SUMMARY
We report that in the rat hippocampus learning leads to a significant increase in extracellular lactate levels, which derive from glycogen, an energy reserve selectively localized in astrocytes. Astrocytic glycogen breakdown and lactate release are essential for long-term but not short-term memory formation, and for the maintenance of long-term potentiation (LTP) of synaptic strength elicited in-vivo. Disrupting the expression of the astrocytic lactate transporters monocarboxylate transporter 4 (MCT4) or MCT1 causes amnesia, which, like LTP impairment, is rescued by lactate but not equicaloric glucose. Disrupting the expression of the neuronal lactate transporter MCT2 also leads to amnesia that is unaffected by either L-lactate or glucose, suggesting that lactate import into neurons is necessary for long-term memory. Glycogenolysis and astrocytic lactate transporters are also critical for the induction of molecular changes required for memory formation, including the induction of phospho-CREB, Arc and phospho-cofilin. We conclude that astrocyte-neuron lactate transport is required for long-term memory formation.
INTRODUCTION
Astrocytes have been generally believed to have a mainly supportive function for neurons in the central nervous system (Kettenmann and Ransom, 2005). However, a growing body of evidence suggests that they play many more active roles, including information processing, signal transmission and regulation of neural and synaptic plasticity (Halassa and Haydon, 2010; Henneberger et al., 2010; Perea et al., 2009). Thus, brain functions, including perhaps cognitive ones, may result from the concerted action of a neuron–glia network. Little is known about the physiological contribution and relative mechanisms of astrocytes to cognitive functions. Astrocytic adenosine modulates sleep homeostasis and cognitive consequences of sleep loss (Halassa et al., 2009), and some studies have begun investigating the role of astrocytes in learning and memory. Spatial learning and working memory in rats appears to increase the number of astrocytes (Jahanshahi et al., 2008a; 2008b) and bead discrimination memory in a day-old chick requires glycogenolysis (Gibbs et al., 2006), which is known to occur in astrocytes and not in neurons (Magistretti, 2008), suggesting that byproducts of glycogen metabolism are critical for memory formation.
One mechanism through which astrocytes and neurons may actively be linked is through metabolic coupling, particularly as a result of neuronal activity (e.g. Magistretti, 2008; Tsacopoulos and Magistretti, 1996). An important source of energy for the brain is glucose entering via transport across the blood brain barrier into both neurons and astrocytes. Astrocytes, but not neurons, store glycogen (Brown et al., 2004; Magistretti, 2008; Vilchez et al., 2007) which can be rapidly converted to pyruvate/lactate and metabolized in the TCA cycle or used for biosynthesis of glutamate (Hamprecht et al., 2005), as well as also potentially produce glucose (Ghosh et al., 2005). Both glucose and lactate can then be exported to neurons as fuel (Brown et al., 2004; Dringen et al., 1993). Alternatively, astrocytes may utilize glycogen, and spare the glucose for neurons' use (DiNuzzo et al., 2010; Swanson, 1992). Glycogenolysis also results in lactate release from astrocytes (Brown et al., 2004; Dringen et al., 1993), and lactate transport between astrocytes and neurons has been shown from glutamate-stimulated glycolysis (Magistretti et al., 1999; Pellerin and Magistretti, 1994) and/or glycogenolysis stimulated by neuromodulators such as noradrenaline and vasoactive intestinal peptide (VIP) (Magistretti, 2006; Magistretti and Morrison, 1988). The transport of lactate occurs via monocarboxylate transporters (MCTs), proton-linked membrane carriers that transport monocarboxylates including lactate, pyruvate and ketone bodies across the cell membrane. MCT4 is expressed mainly by astrocytes, whereas MCT2 is primarily neuronal and MCT1 is expressed in astrocytes, endothelial cells of microvessels ependymocytes and oligodendrocytes (Pierre and Pellerin, 2005; Rinholm et al., 2011). The hypothesis of an astrocyte-neuron metabolic coupling is attractive because it would underscore the importance of functionally connecting these two cell types during activity subserving brain functions, including perhaps cognitive ones.
New learning initiates a cascade of events in the brain that can lead to short and long-term memories. While short-term memories require post-translational modifications, the consolidation, or stabilization of long-term memories depends upon the activation of a gene cascade and downstream modifications in neurons that store the acquired information (Dudai, 2004; Kandel, 2001; Klann and Sweatt, 2008). Among the best characterized and widely proven gene expression mechanisms known to underlie memory consolidation are the activation of CREB (cAMP response element binding protein)-dependent gene expression (Alberini, 2009; Kandel, 2001) and the translation, at activated synapses, of the immediate early gene Arc (activity-regulated cytoskeletal protein), which is believed to play a key role in actin cytoskeletal dynamics and regulate the membrane expression of AMPA receptors (Bramham et al., 2008). Long-term synaptic plasticity and memory are accompanied by synaptic structural changes, which involve actin polymerization (Chen et al., 2007; Fisher et al., 2004; Mantzura et al., 2009) associated with the phosphorylation of the p21-activated kinase-cofilin cascade. Phosphorylated cofilin is one of the major regulators of F-actin dynamics in spines, promotes cytoskeleton assembly and regulates spine morphology (Bamburg et al., 1999; Chen et al., 2007; Fedulov et al., 2007).
Thus, long-term memory formation appears to have high metabolic demands within the underlying active neuronal network. Here we employed rat inhibitory avoidance (IA) to test the hypothesis that astrocyte-neuron metabolic coupling mechanisms play a critical role in memory formation.
RESULTS
Astrocytic glycogen metabolism is required in the hippocampus for long-term memory formation
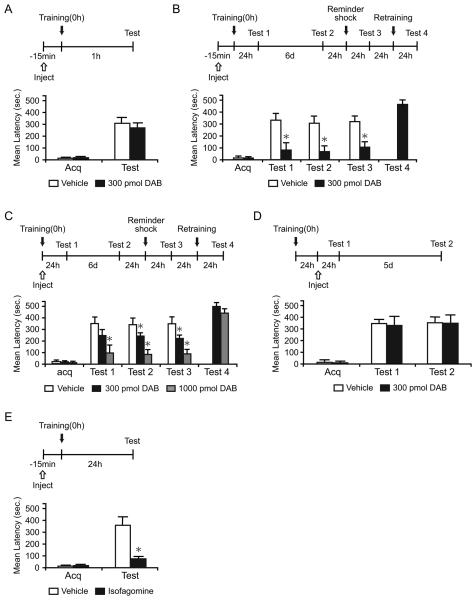
To test whether astrocytic glycogenolysis in the hippocampus affects short- and/or long-term memory retention, we bilaterally injected 300 μM (300 pmol) of the inhibitor of glycogen phosphorylation 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) (Walls et al., 2008), either 15 min before or immediately after IA training (Figure 1A-1C). Chicago sky blue injections showed the diffusion, which spread into the dorsal hippocampus approximately 1.7 mm across the injection site (Figure S1). To test for a dose-response effect, 1000 μM (1,000 pmol) of DAB, injected immediately after training, was also investigated (Figure 1C).
Figure 1. DAB and isofagomine disrupt long-term memory.
