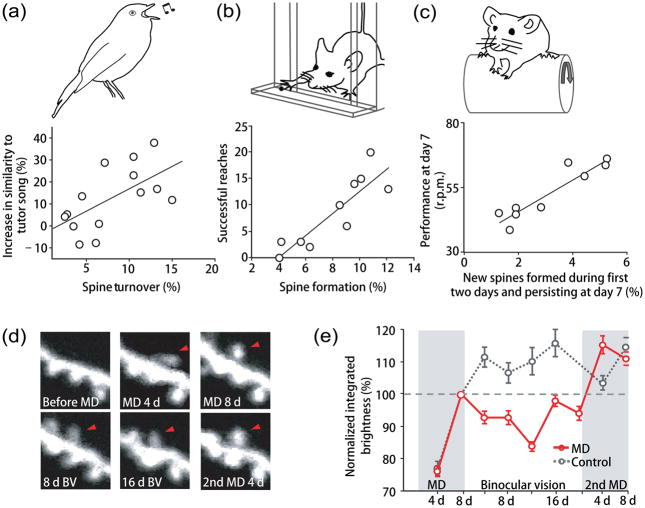
Figure 2.
Spine dynamics and morphological changes associated with learning. (a) Intrinsic dendritic spine turnover correlates with the ability to learn new tasks. In zebra finches (upper panel), the forebrain nucleus HVC is the proximal site where auditory information merges with an explicit song motor representation. Labeled with a lentivirus expressing EGFP, spine turnover of HVC neurons in juvenile songbirds was measured over two hours the night before the first exposure to a song tutor [48]. The scatter plot illustrates that the bird’s capacity of subsequent song imitation correlates with its degree of spine turnover before learning. Each circle represents a single bird. The black line is fitted by linear regression, r=0.63. Modified, with permission, from [48]. (b) Dendritic spine formation during learning correlates with learning performance. YFP-H transgenic mice were trained in a single-pellet reaching task (upper panel). The apical dendrites of L5 neurons in the motor cortex contralateral to the trained limb were imaged the day before and immediately following the first training session. The scatter plot shows that the number of successful reaches during the first training session correlates with the degree of spine formation. Each circle represents one mouse. The black line is fitted by linear regression, r=0.88. Modified, with permission, from [37]. (c) Fractions of stabilized new spines correlate with performance in the re-train session. YFP-H transgenic mice were trained on an accelerating rotarod (upper panel). Dendritic spines of the L5 neurons in motor cortex were imaged at three time points: before training, after 2 days of training, and before re-training on day 7. The scatter plot shows that an animal’s performance in the re-train session on day 7 correlates with stabilized new spines formed in the first 2 days of training. Each circle represents one mouse. The black line is fitted by linear regression, r=0.93. Modified, with permission, from [38]. (d–e) Changes in dendritic spine sizes during visual manipulation. Adolescent GFP-M transgenic mice underwent two 8-day monocular deprivations (MD) interrupted by a 20-day period of binocular vision (BV). Dendritic spines of L5 pyramidal neurons in the binocular region of the visual cortex were imaged every four days. (d) A spine formed during the first four days of MD (red arrowhead) decreases its brightness/size during timepoints within the interval BV period, and then increases its brightness/size during the second period of MD. (e) Average integrated brightness of persistent new spines formed during MD (red) or during the corresponding time period in non-deprived control animals (grey), normalized to the spine brightness value at the end of the first MD or the equivalent time point in control mice. Mean ± s.e.m. Modified, with permission, from [30].
Similarly, sensory manipulation via whisker trimming or environmental enrichment also affects spine motility and turnover in the rodent barrel cortex. During early postnatal development in rats, trimming all whiskers unilaterally decreases both spine and filopodium motility in the contralateral barrel cortex during a brief time window (P11–13), but has no effect on their density, length or shape [17]. In adolescent mice (1–3 months), while sensory deprivation induced by plucking all whiskers on one side reduces spine elimination and delays spine pruning in the contralateral barrel cortex [27], sensory stimulation induced by either an enriched environment or unilateral chessboard plucking (plucking every other whisker, to produce an imbalance in the activation of neighboring whisker columns) increases spine turnover in this same brain region [19, 29, 38]. Furthermore, novel sensory experience promotes new spine stabilization selectively in a subclass of cortical neurons: new spines are preferentially added onto L5 pyramidal neurons with complex apical tufts rather than those with simple tufts [29]. In adult mice (>4 months), sensory manipulation continues to modify spine dynamics, but less robustly than in younger animals, suggesting a more rigid neuronal circuit in adult animals [27].
