Abstract
Background
The Occluded Artery Trial (OAT) showed no difference in outcomes between percutaneous coronary intervention (PCI) vs. optimal medical therapy (MED) in patients with persistent total occlusion of the infarct related artery (IRA) 3–28 days post-MI. Whether PCI may benefit a subset of patients with preservation of infarct zone (IZ) viability is unknown.
Methods and Results
The OAT nuclear ancillary study hypothesized that; 1) IZ viability influences left ventricular (LV) remodeling, and that 2) PCI as compared to MED attenuates adverse remodeling in post-MI patients with preserved viability. Enrolled were 124 OAT patients, who underwent resting nitroglycerin-enhanced 99mTc sestamibi SPECT prior to OAT randomization, with repeat imaging at 1 year. All images were quantitatively analyzed for infarct size, IZ viability, LV volumes and function in a core lab. At baseline, mean infarct size was 26%±18 of the LV, mean IZ viability was 43%±8 of peak uptake, and the majority (70%) of patients had at least moderately retained IZ viability. There were no significant differences in 1-year EDV or ESV change between those with severely reduced vs. moderately retained IZ viability, or when compared by treatment assignment PCI vs. MED. In multivariable models, increasing baseline viability independently predicted improvement in EF (p=0.005). There was no interaction between IZ viability and treatment assignment for any measure of LV remodeling.
Conclusions
In the contemporary era of optimal medical therapy, PCI of the IRA compared to medical therapy alone does not impact LV remodeling irrespective of IZ viability.
Keywords: Acute Coronary Syndrome, Total Occlusions, Percutaneous Coronary Intervention, Coronary Flow, Viability, Remodeling
The Occluded Artery Trial (OAT) demonstrated no difference in clinical outcomes over five years between patients randomized to percutaneous coronary intervention (PCI) versus optimal medical therapy alone (MED) in stable patients with an occluded infarct related artery (IRA) 3–28 days post-MI 1. Data from previous observational and small nonrandomized studies have suggested that preservation of myocardial viability within the infarct zone (IZ) is associated with attenuation of adverse left ventricular (LV) remodeling months after MI 2–5.However, they have been limited by variable testing protocols, small sample sizes, short follow up durations and nonrandomized treatment designs. The potentially interactive effect of revascularization and IZ viability and whether PCI may benefit a subset of OAT patients with relative preservation of IZ viability, is unknown.
The aims of the OAT Nuclear Viability ancillary study (OAT-NUC) were to 1) examine the influence of retained IZ viability on extent of LV remodeling in post MI pts with occluded IRAs; 2) evaluate the influence of revascularization with PCI versus MED on the extent of subsequent LV remodeling, and 3) assess whether OAT patients assigned to PCI with more preserved viability would have less adverse remodeling than similar patients assigned to MED alone.
METHODS
Study Population
Patients in OAT-NUC had identical screening procedures, eligibility criteria, and clinical follow-up as the main OAT protocol previously described6. Briefly, patients had to have a confirmed index MI (2 out of 3 criteria: ischemic symptoms, elevated cardiac markers, typical MI ECG changes), total occlusion (TIMI 0 or 1 flow) of the IRA on angiography on calendar days 3–28 (minimum 24 hours) post MI; and increased risk (EF < 50% and/or proximal occlusion of a major vessel with a large LV risk region). Major exclusion criteria were as in OAT. Each participating center had Institutional Review Board approval, and patients were provided separate additional written informed consent for the OAT-NUC study protocol. The trial is registered as Clinical trials.gov identifier NCT00119847.