Acquisition (Acq) and retention are expressed as mean latency ± SEM (in seconds, sec). Latency scores, n and detailed statistic are reported in Table S1. See also Figure S1. (A) Hippocampal injections of DAB 15 min before IA training had no effect on short-term memory tested at 1 hr after training (n=7/group).
(B and C) Hippocampal injections of DAB 15 min before (B, n=11/group) or immediately after training (C, n=7-9/group) disrupted long-term memory at 24 hr (Test 1). The disruption persisted 7 days after training (Test 2), and memory did not recover after a reminder shock (Test 3). DAB-injected rats had normal retention after retraining (Test 4).
(D) Hippocampal injections of DAB 24 hr after IA training did not affect long-term memory (n=8/group).
(E) Hippocampal injections of isofagomine 15 min before training disrupted long-term memory (n=8-9/group). * p < 0.05.
Student's t-test showed that DAB injected before training did not affect IA acquisition, as the mean latencies to enter the shock compartment during training (acquisition) were similar in all groups (p > 0.05). DAB did not affect short-term memory 1 hr after training (p > 0.05, Student's t-test; Figure 1A). However, DAB injected either before (Figure 1B) or immediately after training (Figure 1C) significantly blocked long-term memory tested at 24 hr (Test 1) in a dose-dependent manner. Re-testing 6 days after Test 1 showed that the memory loss persisted [Test 2, p < 0.05, two-way repeated measure analysis of variance (ANOVA) followed by Newman-Keuls post hoc test; Figure 1B and 1C].
The disrupted memory did not reinstate after a footshock reminder given one day after Test 2 and tested 24 hr later (Test 3, 15 min before, p < 0.05, Student's t-test; immediately after, p < 0.05, one-way ANOVA followed by Newman-Keuls post hoc test). Re-training of the DAB-injected group, 24 hr after Test 3, resulted in normal memory retention 24 h later (Test 4), indicating that the hippocampus was functionally intact (Figure 1B and 1C).
To begin determining the time course of the DAB effect following training, rats were injected with 300 pmol of DAB 24 hr after training and tested 24 hr (Test 1) and 5 days (Test 2) later (Figure 1D). At both Tests, DAB and vehicle-injected rats had comparable memory retention (P > 0.05, two-way repeated measure ANOVA), indicating that DAB disrupts long-term memory formation during a limited time window: it has a maximal effect when injected shortly before training but no effect when injected 24 hr after training.
Similarly, bilateral hippocampal injections of another glycogen phosphorylase inhibitor, isofagomine (Waagepetersen et al., 2000), 15 min before training, significantly disrupted memory retention 24 hr later compared to vehicle (p < 0.05, Student's t-test; Figure 1E).
Thus, astrocytic hippocampal glycogen mobilization is required for long-term, but not short-term, memory formation.
Learning-induced astrocytic release of lactate is required for memory formation
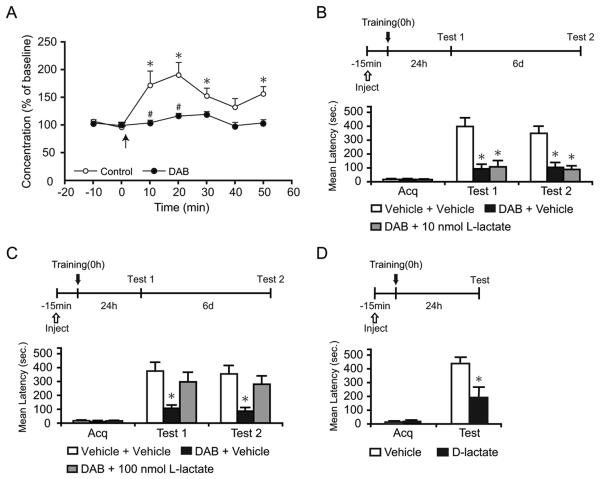
In vitro studies have suggested that glycogenolysis results in lactate release (Brown et al., 2004; Dringen et al., 1993). To test whether glycogenolysis leads to lactate release in vivo after learning, we determined the levels of lactate in the hippocampus of rats before and after training using in vivo microdialysis (Rex et al., 2009). Basal lactate dialysate levels remained constant over time before training (Vehicle, 197.95 ± 55.95 μM; DAB, 216.14 ± 43.62 μM). Training led to a statistically significant increase in lactate dialysate levels compared to baseline concentrations (170-190%, p < 0.05, two-way repeated measure ANOVA followed by Bonferroni post hoc test; Figure 2A), which was completely abolished by DAB in the perfusion medium, indicating that it resulted from glycogenolysis (p < 0.05, two-way repeated measure ANOVA followed by Bonferroni post hoc test; Figure 2A). Retention latencies of both DAB and vehicle-injected groups are shown in Figure S2.
Figure 2. L-lactate rescues the DAB-induced memory impairment.
Lactate concentration, latency scores, n and detailed statistic are reported in Table S2. (A) Dorsal hippocampal extracellular lactate in freely moving rats infused with either vehicle or DAB. Baseline was collected for 20 min before training (0 min, ↑) and continued for 50 min. Training resulted in a significant increase in lactate levels compared to baseline (* p < 0.05) that was completely blocked by DAB (# p < 0.05).
Data are expressed as % of baseline ± SEM (mean of the first 2 samples set 100%). See also Figure S2.
(B-D) Acquisition (Acq) and retention are expressed as mean latency ± SEM (in seconds, sec).
(B and C) Hippocampal injection of DAB or vehicle in combination with 10 nmol (B, n = 7/group), 100 nmol L-lactate (C, n = 12/group) or vehicle were performed 15 min before training and memory was tested at 24 hr. 100 nmol but not 10 nmol of L-lactate rescued the memory impairment by DAB (Test 1). The effect persisted at 7 days after training (Test 2).
(D) Hippocampal injections of D-lactate 15 min before training disrupted long-term memory (n=7-8/group). * p < 0.05.
We then asked whether the DAB-induced amnesia could be rescued by the administration of exogenous lactate. Fifteen min before training, rats were bilaterally injected into the hippocampus with a combination of DAB or vehicle and either 10 nmol (Figure 2B), 100 nmol L-lactate (Figure 2C) or vehicle. Memory was tested 24 hr (Test 1) and 7 days (Test 2) after training (Figure 2). Acquisition was not affected by treatment (p > 0.05; one-way ANOVA). DAB injection with vehicle disrupted long-term memory retention, confirming our previous results (Figure 1B). Conversely, 100, but not 10 nmol of L-lactate co-administered with DAB significantly rescued the memory loss. Retesting the rats 7 days after training revealed that the effects of treatments persisted (p < 0.05 for both tests, two-way repeated measure ANOVA followed by Newman-Keuls post-hoc test). To additionally prove that the transport of L-lactate into neurons is critical for the formation of long-term memory, the competitive effect of the inactive isomer D-lactate was tested. Bilateral hippocampal injection of 20 nmol of D-lactate, 15 min before IA training, significantly blocked memory retention 24 h after training compared to vehicle (p < 0.05; Student's t-test; Figure 2D).