Recent studies in the mouse motor cortex provide further insights into spine changes that occur during learning. By training mice with a forelimb reaching task, it has been demonstrated that new spines form rapidly (within one hour of the first training trial) in apical dendrites of L5 pyramidal neurons in the forelimb motor cortex contralateral to the trained arm [37] (Figure 3a,b). This rapid spinogenesis is followed by enhanced spine elimination, resulting in a similar overall spine density to that observed in control mice. Furthermore, elimination during subsequent training is largely restricted to spines that existed before training, while the new spines induced during learning are found to be preferentially stabilized, even long after training stops (i.e., up to 4 months) [37] (Figure 3c). A recent fixed tissue study further shows that learning-related structural modifications (e.g., increases in spine density and dendritic complexity) are restricted to the corticospinal neurons associated with control of distal forelimb that is required for skilled grasping in rats [47]. Given the fact that distal and proximal forelimb corticospinal neurons are intermingled within the rat motor cortex [47], this finding indicates that structural changes during learning are restricted to discrete subsets of neurons preferentially involved in novel motor experience.
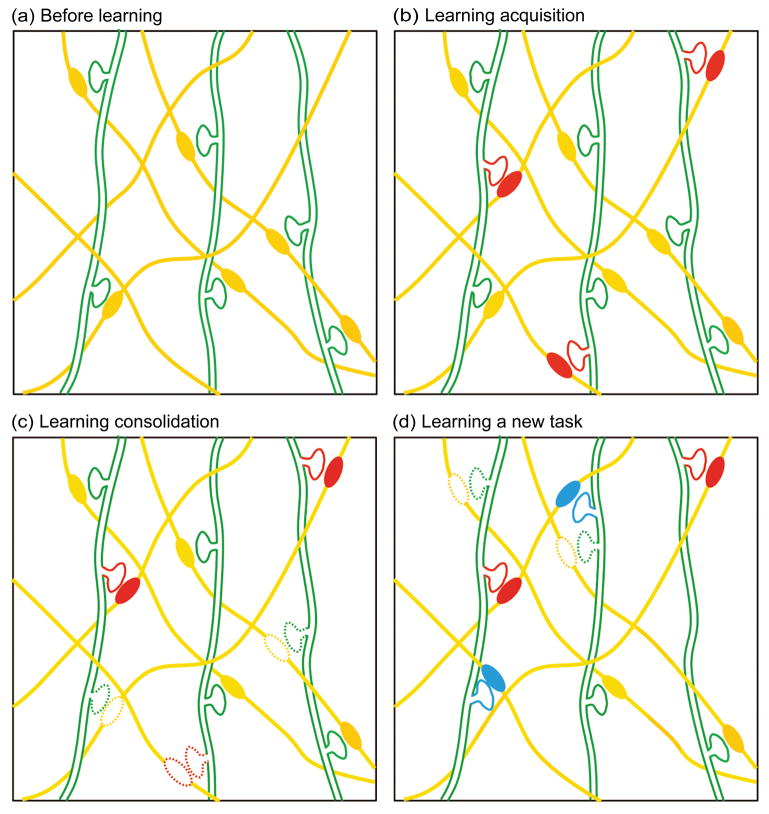
Figure 3.
Schematic drawing illustrating spine remodeling and maintenance in the mouse motor cortex when a mouse is trained with different forelimb motor tasks sequentially (based on experimental data from [37]). (a) Three spiny dendrites (green) connect with their neighboring axons (yellow) at 6 different synaptic sites (identified as spines and boutons, respectively). (b) New synaptic connections (red) form rapidly during the acquisition phase of learning the first motor task (i.e., a single-pellet reaching task). (c) During the learning consolidation phase of the same motor task, a majority of new synapses formed during learning acquisition are selectively stabilized, while synapses that existed prior to learning are preferentially eliminated (with dotted outlines). This results in a rewiring of the circuit without overall changes in synaptic density. (d) Learning a different forelimb motor task (i.e., a pasta handling task) later in life remodels a different set of synapses (blue).