SPECT Imaging and Outcome Measures
Patients were administered 0.4mg sublingual nitroglycerin followed by 25–30mCi of 99mTc-sestamibi 5–10 minutes later, and then imaged at rest 1 hour later using standard algorithms optimized for each camera and computer system. All images were analyzed centrally at the Cardiac Imaging Core Laboratory at Tufts Medical Center, blinded both to OAT treatment assignment and to SPECT study timing (baseline vs. 1-year), using standard quantitative techniques from validated commercially available software (4DM SPECT)7, 8. The IZ was identified by areas falling below 60% of peak myocardial uptake in the OAT IRA territory confirmed by the OAT Angiographic Core Laboratory. Infarct size was the percent of the total LV profile falling <60% peak uptake, a measure previously correlated with other clinically relevant measures9–12. For IZ viability, a severity index was calculated by the software based on the counts within the IZ and prospectively classified as “moderately-retained” for areas with average IZ uptake ≥40% of peak uptake, and “severely reduced” for areas <40% (Figure 1). Patients with no identifiable IZ region (i.e. no regions with <60% peak uptake) were included as having moderately-retained viability. LV ejection fraction (EF)(%), LV end-diastolic (EDV) and end-systolic volumes (ESV)(ml) were calculated with an automated software program8. The use of gated SPECT for the determination of LV EF and volumes has been extensively validated13–15. Wall motion was scored by one observer blinded to randomization group, using the standard 17 segment model, assigning scores ranging from 0 – 4 for wall motion ranging from normal to akinetic.
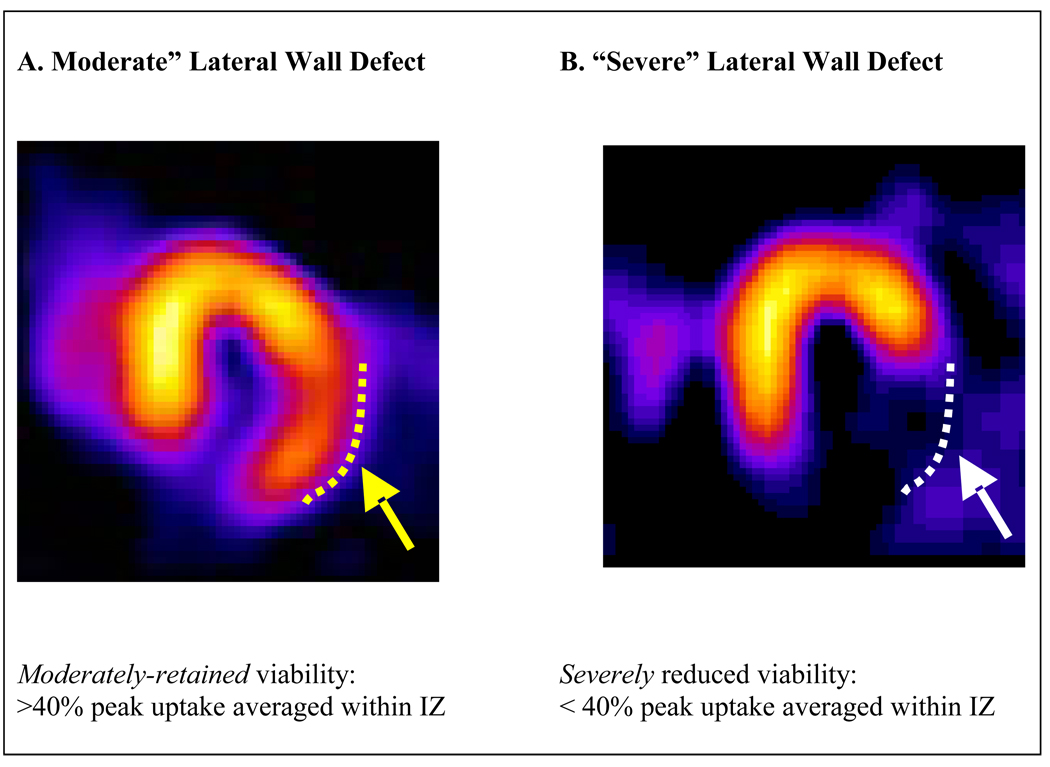
Figure 1.
Examples of horizontal long-axis view of resting SPECT images from two patients in the OAT-NUC trial illustrating the viability classification.
LEFT- A lateral wall infarct, but with some preservation of uptake in the lateral wall (yellow dotted line and arrow), classified as moderately-retained infarct zone viability.
RIGHT - A lateral wall infarct, but a very severe reduction in uptake in the lateral wall (white dotted line and arrow) consistent with severely reduced infarct zone viability.