Hence, training results in a rapid glycogenolysis and consequent efflux of lactate in the hippocampus, which is critical for long-term memory formation.
Blocking glycogen metabolism with DAB disrupts maintenance of hippocampal LTP in vivo and the impairment is rescued by L-lactate
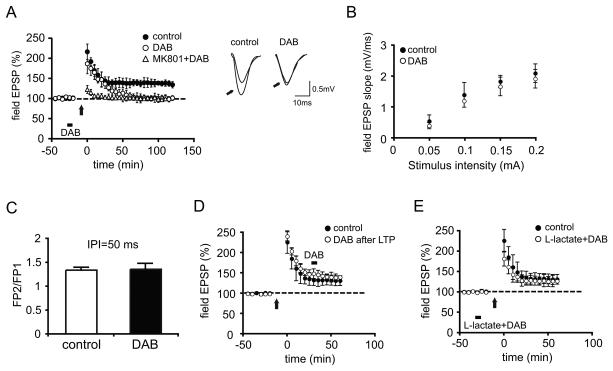
Long-term potentiation (LTP) is a leading cellular model for memory formation and is induced at CA1 synapses by IA (Whitlock et al., 2006). Accordingly, we next tested the effect of blocking glycogen metabolism on LTP in area CA1 of anesthetized young adult rats. After establishing a baseline of responses at Schaffer collateral-area CA1 (SC-CA1) synapses, LTP of SC-CA1 synapses was induced by a high-frequency tetanic stimulation protocol in the absence or presence of DAB delivered locally to the CA1 neuropil through the recording pipette. In the absence of DAB (the control group), robust LTP was induced (fEPSP slope: 133.85 ± 7.9% of baseline at 120 min post-tetanus; Figure 3A, closed circles) as expected (Bozdagi et al., 2007). In contrast, in the presence of DAB, tetanic stimulation produced a strong initial potentiation, but thereafter, potentiation decayed rapidly to baseline by 75 min in the DAB injected group (Figure 3A, open circles), indicating that DAB blocks the maintenance but not the induction of LTP. To rule out that the effect of DAB on LTP maintenance resulted from non-specific general metabolic demands of the high-frequency tetanic stimulation per se, we tested the effect of DAB on baseline neurotransmission in a group of rats in which LTP was blocked by an i.p, injection of the N-methyl-d-aspartate receptor (NMDAR) antagonist MK-801 prior to stimulation. Figure 3A shows that when LTP was blocked by MK-801, DAB had no effect on baseline over 120 min following stimulation, supporting the ides that DAB blocked LTP maintenance selectively. We verified that DAB alone, in the absence of tetanic stimulation, had no effects on basal synaptic transmission by examining parameters that reflect normal synaptic function, including the relationship between stimulus strength and the size of the postsynaptic response (input-output relationship; Figure 3B) or paired-pulse facilitation (PPF, Figure 3C).
Figure 3. DAB impairs in vivo hippocampal LTP and the impairment is rescued by L-lactate.
(A) Average Field EPSP data recorded for 120 min post-tetanus shows that DAB injection (bar) before high frequency stimulation (arrow) blocks LTP (p < 0.05 vs controls at 120 min, n = 4/group). LTP is abolished in animals receiving an intraperitoneal injection of the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 (3mg/kg) 30 min prior to tetanus (open triangles). Inset: Representative EPSP traces were recorded before and 120 min after (indicated by arrows) LTP induction. Left panel, control; right panel, in the presence of DAB.
(B and C) No effect on the relationship between stimulus strength and the size of the postsynaptic response (input-output relationship, B, n = 4/group, p > 0.05) or paired-pulse facilitation (PPF, C, n = 4/group, p > 0.05).
(D) DAB injected 30 min after high frequency stimulation did not affect synaptic potentiation (n = 4/group).
(E) L-lactate reversed the blocking effect of DAB on LTP (n = 4/group). All data are expressed as mean values ± SEM.
To determine whether DAB has an effect on LTP once it is established, we injected DAB into CA1 30 min following LTP induction. There was no effect of DAB on synaptic potentiation once established, indicating that glycogen phosphorylase function is required during the induction or early phases of LTP (Figure 3D) (average percentage of baseline 60 min after LTP induction: control, 131.39 ± 11.01%, DAB, 139.86 ± 6.8%, p > 0.05, one-way ANOVA).
To test if the effect of DAB was due to the astrocytic lactate production, we injected lactate together with DAB prior to LTP induction. Under these conditions, lactate abrogated the blocking effect of DAB on LTP. In the animals injected with lactate and DAB, the increase in fEPSP slope after LTP induction was not significantly different compared to that of control animals (control: 131.39 ± 11.01%, lactate+DAB: 127 ± 13%, of baseline at 60 min post-tetanus, p > 0.05, one-way ANOVA; Figure 3E).
Lactate transport through MCT1 and MCT4 plays an essential role in long-term memory formation
We then investigated whether the effect we found with DAB was indeed due to the astrocytic release of lactate. Toward this end, we tested the effects of knocking down the hippocampal expression of the lactate transporters MCT1 and MCT4 using antisense oligodeoxynucleotides (ODNs). In the brain, the MCT1 is expressed mainly in endothelial cells of microvessels, ependymocytes astrocytes and oligodendrocytes but not in neurons, whereas the MCT4 is expressed virtually exclusively in astrocytes (Pierre and Pellerin, 2005). Western blot analyses of synaptoneurosomal preparations (Villasana et al. 2006) showed that MCT4 and MCT2 are highly enriched in the synaptic fraction compared to the total lysate; MCT1, although not enriched compared to total lysate, is clearly present (Figure 4A). Hence, all these MCTs may be involved in lactate transport across the astrocytic-synaptic compartments. Rats were bilaterally injected into the hippocampus with MCT1-ODN or a relative scrambled control ODN containing the same base pair sequence but in a randomized order (scrambled-ODN, SC1-ODN) 1 hr before training and were euthanized 12 hr after training. Control rats received the same injections but did not undergo training. An ODN injection into the dorsal hippocampus targets and remains confined to this region (e.g. Taubenfeld et al., 2001a), as also shown by the Chicago blue diffusion shown in Figure S1. Dorsal hippocampal extracts obtained from these animals were analyzed for MCT1 expression levels using quantitative western blot analyses. A one-way ANOVA followed by Newman-Keuls post-hoc test showed that the expression of MCT1 was significantly higher in trained compared to untrained rats injected with SC1-ODN (p < 0.05). This induction was completely and selectively blocked by MCT1-ODN (p < 0.05; Figure 4B). In fact, no change in MCT4 and MCT2 concentrations was found across the same samples (MCT2, p > 0.05; MCT4, p > 0.05; one-way ANOVA followed by Newman-Keuls post-hoc test; Figure 4B). Thus, MCT1, but not MCT4 or MCT2 expression is significantly upregulated upon training and MCT1-ODN selectively blocks this enhancement.
Figure 4. MCT1 is induced by training. Disruption of MCT1 expression impairs long-term memory. Memory is rescued by L-lactate.
Mean %, n, latency and detailed statistic are reported in Table S3.