Similar increases in spine turnover have been observed in mice performing other motor tasks, including the rotarod [38] and pasta handling [37] tasks. Interestingly, the degree of spine formation is closely associated with the degree of learning acquisition [37] (Figure 2b), while survival of new spines correlates with the maintenance of the motor skill [37] (Figure 2c). In adults, motor learning increases spine turnover in naïve mice, but not in mice that have been trained with the same task in adolescence (pre-trained mice) [37], suggesting that the circuit required for such a task is established during adolescent training and maintained into adulthood. Furthermore, subsequent training of pre-trained mice with a novel motor skill continues to induce a robust spinogenesis in the adult motor cortex, indicating that different motor skills are likely encoded by different subpopulations of synapses in the motor cortex [37] (Figure 3d).
Taking advantage of the unique tutored song learning behavior in songbirds, another study has investigated how instructive experience acts on the juvenile brain to trigger learning [48]. In songbirds, auditory information and motor representation are integrated in the forebrain nucleus, HVC, with a critical period of sensorimotor correlation around P45-90. Using a lentivirus expressing EGFP that was intracranially injected into the HVC nucleus, this study demonstrated that spine dynamics of HVC neurons decreased with age, and song learning around P60 triggered rapid dendritic spine stabilization of HVC neurons [48]. Interestingly, a higher degree of spine turnover before song learning was found to correlate with a greater capacity for subsequent song imitation, suggesting that structural dynamics in sensorimotor circuits may greatly contribute to behavioral outcomes [48] (Figure 2a).
Taken together, the above studies show that, despite the absence of large-scale remodeling of dendrites, rearrangement of cortical connections in the form of de novo growth and loss of dendritic spines provides a structural substrate for experience-dependent plasticity. Compared with synaptic strength changes alone, new synapse formation could greatly increase the memory storage capacity of the brain [49–51]. In addition, stabilized new spines outlast experiences per se and may serve as structural traces for earlier memories, therefore preparing the brain for quicker adaptation to similar experiences in the future [30, 37]. If this is indeed the case, how are different memories stored in the brain? One hypothesis is that different memories are stored in different subsets of neurons or in different subsets of synapses from the same neuron [52]. Supporting this, it has been shown that a subpopulation of lateral amygdala neurons with elevated cyclic adenosine monophosphate response element–binding protein (CREB) levels are preferentially activated during fear conditioning [53]. In addition, different subsets of synapses on motor cortex pyramidal neurons are selectively reorganized for different forelimb motor learning tasks [37]. While ablation/inactivation of CREB neurons has been shown to selectively erase fear memory [54, 55], it remains unknown if destabilizing learning-induced spines can erase motor memory as well. Furthermore, learning-induced spine formation occurs at the expense of spines that exist before learning [37], suggesting that homeostatic regulation could play an important role in maintaining stable spine density in the adult brain.
Dendritic plasticity of inhibitory interneurons
Inhibitory interneurons make up 20–30% of the neuronal population in the mature cortex. They arborize locally, and play important roles in local circuit regulation [56]. So far, few studies have investigated structural dynamics of interneurons in vivo. All of these studies have used the thy1-GFP-S line, which labels both pyramidal neurons and interneurons sparsely in the superficial layers of the mouse cortex, and post-imaging three-dimensional (3D) reconstruction and immunostaining have been used to identify and locate the imaged interneurons. Live imaging of interneuron dendritic arbors in young adult mice (older than 6 weeks) demonstrated that, unlike stable dendritic branches observed in pyramidal neurons, dendritic branches of inhibitory interneurons in the superficial layers of the cortex are very dynamic [57] (Figure 1g). Interestingly, remodeled interneurons were determined to be spatially restricted to a specialized dynamic zone in the cortex, corresponding to a superficial strip of L2/3 [58]. Furthermore, sensory manipulations, in the form of monocular and binocular deprivation, have been shown to increase the fraction of dynamic branches in cortical interneurons by around 3-fold in adult mice [10]. The distribution of dynamic branches and the timeline of remodeling were also found to be laminar dependent [10]. Given the fact that synapses are located along dendrites of aspiny interneurons, formation and elimination of synapses are likely to accompany dendritic rearrangements. It has been estimated that there is ~10% turnover of interneuron synapses per week during visual deprivation [10]. This is on the same order of dendritic spine turnover observed in L5 pyramidal neurons during sensory manipulation and motor learning [30, 37, 38], suggesting comparable degrees of structural remodeling of excitatory and inhibitory synapses in the adult cortex.