Statistical Analysis
Data management and statistical analyses were performed by an independent data coordinating center. Baseline clinical and index MI characteristics including medication use at baseline hospital discharge and 1-year were assessed as frequencies/proportions or mean±SD, and compared to the remaining main OAT patients and by treatment group PCI vs. MED using Chi-Square or Fisher tests. The primary endpoint of LV remodeling was assessed by change in LV end-diastolic volume from baseline to 1 year. To evaluate the influence of retained IZ viability on extent of LV remodeling, change in LV volumes and EF were compared by baseline viability. To evaluate the influence of treatment on LV remodeling, changes in LV volumes were compared by treatment assignment PCI vs. MED. To examine the interaction between baseline IZ viability and treatment on extent of remodeling, the effect of PCI vs. MED on change in LV volumes was compared among those with moderately-retained versus severely-reduced viability. Post-hoc analyses were also performed with viability as a continuous function. Intention-to-treat principle was used for all treatment related analyses except for an as-treated sensitivity analysis. Two-sided two-group t-tests at alpha=0.05 were done for comparisons of changes from baseline to 1-year. Student's t-test to determine if the change from baseline to 1 year was different from 0 within each treatment and/or viability group. It was estimated that with a targeted total sample size of 200 pts and a 25ml predicted standard deviation for 1-year EDV change there would be 80% power to detect a 14ml difference in EDV change between viability groups and between treatment groups with up to a 25% crossover and a 15% drop-out rate. As described below, this sample size target was not reached, and with the observed SD of 26.9ml and final achieved sample size of 124, post-hoc analysis estimated that there was 80% power to detect a difference of −15.0ml for the primary outcome of change in LV EDV from baseline to 1 year. Reasons for not achieving the projected sample size included: a) OAT NUC ancillary study began recruitment well after the parent OAT study, b) study start-up at many sites was more prolonged than anticipated, c) recruitment was slower than anticipated, and d) the main OAT recruitment ended before the final OAT-NUC sample size was achieved, thus recruitment in OAT-NUC was required to stop as well.