(A) MCTs expression performed on total (T) or synaptoneurosomal (S) extracts of dorsal hippocampus. Postsynaptic density-95 (PSD95) is used to show synaptoneurosmal enrichment. Glial fibrillary acidic protein (GFAP) is used as an astrocytic marker.
(B) Examples and densitometric quantitative western blot analysis of MCT1, MCT2 and MCT4 carried out in dorsal hippocampal extracts from trained and untrained rats (n=6/group) injected with MCT1- or SC1-ODN and euthanized 12 hr after training. MCT1, but not MCT2 or MCT4, was significantly induced by training. This induction was selectively blocked by MCT1-ODN injection. Data are expressed as mean percentage ± SEM of the untrained-SC1-ODN (100%) mean values. All MCTs values were normalized to those of actin.
(C) Examples and densitometric analysis of MCT1, MCT2 and MCT4 quantitative western blots of extracts from dorsal hippocampal punches taken around the needle placement from trained rats that received hippocampal MCT1- or SC1-ODN injections and were euthanized 12 h after training (n=4/group). Data are expressed as mean percentage ± SEM of trained-SC1-ODN (100%) mean values. All MCTs values were normalized to those of actin.
(D-G) Memory acquisition (acq) and retention are expressed as mean latency ± SEM (in seconds, sec).
(D) Hippocampal injections of MCT1-ODN 1 hr before training did not affect short-term memory (n=7/group).
(E) Hippocampal injection of MCT1-ODN disrupted long-term memory. MCT1- or SC1-ODN were injected 1 hr before training and rats were tested 24 hr after training (Test 1) and 6 days later (Test 2). The memory disruption persisted at Test 2, and memory did not recover following a reminder shock (Test 3). MCT1-ODN amnesic rats showed normal retention after re-training (Test 4) (n=10-11/group).
(F) L-lactate but not glucose rescued the memory impairment induced by blocking MCT1 expression (n=7-13/group). MCT1- or SC1-ODN were injected 1 hr before training. Llactate, glucose or vehicle (PBS) were injected 15 min before training. Rats were tested 24 hr after training (Test 1) and 6 days later (Test 2).
(G) High concentration of glucose transiently rescued the MCT1-ODN-induced memory impairment (n = 8-11/group). MCT1- or SC1-ODN were injected 1 hr before training. 150 nmol of glucose or vehicle (PBS) were injected 15 min before training. Rats were tested 24 hr after training (Test 1) and 6 days later (Test 2). * p < 0.05.
The efficacy of the MCT1-ODN knock-down was further confirmed with quantitative western blot analyses of hippocampal extracts of trained rats obtained from the area targeted by the injection, that is 1.7 mm around the needle placement. As shown in Figure 4C, MCT1 level was significantly reduced by the MCT1-ODN compared to SC1-ODN (p < 0.05; Student's t-test), whereas no change in MCT2 and MCT4 were found (MCT2, p > 0.05; MCT4, p > 0.05; Student's t-test).
The effect of MCT1-ODN-mediated knock down was then assessed on memory retention (Figure 4D and 4E). Hippocampal injection of MCT1-ODN, compared to SC1-ODN, 1 hr before training, had no effect on acquisition (p > 0.05; Student's t-test or oneway ANOVA) or short-term memory tested 1 hr after training (p > 0.05, Student's t-test; Figure 4D). However, it completely disrupted long-term memory 24 hr after training (Test 1). This disruption persisted 7 days after training (Test 2, p < 0.05, two-way repeated measure ANOVA followed by Newman-Keuls post-hoc test; Figure 4E), and memory did not reinstate following a reminder footshock (Test 3, p < 0.05; Student's t-test). However, after re-training, the MCT1-ODN-injected rats had normal memory retention, indicating that their hippocampi were functioning normally. Thus, blocking the induction of MCT1 evoked by IA training disrupts long-term memory.
Given the evidence for a role of lactate in long-term memory formation we next asked whether exogenous L-lactate could rescue the MCT1-ODN-induced amnesia. Because a memory-enhancing effect has been reported for glucose (McNay and Gold, 2002; Messier, 2004) we also tested the potential rescuing effect of this energy substrate. Rats were injected with MCT1- or SC1-ODN 1 hr before training and again injected, 15 min before training, with either 100 nmol L-lactate, equicaloric 50 nmol glucose or vehicle (Figure 4F). Memory was tested 24 hr (Test 1) and 7 days (Test 2) after training. The acquisition latencies were similar in all groups (p > 0.05; one-way ANOVA). A two-way repeated measure ANOVA followed by Newman-Keuls post hoc test indicated that MCT1-ODN with vehicle significantly impaired memory at both Test 1 and Test 2 (p > 0.05), thus confirming our previous results shown in Figure 4E. Furthermore, rats injected with SC1-ODN and glucose had a significant increase in memory retention at 24 hr after training compared to SC1-ODN-vehicle-injected rats (p < 0.05). Notably, lactate but not glucose significantly rescued memory retention caused by MCT1-ODN (p < 0.05).
Because glucose enhanced memory retention, we tested whether higher concentrations of glucose could rescue/enhance the MCT1-induced amnesia. Rats were injected with MCT1-ODN or SC1-ODN 1 hr before training and again injected, 15 min before training, with 150 nmol of glucose or vehicle (Figure 4G). A two-way repeated measure ANOVA followed by Newman-Keuls post hoc test revealed that, as with previous results, MCT1-ODN significant impaired memory at both Test 1 and Test 2 (p < 0.05) and glucose significantly rescued memory impairment at Test1 (p < 0.05). However, the effect was transient, as memory was again impaired at Test 2 (p < 0.05).
We next determined the effect of knocking down the expression of the astrocytic MCT4 on memory formation (Figure 5). Hippocampal injection of MCT4-ODN 1 hr before training significantly and selectively knocked down MCT4 concentration 12 hr after training, compared to scrambled-ODN (SC4-ODN), as revealed by quantitative western blot analyses of extracts obtained from 1.7 mm around the needle placement (Figure 5A, p < 0.05; Student's t-test). No change in MCT1 and MCT2 expression levels was found across samples (MCT1, p > 0.05; MCT2, p > 0.05; Student's t-test). MCT4-ODN injected 1 hr before training significantly impaired memory retention at both 24 hr (Test 1) and 7 days (Test 2) after training compared to SC4-ODN (p < 0.05, two-way repeated measure ANOVA followed by Newman-Keuls post-hoc test, Figure 5B). Notably, the memory of MCT4-ODN-injected rats was significantly rescued by 100 nmol of L-lactate but not of equicaloric, 50 nmol glucose injected 15 min before training (p < 0.05). There was no effect of treatment on acquisition (p > 0.05; one-way ANOVA). These data suggest that lactate transport through MCT1 and MCT4 is critical for long-term memory formation.
Figure 5. Disruption of MCT4 expression also impairs long-term memory.
Mean %, n, latency and detailed statistic are reported in Table S4.
(A) Examples and densitometric analysis of MCT4, MCT1 and MCT2 quantitative western blots of extracts from dorsal hippocampal punches taken around the needle placement from trained rats that received hippocampal MCT4- or SC4-ODN injections and were euthanized 12 h after training (n=4/group). Data are expressed as mean percentage ± SEM of trained-SC4-ODN (100%) mean values. All MCTs values were normalized to those of actin.