Axonal plasticity
Axons are the output of neurons. Axonal boutons are presynaptic structures that can have a variety of morphologies: en passant boutons are small varicosities along axons, while terminaux boutons look like dendritic spines with a bulbous head at their tips [59]. During postnatal development (i.e., the first 3 weeks in mice), it has been found that axonal growth is cell-type specific. Cajal-Retzius axons grow slowly and follow tortuous paths, while thalamocortical axons grow quickly and straight [60]. At this age, both axon types favor an overall growth in the superficial cortical layers, with slightly more terminal extension than retraction [60]. In adult mice, axonal branches are mostly stable [59]. However, boutons on different types of axons display different morphologies and dynamics [59] (Figure 1e,f). Thalamocortical axons contain large terminaux boutons and en passant boutons, which are mostly stable with 85% boutons persisting over a month [59]. In contrast, axons from mouse cortical L6 neurons contain a high density of small terminaux boutons, which are highly dynamic with ~60% turnover during the same period [59]. Intracortical axons from L2/3 and L5 neurons contain mostly en passant boutons, which show an intermediate level of dynamics [59]. Axon dynamics have also been investigated in the adult monkey visual cortex, using a nonreplicative adeno-associated virus (AAV) that expresses EGFP under the control of the cytomegalovirus enhancer-promoter [61]. This study found that there was ~7% turnover of axonal boutons per week, with no net change in overall density, demonstrating that axonal branches are largely stable in the adult monkey cortex [61].
Many lines of evidence have shown that lesions and sensory manipulations alter dynamics of axonal arborization, even in adult animals. In monkeys, focal binocular lesions trigger a robust growth of axons towards the center of the lesion projection zone initially, followed by a delayed retraction that returns axonal density to the pre-lesion level [62]. Such changes are accompanied by an increase of axonal bouton turnover, resulting in rewiring of neuronal circuits [62]. Similarly, plucking 2 rows of whiskers leads to a rapid and massive remodeling of axons from both excitatory and inhibitory neurons in the mouse barrel cortex [63]. While excitatory neurons show a net increase of axonal projections from non-deprived whisker barrels to deprived barrels, inhibitory neurons in deprived whisker barrels extend their axons towards non-deprived whisker barrels. Such reciprocal sprouting of axons of excitatory and inhibitory neurons suggests that the excitation/inhibition balance in deprived and non-deprived barrel columns contribute to the sensory deprivation-induced topographic remapping [63].
In summary, cortical axons undergo rapid and large-scale rearrangements both during development and in response to injuries. Like dendrites, axons from different types of neurons vary dramatically in their morphology and dynamics. When dendrites and axons imaged under similar experimental conditions are compared, dynamics of dendritic spines are somewhat higher than those of axonal boutons [59]. This discrepancy could be explained either by the failure of some new spines to make synapses, or by some new spines making synapses on existing boutons. Alternatively, the imaged axons and dendrites may not form synapses together. These questions can only be addressed by imaging pre- and post-synaptic pairs simultaneously in the same animal.
Structural plasticity and functional synapses
The imaging studies discussed above depict a dynamic picture of structural plasticity for cortical neurons. This raises the question as to how these neuronal structural changes represent synaptic connectivity changes and thus, the rewiring of neuronal circuits. Live imaging in combination with postmortem ultrastructural examinations has been utilized to address this question.