In OAT and the OAT ancillary studies, a p < 0.01 was pre-specified for significance for all secondary analyses to limit Type I error. Separate multivariable models were constructed for analysis of the change between baseline and 1 year in LV volumes, and EF. The baseline values of ES Volume and EF were included in the first step of each corresponding stepwise model and treatment group (PCI vs. MED) was included in each step of the process. For ED volume, the baseline measure was also included in each step. Baseline clinical and angiographic characteristics that were associated in univariate analysis for each outcome measure (i.e. p-value <0.10) were included in the corresponding multivariable model. For multivariable models, stepwise linear regression with backward elimination was conducted, with a p value of 0.01 required for a variable to be retained. Baseline infarct size and viability tested for possible inclusion in the models as both categorical and continuous functions. Because the models for 1-year change in LV volumes, and EF had normally distributed residuals, these measures were suitable for analysis with linear regression.
Changes in ED volume, ES volume, WM index and WM score were not normally distributed and were winsorized then analyzed with the t-test. Winsorization is a method to control the variability introduced by extreme values. This method typically identifies the top and bottom 5% and reduces them to the next highest (or lowest) value. This method permits the use of procedures that require a normal distribution (i.e. multiple regression), while controlling the influence of these extreme values.16
RESULTS
Baseline Characteristics
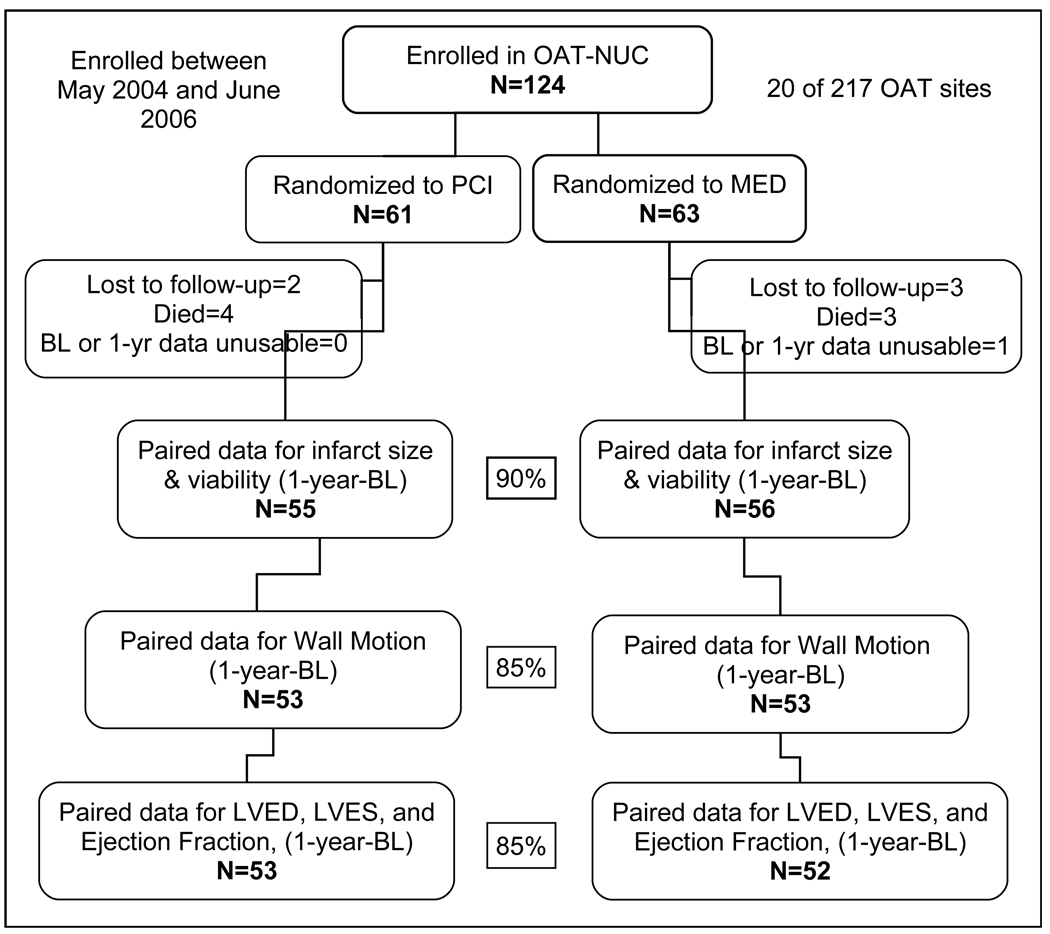
Between May 2004 and June 2006, there were 124 patients enrolled in OAT-NUC at 20 sites in Australia, Austria, Brazil, Canada, Poland, and the United States (Figure 2). The patients in OAT-NUC were similar in clinical history to the rest of the main OAT study population at baseline except for a higher proportion of cerebrovascular disease (Table 1).
Figure 2.
OAT-NUC Study Enrollment and Patient Flow
TABLE 1.
Baseline Characteristics of Study Cohort
| OAT-NUC | Remaining OAT | p | |||
|---|---|---|---|---|---|
| N=124 | N=2077 | ||||
| DEMOGRAPHICS | N | N | |||
| Age (mean, years) | 124 | 59±11 | 2077 | 59±11 | 0.95 |
| Female (%) | 26 | 21.0 | 458 | 22.0 | 0.78 |
| Race Black (%) | 3 | 2.4 | 66 | 3.2 | |
| Hispanic (%) | 17 | 13.7 | 260 | 12.5 | |
| Other (%) | 5 | 4.0 | 87 | 4.2 | 0.95 |
| CLINICAL HISTORY | |||||
| Hypertension (%) | 66 | 53.2 | 1005 | 48.4 | 0.30 |
| Diabetes (%) | 21 | 16.9 | 433 | 20.8 | 0.30 |