(B) Memory acquisition (acq) and retention are expressed as mean latency ± SEM (in seconds, sec). Hippocampal injection of MCT4-ODN disrupted long-term memory and L-lactate but not glucose, rescued it. MCT4- or SC4-ODN were injected 1 hr before training. L-lactate, glucose or vehicle (PBS) were injected 15 min before training. Rats were tested 24 hr after training (Test 1) and 6 days later (Test 2). (n=10-12/group). * p < 0.05
Lactate transport through MCT2 is also required for long-term memory formation
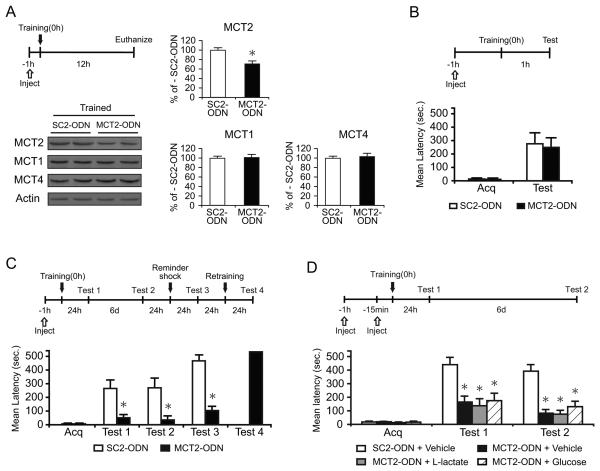
We next tested the effect of antisense-mediated knock-down of the neuronal lactate transporter MCT2 on memory formation (Figure 6). Rats received a bilateral hippocampal injection of MCT2-ODN or scrambled-ODN (SC2-ODN) 1 hr before training and were euthanized 12 hr after training. Quantitative western blots of the hippocampal area targeted by the injection (1.7 mm radius) showed that MCT2 concentrations were significantly reduced by MCT2-ODN compared to SC2-ODN (Figure 6A, p < 0.05; Student's t-test). No change in MCT1 and MCT4 concentrations was found across samples (MCT1, p > 0.05; MCT4, p > 0.05; Student's t-test), indicating that MCT2-ODN selectively downregulates the expression of MCT2.
Figure 6. Disruption of MCT2 expression impairs long-term memory.
Mean %, n, latency and detailed statistic are reported in Table S5.
(A) Examples and densitometric analysis of MCT2, MCT1 and MCT4 quantitative western blots of extracts from dorsal hippocampal punches taken around the needle placement from trained rats that received hippocampal MCT2- or SC2-ODN injections and were euthanized 12 h after training (n=4/group). Data are expressed as mean percentage ± SEM of trained-SC2-ODN (100%) mean values. All MCTs values were normalized to those of actin.
(B-D) Acquisition (acq) and retention are expressed as mean latency ± SEM (in seconds, sec).
(B) Hippocampal injections of MCT2-ODN 1 hr before training did not affect short-term memory tested 1 hr later (n=8/group).
(C) Hippocampal injections of MCT2-ODN disrupted long-term memory. MCT2- or SC2-ODN were injected 1 hr before training. Rats were tested 24 hr after training (Test 1) and 6 days later (Test 2). The memory disruption persisted at Test 2, and memory did not recover following a reminder foot shock (Test 3). The amnesic rats that received the MCT2-ODN showed normal retention after re-training (Test 4) (n = 8/group).
(D) Neither L-lactate nor glucose rescued the memory impairment induced by MCT2 disruption (n = 6-8/group). MCT2- or SC2-ODN were injected 1 hr before training. Llactate, glucose or vehicle (PBS) were injected 15 min before training. Rats were tested 24 hr after training (Test 1) and 6 days later (Test 2). * p < 0.05.
We then examined the effect of knocking down of MCT2 on memory retention. Rats were injected with MCT2-ODN or SC2-ODN 1 hr before training. Memory retention was tested 1 or 24 hr after training. Acquisitions were similar in all groups (p > 0.05; Student's t-test). MCT2-ODN did not affect short-term memory at 1 hr after training (p > 0.05; Student's t-test; Figure 6B). However, it significantly disrupted long-term memory retention at 24 hr after (Test 1) and 7 days after training (Test 2, p < 0.05, two-way repeated measure ANOVA followed by Newman-Keuls post-hoc test; Figure 6C). Memory did not recover following a reminder foot shock (Test 3, p < 0.05; Student's t-test), but the memory of MCT2-ODN-injected rats was normal after retraining (Figure 6C). Hence, blocking the function of MCT2 impairs long-term memory.
To determine whether L-lactate and/or glucose rescues the MCT2-ODN-induced memory impairment, MCT2- or SC2-ODN were injected 1 hr before training and 100 nmol of L-lactate, 50 nmol of glucose or vehicle were injected 15 min before training. Memory was tested 1 (Test 1) and 7 days (Test 2) after training. A two-way repeated measure ANOVA followed by Newman-Keuls post hoc test showed that, confirming our previous data (Figure 6C), MCT2-ODN with vehicle significantly impaired memory retention at both Test 1 and Test 2 (p > 0.05), and both L-lactate and glucose failed to rescue this amnesia (P > 0.05; Figure 6D). These results confirm that glucose does not rescue the memory impairment due to astrocytic export of lactate and indicates that lactate import through MCT2 is essential for long-term memory formation.
Training-dependent induction of Arc, pCREB and pcofilin are completely blocked by DAB and significantly rescued by L-lactate
We tested whether disrupting astrocytic glycogen mobilization influences neuronal hippocampal molecular changes known to be required for memory consolidation in a variety of tasks. Toward this end, we investigated the induction of Arc expression, ser 133 pCREB, which is known to be critical for CREB-dependent gene expression regulation (Alberini, 2009; Mayr and Montminy, 2001) and the phosphorylation of cofilin (pcofilin).
The change in expression levels of these markers following training, as well as the effect of hippocampal DAB and L-lactate administrations were determined. Rats were either trained in IA or remained in the homecage (untrained controls). Untrained controls, or rats 15 min before training, received a bilateral injection into the hippocampus of either 300 pmol DAB or vehicle, or DAB and 100 nmol L-lactate. All animals were euthanized either 30 min or 20 hr after training. Thirty minutes after training was used to examine Arc expression, since Arc is known to be rapidly induced (Bramham et al., 2008). Twenty hours after training was used to determine pCREB and pcofilin changes, since they are known to accompany the formation of long-term memory and plasticity for several hours or days (Alberini, 2009; Fedulov et al., 2007). The homecage control rats that received similar treatments were euthanized at paired timepoints (Figure 7). Quantitative western blot analyses were used to determine Arc, pCREB, CREB, pcofilin and cofilin concentrations. Quantitative western blot analyses indicated that, as expected, rats that received vehicle injection and underwent IA training (vehicle-trained) had a significant increase in hippocampal Arc compared with untrained vehicle-injected rats (p < 0.05, one-way ANOVA followed by Newman-Keuls post hoc test, Figure 7A and 7B). This increase was completely blocked by DAB (p < 0.05). L-lactate significantly rescued this DAB effect compared to vehicle (p < 0.05).