In the case of dendritic spines, evidence from classic electron microscopy (EM) studies has demonstrated that spine volume correlates with the size of the postsynaptic density and the number of presynaptic vesicles (see reviews [23, 64]), suggesting that larger spines have stronger synapses. In organotypic hippocampal cultures, spines older than 15–19 hours consistently show ultrastructural hallmarks of typical synapses [33]. Moreover, retrospective 3D EM reconstruction following in vivo time-lapse imaging has demonstrated that although a proportion of newly formed (i.e., less than 4 days old) spines lack synapses, both pre-existing and persistent new spines exhibit ultrastructural hallmarks of typical synapses [19, 35]. Furthermore, a recent electrophysiology study using rat hippocampal slice culture demonstrates that a few hours after spine growth, both AMPA-and NMDA-type glutamate receptor currents are indistinguishable from those of mature spines of comparable volumes, further suggesting active functional properties of new spines [65].
For axons and inhibitory neurons, it is also thought that structural changes likely represent changes in synaptic connectivity. With the estimated synaptic density of one synapse per micron of dendrite, a significant synaptic reorganization could accompany the substantial dendritic rearrangement of interneurons [10]. For axons, earlier EM studies have shown that when boutons are identified under light microscopy and subsequently examined with EM, almost all boutons have synapses (see e.g., [66]). This idea is further supported in a recent study combining time-lapse in vivo imaging with restrospective EM reconstruction, showing that en passant boutons, terminaux boutons, and boutons at tips of axons in the adult mouse cortex, all have visible synaptic contacts [59]. Cumulatively, the above studies support the idea that synaptic structural changes observed by in vivo imaging serve as a good indicator for reorganization of the functional synaptic connections, although further studies are required to directly demonstrate causality.
Concluding remarks
In summary, it is now generally concluded that synaptic structures remodel rapidly in developing animals. In adults, despite the global stability of axonal and dendritic stability, a small subset of synaptic structures have been observed to turn over rapidly. Experience modifies neuronal circuits in a regional, laminar and cell type-specific manner. Nevertheless, despite the rapid technical advances in this field, many questions remain unanswered (Box 3). Advancing our knowledge of such issues will not only lead to a better understanding of experience-dependent synaptic plasticity, but also shine light on how synaptic alterations contribute to functional defects in injuries and neurological diseases.
Box 3. Questions for future research.
How do synaptic structures change in deeper cortical layers? Due to the limitation of current two-photon laser scanning systems, in vivo structural imaging is currently limited to superficial cortical layers (mostly L1 and L2). Given the different connectivity between the different cortical layers, it may not be appropriate to simply extend the present understanding obtained from superficial layers to deeper layers of the cortex. To solve this problem, new developments in imaging techniques are necessary, such as fiber optics and adaptive optics [82].
How do we study cell-type specific and synapse specific structural changes? Many lines of evidence suggest that experience-dependent structural plasticity happens in selective cell types of well-defined circuits. Thus, it is important to label and image in a cell-type specific manner. So far, the majority of in vivo imaging has been performed using thy1 transgenic lines, which express fluorescent proteins sparsely in a subset of cortical neurons, often with mixed cell populations, including both pyramidal neurons and interneurons [18]. While most cell-type-specific lines label the whole population of target cell types and are useful for molecular examination and manipulation, imaging them is challenging due to the high density of neurons within the cortex and/or dim expression of the EGFP reporter gene. The field is in need of new molecular approaches to label a specific cell type sparsely but brightly. The Gene Expression Nervous System Atlas (GENSAT) project has generated hundreds of mouse lines that express EGFP under different bacterial artificial chromosomes (BACs) [83, 84]. A recent screen using these lines has identified several mouse lines selectively labeling inhibitory neurons in unique retinal circuits [85]. A similar screen in the cortex could lead to the identification of cell-type specific mouse lines that will be valuable for in vivo imaging. More importantly, not only is experience-dependent structural reorganization cell-type selective, such reorganization is possibly limited to specific synapses. Spinogenesis has been observed when pre-trained mice are challenged with learning a new skill with the same forelimb, suggesting that different motor skills are encoded by different sets of synapses in the motor cortex [37]. Therefore, identifying synapses on the same cell that form different networks (potentially with different presynaptic partners) will be another challenging question to address for future studies.