| On Insulin (%) | 7 | 5.6 | 117 | 5.6 | 1.00 |
| Hypercholesterolemia (%) | 64 | 51.6 | 1078/2076 | 51.9 | 0.95 |
| Angina (%) | 27 | 21.8 | 468 | 22.5 | 0.84 |
| Prior MI* (%) | 8 | 6.4 | 239 | 11.5 | 0.08 |
| Prior CHF† (%) | 3 | 2.4 | 49/2075 | 2.4 | 1.00 |
| Cerebrovascular Disease (%) | 11 | 8.9 | 71 | 3.4 | 0.005 |
| Prior Stroke (%) | 9 | 7.3 | 54 | 2.6 | 0.008 |
| Peripheral Vascular Disease (%) | 2 | 1.6 | 81/2075 | 3.9 | 0.33 |
| Family History CAD‡ (%) | 52 | 41.9 | 831 | 40.0 | 0.67 |
| Current Smoker (%) | 51 | 41.1 | 808 | 38.9 | 0.62 |
| History of PCI§ (%) | 3 | 2.4 | 102 | 4.9 | 0.21 |
| Renal Insufficiency (%) | 2 | 1.6 | 28 | 1.4 | 0.68 |
| CLINICAL MEASURES_ | |||||
| BMI‖ (kg/m2) | 123 | 28 ± 5 | 2062 | 29 ± 5 | 0.10 |
| Heart Rate (bpm) | 124 | 70 ± 11 | 2074 | 72 ± 12 | 0.12 |
| Systolic BP# (mmHg) | 124 | 120 ± 15 | 2075 | 121 ± 18 | 0.76 |
| Diastolic BP (mmHg) | 124 | 74 ± 10 | 2075 | 72 ± 11 | 0.05 |
| INDEX MI | |||||
| Days MI to Randomization (Median[IQR}) | 124 | 14.5 [7–23] | 2077 | 8.0 [5–16] | <0.001 |
| Ejection Fraction (mean, %) | 122 | 48 ± 11 | 2063 | 48 ± 11 | 0.61 |
| EF** ≥ 50% (%) | 53/122 | 43.4 | 962/2063 | 46.6 | 0.49 |
| Highest Killip Class II–IV (%) | 23 | 18.6 | 394/2068 | 19.0 | 0.89 |
| STE / Q waves / R wave loss (%) | 104 | 83.9 | 1801 | 86.7 | 0.37 |
| ANGIOGRAPHY | |||||
| Infarct Related Artery-LAD†† (%) | 39 | 31.5 | 754 | 36.3 | |
| - LCX‡‡ (%) | 21 | 16.9 | 314 | 15.1 | |
| - RCA§§ (%) | 64 | 51.6 | 1009 | 48.6 | 0.54 |
| Multivessel Disease(%) | 25/123 | 20.3 | 354/2060 | 17.2 | 0.37 |
| Collaterals - Grade 1 or 2 (%) | 108/123 | 87.8 | 1814/2050 | 88.5 | 0.82 |
| Collaterals - Grade 2 (%) | 10/123 | 8.1 | 364/2050 | 17.8 | 0.006 |
MI=myocardial infarction,
CHF=congestive heart failure,
CAD=coronary artery disease,
PCI=percutaneous coronary intervention,
BMI=body mass index,
BP=blood pressure,
EF=ejection fraction,
LAD=left anterior descending artery,
LCX= left circumflex coronary artery,
RCA= right coronary artery.
Among the OAT-NUC patients, those randomized to PCI versus MED were also similar except for a trend toward more Killip Class II–IV in the PCI group (Table 2), more thienopyridine use among PCI patients and more beta blockers use in the MED group at 1 year (Table 2).
TABLE 2.
OAT-NUC Patient Characteristics by Treatment Assignment
| PCI* | MED† | ||||
|---|---|---|---|---|---|
| (N = 61) | (N = 63) | p-value | |||
| % or | % or | ||||
| n | Mean±SD | n | Mean±SD | ||
| DEMOGRAPHICS | |||||
| Age (mean, years) | 61 | 58.5±11.5 | 63 | 58.6±10.6 | 0.93 |