Figure 7. Training-induced increase of Arc, pCREB and pcofilin are completely blocked by DAB and significantly rescued by L-lactate.
Mean %, n and detailed statistic are reported in Table S6.
(A-F) Examples (A) and densitometric western blot analysis of Arc (B), pCREB (C), CREB (D), pcofilin (E) and cofilin (F) performed on dorsal hippocampal extracts from trained and untrained rats injected 15 min before training with vehicle, DAB+vehicle or DAB+L-lactate and euthanized 30 min (for Arc) or 20 hr (for all other markers) after training. See also Figure S3.
Arc (B), pCREB (C) and pcofilin (E) expression were significantly increased after training. This increase was completely blocked by DAB and rescued by L-lactate. There is no change in expression of CREB (D) and cofilin (F) across samples (n=4-7/group). Data are expressed as mean percentage ± SEM of untrained, vehicle-injected control (100%) mean values. All proteins values were normalized to those of actin. * p < 0.05.
Similarly, at 20 hr after training, pCREB and pcofilin were significantly increased in the hippocampus of vehicle-injected, trained rats compared to vehicle-injected untrained (p <0.05, one-way ANOVA followed by Newman-Keuls post-hoc test, Figure 7A and 7C-7F), whereas the total concentration of CREB and cofilin, respectively, remained unchanged (p > 0.05). Both increases were completely blocked by DAB (p < 0.05). L-lactate injection rescued the effect of DAB and restored a high pCREB and pcofilin expression, which was comparable to that of trained rats that received vehicle injection (p > 0.05). These results were further confirmed by MCT1 knock down treatments. Bilateral hippocampal MCT1-ODNs injection significantly blocked pCREB induction 12 h after training, compared to SC1-ODN (p < 0.05, one-way ANOVA followed by Newman-Keuls post hoc-test, Figure S3).
Together, these data indicate that both DAB and down regulation of MCT1 expression block molecular changes induced by learning and required for long-term memory formation as well as relative synaptic structural changes, and that L-lactate is sufficient to fully rescue the effect.
DISCUSSION
Little is known about the contribution of astrocytes to cognitive functions. The results of this study lead to the conclusion that astrocyte-neuron lactate transport plays a critical role in long-term memory formation.
The critical role of glycogen metabolism-derived lactate in long-term plasticity and memory formation
Our results on the DAB-dependent hippocampal lactate release after training, DAB- and isofagomine-induced amnesia rescued by exogenous L-lactate and D-lactate-induced amnesia indicate that learning leads to astrocytic glycogenolysis and lactate release that is critical for long-term memory formation, but dispensable for learning and short-term memory. The DAB-dependent effects are astrocytic and not neuronal because although glycogen can be found in fetal, neonatal and postnatal juvenile neurons up to one month of age (McKenna et al, 2006), it is absent from neurons of the hippocampus and neocortex of adult brain and only found occasionally in large neurons in the brainstem or in the peripheral nervous system (Sotelo and Palay 1968; Magistretti 2008).
An amnestic effect of DAB on memory retention at 30 to 70 min after training has been previously reported by Gibbs et al. (2006) in a day-old chick bead-discrimination learning paradigm. This amnesia was rescued by lactate, glutamine or acetate plus aspartate. Glucose also had an effect in rescuing DAB-induced memory impairment, but it was transient and found only immediately after the injection but not later (Gibbs et al. 2006). The authors concluded that glycogenolysis is important to provide neurons with glutamine as the precursor for neuronal glutamate and GABA and that glycogen is a preferred glutamate precursor during learning in chick (Gibbs et al., 2007).
Our results extend these findings to an adult rat hippocampal memory and show that the hippocampus is an anatomical site where glycogenolysis, around the time of training but not 24 hours later, is required for long-term memory formation. Furthermore, we show, for the first time to our knowledge, that learning results in glycogenolysis-dependent lactate release in the hippocampus critical for memory formation. In line with our data, sensory and cognitive stimuli in humans and animals produce an increase in brain lactate (Fray et al., 1996; Urrila et al., 2004). The DAB-induced amnesia did not reverse after a footshock reminder, suggesting that disrupting glycogen metabolism around the time of training persistently impairs memory consolidation or storage.
Our results do not exclude that, in addition to L-lactate, other substrates that crosstalk with or are downstream of lactate production may rescue or enhance the impaired memory caused by blocking glycogenolysis. For example, glutamine and GABA may rescue the DAB-induced amnesia, as one of the end-results of training-induced lactate production may be indeed the increase of glutamine and GABA. Glucose uptake may also result in lactate increase that may contribute to memory consolidation or enhancement. Our data, confirming previous studies (McNay and Gold, 2002; Messier, 2004), show, in fact, that glucose can enhance memory. However, glucose is much less efficient in rescuing the amnesia caused by DAB and its effect is transient, indicating that the end mechanisms of lactate or glucose might be different or at least have different kinetics. Dissecting the effects of lactate downstream to its uptake into neurons shall be the focus of future investigations.
We also found similar outcomes when we investigated in vivo hippocampal LTP and conclude that maintenance but not induction of Schaffer collateral-CA1 LTP requires glycogenolysis and is mediated by lactate. Although our results indicate that glycogenolysis is required for LTP and not baseline activity, they do not inform about the nature of the targeted mechanisms and further experiments shell shed light on this issue. These findings add to the evidence provided by a number of other studies that investigated other types of astrocytic mechanisms, including release of d-Serine (Henneberger et al., 2010; Panatier et al., 2006; Zhang et al., 2008), ATP-dependent vesicular release of glutamate (Jourdain et al., 2007) and astroglial gap junctions-mediated delivery of energetic metabolites from blood vessels to distal neurons (Rouach et al., 2008).
The critical role of lactate transport between astrocytes and neurons in memory formation
Disrupting the expression of both the astrocytic and neuronal lactate transporters MCT4 and MCT2, which are highly enriched at synaptic sites, result in amnesia, which is rescued by L-lactate after MCT4 but not MCT2 expression disruption. These results together with the evidence of a necessary role for glycogenolysis-dependent lactate release in the hippocampus strongly support the conclusion that an astrocytic-neuronal lactate transport critically mediates long-term memory formation.