How does imaged structural plasticity relate to functional changes at synapses? One approach to identify functional synapses under the light microscopy is to label pre- and post-synaptic elements with different colored fluorescently-labeled marker proteins. However, given the resolution limit of light microscopy, retrospective EM following repetitive in vivo imaging is still the ultimate proof that the observed synapses are functional. Recent developments in high-throughput, automated sectioning and imaging overcome this impasse and potentially make ultrastructural analysis of imaged tissues routine [86, 87].
How do changes in synaptic structures contribute to changes in neuronal circuits? With development of in vivo functional imaging, a few studies have attempted to examine structural and functional changes in the same animals. For example, intracortical microstimulation following in vivo imaging indicates that reaching task-induced synaptic reorganization is restricted in the contralateral forelimb motor cortex [37]. In addition, remodeling of dendritic spines has been shown to be closely associated with functional re-organization in the cortex following retina injury or in the post-stroke brain [46, 88]. Further studies of the relationship between structural remodeling and functional reorganization will provide valuable information to help answer this question. The recent development of genetically encoded calcium indicators [89–91] and innovations in imaging neuronal activity in awake, head-fixed mice [92–96] have opened new opportunities toward this direction. For instance, the functional imaging of individual hippocampal pyramidal neurons during spatial navigation in behaving mice has recently been described [96].
Are neuronal structural dynamics the cause or the consequence of behavioral modification? Despite the exciting recent findings of structural remodeling associated with learning [37, 38, 48] (see also Figure 2a–c), all studies so far have been limited to showing a correlation, rather than a causal relationship. An ambitious goal is to selectively mark the population of synapses formed during a particular behavior, such as learning, then to perturb synaptic function and examine the behavioral readout. However, a better understanding of synaptic molecular mechanisms is crucial for designing such experiments.
Acknowledgments
We thank David States, Xinzhu Yu and Drs. Denise Garcia, Cris Niell, Sunil Gandhi for critical comments on this manuscript. This work was supported by grants from the Ellison Medical Foundation, the Dana Foundation, and the National Institute on Aging to Y.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James W. The Principles of Psychology. Vol. 1 Henry Holt & Co; 1890. [Google Scholar]
- 2.Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20:401–412. doi: 10.1016/s0896-6273(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 3.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubel DH, et al. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 5.Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 6.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 7.Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult Mammalian cortex. Neuron. 2002;35:1015–1017. doi: 10.1016/s0896-6273(02)00903-0. [DOI] [PubMed] [Google Scholar]
- 8.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 9.Bavelier D, et al. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JL, Nedivi E. Neuronal structural remodeling: is it all about access? Curr Opin Neurobiol. 2010;20:557–562. doi: 10.1016/j.conb.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X, Zuo Y. Spine plasticity in the motor cortex. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 13.Draft RW, Lichtman JW. It’s lonely at the top: winning climbing fibers ascend dendrites solo. Neuron. 2009;63:6–8. doi: 10.1016/j.neuron.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtman JW, Fraser SE. The neuronal naturalist: watching neurons in their native habitat. Nat Neurosci. 2001;4(Suppl):1215–1220. doi: 10.1038/nn754. [DOI] [PubMed] [Google Scholar]
- 15.Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system. Nat Rev Neurosci. 2006;7:449–463. doi: 10.1038/nrn1905. [DOI] [PubMed] [Google Scholar]
- 16.Denk W, et al. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 17.Lendvai B, et al. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 18.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 19.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 20.Mizrahi A, Katz LC. Dendritic stability in the adult olfactory bulb. Nat Neurosci. 2003;6:1201–1207. doi: 10.1038/nn1133. [DOI] [PubMed] [Google Scholar]
- 21.Matter C, et al. Delta-catenin is required for the maintenance of neural structure and function in mature cortex in vivo. Neuron. 2009;64:320–327. doi: 10.1016/j.neuron.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow DK, et al. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–118. doi: 10.1038/nn.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- 25.von Bohlen Und Halbach O. Structure and function of dendritic spines within the hippocampus. Ann Anat. 2009;191:518–531. doi: 10.1016/j.aanat.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Nimchinsky EA, et al. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 27.Zuo Y, et al. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 28.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Holtmaat A, et al. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 30.Hofer SB, et al. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilbrecht L, et al. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J Neurosci. 2010;30:4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tropea D, et al. Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex. J Neurosci. 2010;30:11086–11095. doi: 10.1523/JNEUROSCI.1661-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagerl UV, et al. Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. J Neurosci. 2007;27:8149–8156. doi: 10.1523/JNEUROSCI.0511-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiala JC, et al. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knott GW, et al. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- 36.Zuo Y, et al. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang G, et al. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grutzendler J, et al. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 40.Majewska AK, et al. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 42.Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 43.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 46.Keck T, et al. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci. 2008;11:1162–1167. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, et al. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts TF, et al. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chklovskii DB, et al. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 50.Stepanyants A, et al. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 51.Stepanyants A, Chklovskii DB. Neurogeometry and potential synaptic connectivity. Trends Neurosci. 2005;28:387–394. doi: 10.1016/j.tins.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Silva AJ, et al. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han JH, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 54.Han JH, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y, et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 57.Lee WC, et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 2006;4:e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee WC, et al. A dynamic zone defines interneuron remodeling in the adult neocortex. Proc Natl Acad Sci U S A. 2008;105:19968–19973. doi: 10.1073/pnas.0810149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Paola V, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 60.Portera-Cailliau C, et al. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005;3:e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stettler DD, et al. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49:877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Yamahachi H, et al. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marik SA, et al. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLoS Biol. 2010;8:e1000395. doi: 10.1371/journal.pbio.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arellano JI, et al. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zito K, et al. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson JC, Martin KA. Does bouton morphology optimize axon length? Nat Neurosci. 2001;4:1166–1167. doi: 10.1038/nn772. [DOI] [PubMed] [Google Scholar]
- 67.Zhang S, et al. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5:e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy TH, et al. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28:1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown CE, et al. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown CE, et al. Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. J Cereb Blood Flow Metab. 2010;30:783–791. doi: 10.1038/jcbfm.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai J, et al. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 73.Spires TL, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bittner T, et al. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cruz-Martin A, et al. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan F, et al. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuhrmann M, et al. Dendritic pathology in prion disease starts at the synaptic spine. J Neurosci. 2007;27:6224–6233. doi: 10.1523/JNEUROSCI.5062-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu J, et al. Local-Circuit Phenotypes of Layer 5 Neurons in Motor-Frontal Cortex of YFP-H Mice. Front Neural Circuits. 2008;2:6. doi: 10.3389/neuro.04.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holtmaat A, et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang G, et al. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drew PJ, et al. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 2010;7:981–984. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilt BA, et al. Advances in light microscopy for neuroscience. Annu Rev Neurosci. 2009;32:435–506. doi: 10.1146/annurev.neuro.051508.135540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 84.Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7:483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- 85.Siegert S, et al. Genetic address book for retinal cell types. Nat Neurosci. 2009;12:1197–1204. doi: 10.1038/nn.2370. [DOI] [PubMed] [Google Scholar]
- 86.Knott GW, et al. A protocol for preparing GFP-labeled neurons previously imaged in vivo and in slice preparations for light and electron microscopic analysis. Nat Protoc. 2009;4:1145–1156. doi: 10.1038/nprot.2009.114. [DOI] [PubMed] [Google Scholar]
- 87.Knott G, et al. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci. 2008;28:2959–2964. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown CE, et al. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29:1719–1734. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mank M, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5:805–811. doi: 10.1038/nmeth.1243. [DOI] [PubMed] [Google Scholar]
- 91.Wallace DJ, et al. Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nat Methods. 2008;5:797–804. doi: 10.1038/nmeth.1242. [DOI] [PubMed] [Google Scholar]
- 92.Dombeck DA, et al. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andermann ML, et al. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell Neurosci. 2010;4:3. doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Komiyama T, et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 96.Dombeck DA, et al. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]