| Female(%) | 15 | 24.6 | 11 | 17.5 | 0.33 |
| Race Black(%) | 1 | 1.6 | 2 | 3.2 | |
| Hispanic(%) | 9 | 14.8 | 8 | 12.7 | |
| Other(%) | 1 | 1.6 | 4 | 6.4 | 0.65 |
| CLINICAL HISTORY | |||||
| Hypertension(%) | 33 | 54.1 | 33 | 52.4 | 0.85 |
| Diabetes(%) | 8 | 13.1 | 13 | 20.6 | 0.26 |
| On Insulin(%) | 1 | 1.6 | 6 | 9.5 | 0.11 |
| Hypercholesterolemia(%) | 29 | 47.5 | 35 | 55.6 | 0.37 |
| Prior Angina(%) | 12 | 19.7 | 15 | 23.8 | 0.58 |
| Prior MI‡(%) | 3 | 4.9 | 5 | 7.9 | 0.72 |
| Prior PCI*(%) | 1 | 1.6 | 2 | 3.2 | 1.00 |
| Prior CHF§(%) | 2 | 3.3 | 1 | 1.6 | 0.62 |
| Cerebrovascular Disease(%) | 5 | 8.2 | 6 | 9.5 | 0.80 |
| Prior Stroke(%) | 5 | 8.2 | 4 | 6.4 | 0.74 |
| Peripheral Vascular Disease(%) | 2 | 3.3 | 0 | 0.0 | 0.24 |
| Current Smoker(%) | 24 | 39.3 | 27 | 42.9 | 0.69 |
| Family History CAD‖ (%) | 22 | 36.1 | 30 | 47.6 | 0.19 |
| CLINICAL MEASURES | |||||
| BMI# (kg/m2) | 61 | 28.4±4.6 | 62 | 27.2±4.2 | 0.16 |
| Heart Rate (bpm) | 61 | 69.9±10.9 | 63 | 70.4±10.6 | 0.77 |
| Systolic BP** (mmHg) | 61 | 119.0±15.2 | 63 | 121.7±14.5 | 0.31 |
| Diastolic BP (mmHg) | 61 | 73.4±10.3 | 63 | 75.1±9.4 | 0.33 |
| Fasting Glucose(mg/dl) | 57 | 112.0±48.7 | 62 | 109.3±26.8 | 0.72 |
| Estimated GFR†† 30–59(%) | 7 | 11.5 | 7 | 11.1 | |
| 60–89(%) | 35 | 57.4 | 34 | 54.0 | |
| ≥ 90(%) | 19 | 31.2 | 22 | 34.9 | 0.90 |
| INDEX MI | |||||
| Days from MI | 61 | 14.7±8.6 | 63 | 15.5±7.8 | 0.63 |
| Ejection Fraction (mean %) | 61 | 49.0±12.1 | 61 | 47.4±10.6 | 0.44 |
| EF‡‡ ≥ 50%(%) | 29 | 47.5 | 24/61 | 39.3 | 0.36 |
| ST elevation/Q wave/R loss(%) | 52 | 85.3 | 52 | 82.5 | 0.68 |
| Killip Class II–IV(%) | 16 | 26.2 | 7 | 11.1 | 0.03 |
| Mitral Regurgitation(%) | 5/40 | 12.5 | 8/38 | 21.0 | 0.31 |
| ANGIOGRAPHY | |||||
| IRA - LAD§§ (%) | 19 | 31.2 | 20 | 31.8 | |
| - LCX‖‖(%) | 11 | 18.0 | 10 | 15.9 | 0.95 |
| - RCA##(%) | 31 | 50.8 | 33 | 52.4 | |
| Multivessel disease(%) | 9 | 14.8 | 16/62 | 25.8 | 0.13 |
| Collaterals: Grade 1 or 2(%) | 54 | 88.5 | 54/62 | 87.1 | 0.81 |
| Collaterals: Grade 2(%) | 5 | 8.2 | 5/62 | 8.1 | 1.00 |
| MEDICATIONS AT HOSPITAL DISCHARGE | |||||
| Aspirin(%) | 59 | 96.7 | 60 | 95.2 | 1.00 |
| Thienopyridine (clopidogrel or ticlopidine)(%) | 52 | 85.2 | 26 | 41.3 | < 0.001 |
| Warfarin(%) | 3 | 4.9 | 3 | 14.3 | 0.08 |
| Beta Blocker(%) | 56 | 91.8 | 62 | 98.4 | 0.11 |
| Calcium channel Blocker(%) | 0 | 0.0 | 4 | 6.4 | 0.12 |
| Lipid lowering agent(%) | 56 | 91.8 | 62 | 98.4 | 0.11 |
| Spironolactone(%) | 8 | 13.1 | 4 | 6.4 | 0.20 |
| EF <40% - ACE-Inhibitor***/ARB†††(%) | 12/12 | 100.0 | 13/13 | 100.0 | 1.00 |