Furthermore, learning leads to a significant increase in MCT1 expression, whose disruption completely blocks long-term memory. MCT1 is expressed in endothelial cells of microvessels ependymocytes, oligodendrocytes and astrocytes and, interestingly, was found in the synaptoneurosomal fraction, which consists of resealed presynaptic sac (synaptosome) fused to a larger resealed post-synaptic sac (neurosome) as well as resealed astrocytic processes, as confirmed by the presence of GFAP (Villasana et al. 2006, Williams et al. 2009). GFAP has been generally believed to be absent from the very fine perysynaptic astrocytic compartments; hence, a possible explanation for its detection in synaptoneurosomal fractions is that communications between astrocytes and synapses may not be restricted to the fine astrocytic protrusions but also take place in larger, GFAP-positive processes that are sealed to synaptoneurosomal sacs. In addition, it is possible that MCT1 expressed in the other cells also play a critical role in long-term memory formation. Interestingly, rapid regulation of MCT1 expression has been documented in cell lines incubated with lactate (Hashimoto et al., 2007). Further experiments should shed light on whether the learning-related induction of MCT1 is perisynaptic and how MCT1expressed in different cells and compartments contribute to long-term memory formation. L-lactate, but not equicaloric concentrations of glucose, rescues these MCT1- MCT2- or MCT4-knock-down-dependent amnesia, indicating that glucose and lactate are not functionally interchangeable. However, as discussed above, glucose enhances memory retention in control animals, and higher concentrations of glucose can rescue the MCT1-ODN induced amnesia, but only transiently. Possible explanations for these results are: first, that the glucose-mediated memory enhancement might occur via astrocytic glucose uptake and subsequent glycolytic processing leading to lactate release and that an overload of lactate via high glucose concentrations may overcome the temporally-limited effect of the antisense treatments. Second, glucose entering the neuron directly may produce outcomes overlapping with those resulting from the lactate shuttling (e.g. glutamine and GABA increase). Nevertheless, our data point to the conclusion that glucose partially and less efficiently substitutes for lactate.
Thus, we conclude that an astrocyte-neuron lactate transport critically supports neuronal functions, in agreement with what proposed by Pellerin and Magistretti (1994).
The astrocyte-neuron lactate transport hypothesis does not contradict the classical belief that glucose is a fundamental metabolic substrate in mature brain (Clarke and Sokoloff, 1994); it suggests that both glucose and lactate have critical roles for adult brain functioning. On one hand, lactate may represent an essential substrate mediating the astrocyte-neuron metabolic coupling during activity-related high-energy demands; on the other, glucose uptake into neurons also occurs as suggested by the expression of glucose transporters of the glut 3 type (Magistretti, 2008). However, recent data by Herrero-Mendez et al (2009) showed that cultured cortical neurons cannot sustain a high glycolytic rate as glucose is mainly used to maintain their antioxidant status, and studies in the skeletal muscle show that lactate is formed and utilized continuously under fully aerobic conditions and mediates, in addition to energy supply, important functions such as cellular signaling and redox regulation. Thus, in agreement with Brooks (2009), we propose that glycolytic and oxidative pathways should be viewed as linked, as opposed to alternative processes also with the astrocyte-neuron network. Further studies shall shed light on the underlying mechanisms. In agreement with this idea, there is also evidence of compartmentalized energy metabolism between astrocytes and neurons, especially during increased neuronal activity (Kasischke et al., 2004) and of lactate being a crucial aerobic energy substrate that enables neurons to endure activation (Schurr et al., 1999).
The critical role of astrocytes in learning-induced molecular and synaptic changes
We also found that pCREB and Arc expression, as well as pcofilin, which represent essential mechanisms underlying long-term synaptic plasticity and memory formation and their related synaptic structural changes (Alberini, 2009; Bramham et al., 2008) also depend on an intact glycogen metabolism and astrocyte-neuron lactate transport. Both pCREB and Arc are mostly induced in neurons following training or long-term plasticity (Impey et al., 1998; Leutgeb et al., 2005; Taubenfeld et al., 2001b; Vazdarjanova et al., 2006). The complete disruption of both learning-dependent pCREB and Arc by DAB or MCT1-ODN suggests that there is a critical functional link between the astrocytic glycogenolysis and lactate transport and the neuronal gene expression regulation underlying long-term memory formation. The lactate-mediated rescuing indicates that this functional link is mediated by lactate. Hence, it is tempting to speculate that the neurometabolic coupling mediated by lactate supplies the energy needed by activated neurons for their local protein synthesis, degradation and activation of signaling pathways that lead to the regulation of the gene expression cascade that underlies long-term memory. Because lactate has been suggested to play an important role in cellular and organelle redox balance (Brooks, 2009), we may also hypothesize that lactate not only represents an energy source, but also mediates coordinated astrocyte-neuron cell-cell and intracellular responses important for cell signaling (Gordon et al., 2008) and regulation of gene expression.
Finally, our data showing learning-induced increased phosphorylation of cofilin is in agreement with and extends previous studies reporting that unsupervised context learning in rats leads to a 30% increase in spine numbers in the hippocampal CA1 region. These spines express pcofilin and have a larger size compared to the average spines of control naïve rats (Fedulov et al., 2007). Similarly, pcofilin has also been found upregulated following LTP in spines that have larger post-synaptic densities (PSDs) (Chen et al., 2007). Hence, pcofilin represents a correlative marker of spine morphological changes induced during long-term plasticity and memory. We also showed that the increase in pcofilin depends on an intact glycogen metabolism and lactate transport, strengthening the hypothesis that the astrocyte-neuron lactate transport is critical to enable the synaptic molecular and spine morphological changes underlying long-term memory formation.
In conclusion, we propose that astrocyte-neuron lactate transport is essential for long-term synaptic plasticity, long-term memory and its underlying molecular and synaptic changes. These results may have important implications for memory disorders and pathologies carrying memory or cognitive deficits in general, including neurodegenerative conditions such as Alzheimer's disease, aging and dementia.
EXPERIMENTAL PROCEDURES
Animals
All protocols involving the use of animals complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Mt. Sinai School of Medicine Animal Care Committees. See supplemental experimental procedures for details.
Cannulae implants
Bilateral hippocampal surgeries were performed as described by Taubenfeld et al. (2001a). See supplemental experimental procedures for details.
Inhibitory avoidance (IA)
IA was carried out as described previously (Taubenfeld et al. 2001a). See supplemental experimental procedures for details.
Drug and oligodeoxynucleotide injections
DAB, sodium L-lactate, sodium D-lactate, D-glucose (Sigma-Aldrich, St. Louis, MO) and isofagomine (Santa Cruz Biotechnology, Santa Cruz, CA) were dissolved in phosphate-buffered saline (PBS, pH 7.4). Oligodeoxynucleotides were reverse phase cartridge-purified and purchased from Gene Link (Hawthorne, NY). All injections were performed at 1 μl of volume per hippocampal side. See supplemental experimental procedures for details.
Microdialysis
Microdialysis was performed as described in Rex et al. (2009), using a flowrate of 3μl/min and samples were collected every 10 min. Samples were subsequently analyzed using the AbCam Lactate Fluorescence Assay Kit. See supplemental experimental procedures for details.
Synaptoneurosomal preparation
Synaptoneurosomal preparation was carried out as described in Villasana et al. (2006). See supplemental experimental procedures for details.
Western blot analysis
Primary antibodies [rabbit anti-MCT1, rabbit anti-MCT2, rabbit anti-MCT4, rabbit anti-pCREB and rabbit anti-CREB, rabbit anti-cofilin, mouse anti-PSD95 and rabbit anti-GFAP (Millipore, Billerica, MA), rabbit anti-pcofilin (Abcam, Cambridge, MA), and rabbit anti-Arc (Synaptic System, Gottingen, Germany)] were used. Actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used for loading normalization. Western blot were performed as described previously (Taubenfeld et al., 2001b). See supplemental experimental procedures for details.
Electrophysiology
Electrophysiological experiments were carried out as previously described (Bozdagi et al. 2007). DAB (300 μM), lactate (100 mM) or lactate+DAB (2x concentration from each) were applied. See supplemental experimental procedures for details.
Statistical Analysis
Data were analyzed with one- or two-way repeated measure ANOVA followed by Newman–Keuls or Bonferroni post hoc test. When two groups were compared, Student's t test was used.
Supplementary Material
Acknowledgements
This work was supported by grants R01-MH065635 and R01-MH074736 to C.M.A. and T32-MH087004 to S.A.S. We thank Rick Koch, Dhananjay Bambah-Mukku, Dillon Chen, Gabriella Pollonini and Virginia Gao for technical support, and Reginald Miller and the Center for Comparative Medicine and Surgery facility at Mount Sinai School of Medicine for technical support. P.J.M. is the recipient of the Asterion Chair at EPFL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell. Biol. 1999;9:364–370. doi: 10.1016/s0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J. Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkok S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem. Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J. Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DD, Sokoloff L. Circulating and energy metabolism of the brain. In: Siegle GJ, Agranoff BW, Albers RW, Molinoff PB, editors. In Basic Neurosciemistry. Raven Press; New York: 1994. pp. 645–680. [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J. Cereb. Blood Flow Metab. 2010;30:1895–1904. doi: 10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Fedulov V, Rex CX, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence That Long-Term Potentiation Occurs within Individual Hippocampal Synapses during Learning. J. Neurosci. 2007;27:8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J. Neurosci. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray AE, Forsyth RJ, Boutelle MG, Fillenz M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J. Physiol. 1996;496:49–57. doi: 10.1113/jphysiol.1996.sp021664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Cheung YY, Mansfield BC, Chou JY. Brain contains a functional glucose-6-phosphatase complex capable of endogenous glucose production. J. Biol. Chem. 2005;280:11114–11119. doi: 10.1074/jbc.M410894200. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Lloyd HG, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J. Neurosci. Res. 2007;85:3326–3333. doi: 10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B, Verleysdonk S, Wiesinger H. Enzymes of carbohydrate and energy metabolism. In: Kettenmann H, Ransom BR, editors. Neuroglia. 2nd ed. Oxford University Press; New York: 2005. pp. 202–215. [Google Scholar]
- Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat. Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Sadeghi Y, Hosseini A, Naghdi N, Marjani A. The effect of spatial learning on the number of astrocytes in the CA3 subfield of the rat hippocampus. Singapore Med. J. 2008a;49:388–391. [PubMed] [Google Scholar]
- Jahanshahi M, Sadeghi Y, Hosseini A, Naghdi N, Piriaie A. Working memory learning method and astrocytes number of different subfields of rat's hippocampus. Am. J. Anim. Vet. Sci. 2008b;3:28–31. [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. Neuroglia. 2nd ed. Oxford University Press; New York: 2005. [Google Scholar]
- Klann E, Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol. Learn. Mem. 2008;89:247–259. doi: 10.1016/j.nlm.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Frey JU, Behnisch T. Single cell analysis of activity-dependent cyclic AMP-responsive element-binding protein phosphorylation during long-lasting long-term potentiation in area CA1 of mature rat hippocampal-organotypic cultures. Neuroscience. 2005;131:601–610. doi: 10.1016/j.neuroscience.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Brain energy metabolism. In: Squire LR, Berg D, Bloom FE, du Lac S, Ghosh A, Spitzer NC, editors. Fundamental Neuroscience. 3rd ed. Academic Press; San Diego: 2008. pp. 271–293. [Google Scholar]
- Magistretti PJ, Morrison JH. Noradrenaline- and vasoactive intestinal peptide-containing neuronal systems in neocortex: functional convergence with contrasting morphology. Neuroscience. 1988;24:367–378. doi: 10.1016/0306-4522(88)90338-7. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mantzura L, Joelsa G, Lamprecht R. Actin polymerization in lateral amygdala is essential for fear memory formation. Neurobiol. Learn. Mem. 2009;91:85–88. doi: 10.1016/j.nlm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Rolf Gruetter R, Sonnewald U, Waagepetersen HS, Schousboe A. Energy Metabolism of the Brain. In: Siegel G, Albers RW, Brady S, Price DL, editors. Basic Neurochemistry. Elsevier; London: 2006. pp. 531–557. [Google Scholar]
- McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav. Cogn. Neurosci. Rev. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- Messier C. Glucose improvement of memory: a review. Eur. J. Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Rex A, Bert B, Fink H, Voigt JP. Stimulus-dependent changes of extracellular glucose in the rat hippocampus determined by in vivo microdialysis. Physiol. Behav. 2009;98:467–473. doi: 10.1016/j.physbeh.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessarism N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J. Neurosci. 1999;19:34–39. doi: 10.1523/JNEUROSCI.19-01-00034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Palay SL. The fine structure of the lateral vestibular nucleus in the rat. I. Neurons and neuroglial cells. J. Cell Biol. 1968;36:151–179. [PubMed] [Google Scholar]
- Swanson RA. Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can. J. Physiol. Pharmacol. 1992;70:138–144. doi: 10.1139/y92-255. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat. Neurosci. 2001a;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein β and δ co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J. Neurosci. 2001b;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J. Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Häkkinen AM, Lundbom N, Porkka-Heiskanen T. Stimulus-induced brain lactate: effects of aging and prolonged wakefulness. J. Sleep Res. 2004;13:111–119. doi: 10.1111/j.1365-2869.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Vallès J, García-Fojeda B, CriadoGarcía O, Fernández-Sánchez E, Medraño-Fernández I, Domínguez J, et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Villasana LE, Klann E, Tejada-Simon MV. Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. J. Neurosci. Methods. 2006;158:30–36. doi: 10.1016/j.jneumeth.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waagepetersen HS, Westergaard N, Schousboe A. The effects of isofagomine, a potent glycogen phosphorylase inhibitor, on glycogen metabolism in cultured mouse cortical astrocytes. Neurochem. Int. 2000;36:435–440. doi: 10.1016/s0197-0186(99)00146-1. [DOI] [PubMed] [Google Scholar]
- Walls AB, Sickmann HM, Brown A, Bouman SD, Ransom B, Schousboe A, Waagepetersen HS. Characterization of 1,4-dideoxy-1,4-imino-d-arabinitol (DAB) as an inhibitor of brain glycogen shunt activity. J. Neurochem. 2008;105:1462–1470. doi: 10.1111/j.1471-4159.2008.05250.x. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Williams C, Mehrian Shai R, Wu Y, Hsu YH, Sitzer T, Spann B, McCleary C, Mo Y, Miller CA. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer's disease. PLoS One. 2009;4:e4936. doi: 10.1371/journal.pone.0004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gong N, Wang W, Xu L, Xu TL. Bell-shaped D-serine actions on hippocampal long-term depression and spatial memory retrieval. Cereb. Cortex. 2008;18:2391–2401. doi: 10.1093/cercor/bhn008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.