| MEDICATIONS AT 1 YEAR | (N = 41) | (N = 47) | |||
| Aspirin(%) | 39 | 95.1 | 38 | 80.8 | 0.04 |
| Clopidogrel(%) | 8 | 19.5 | 6 | 12.8 | 0.39 |
| Diuretic(%) | 10 | 24.4 | 15 | 31.9 | 0.43 |
| Beta Blocker(%) | 32 | 78.0 | 45 | 95.7 | 0.01 |
| Ca Blocker(%) | 1 | 2.4 | 4 | 8.5 | 0.37 |
| Lipid lowering agent(%) | 37 | 90.2 | 45 | 95.7 | 0.41 |
| Spironolactone(%) | 4 | 9.8 | 5 | 10.6 | 1.00 |
| EF <40% - ACE-Inhibitor***/ARB††† (%) | 7/9 | 77.8 | 7/8 | 87.5 | 1.00 |
PCI=percutaneous coronary intervention,
MED=medical therapy alone,
MI=myocardial infarction,
CHF= congestive heart failure,
CAD=coronary artery disease,
BMI=body mass index,
BP=blood pressure,
GFR= glomerular filtration rate,
EF=ejection fraction,
LAD=left anterior descending artery,
LCX= left circumflex coronary artery,
RCA= right coronary artery,
ACE= Angiotensin-Converting Enzyme,
ARB=Angiotension Receptor Blockers
At baseline, mean infarct size was 26±18% of the LV. Mean IZ viability was 43 ± 8% of peak uptake, and the majority (70%) of patients (N=87), had at least moderately-retained viability with peak uptake ≥40%. Mean EF was 48±11%.
Paired baseline and 1-year data was available for infarct size and viability in 90% of the OAT-NUC study cohort, and in 85% for LV volumes, EF, and wall motion. Those with paired data were generally similar in clinical characteristics compared to those without paired data, though with less prior stroke (5% vs. 31%, p=0.007) and cerebrovascular disease (5% vs. 38%, p=0.002), and a trend toward fewer LCX infarcts (p=0.05).
Influence of Baseline Infarct Zone Viability on Remodeling Over 1 Year
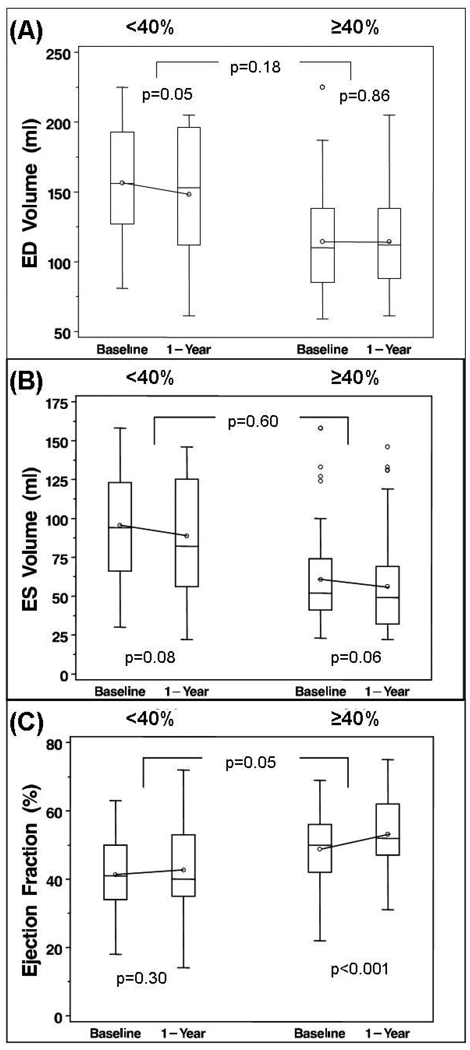
Those with severely-reduced IZ viability had significantly larger mean EDV and ESV and lower EF at baseline and 1 year, compared to those with moderately-retained viability (Figure 3 and Table 3). There were no significant changes in EDV or ESV for either group. There was a trend toward greater improvement in EF among those with moderately-retained viability compared to those with severely reduced viability (between-group difference p=0.05) (Figure 3). Increasing baseline viability as a continuous variable tended to predict improvement in EF (p=0.05), but was not associated with 1-year EDV or ESV change.
Figure 3.
One year changes in end-diastolic (ED) and end-systolic (ES) volumes and ejection fraction, in patients with severe reduction in viability in the infarct zone (“ <40%” of peak tracer uptake), compared to those patients with moderately preserved viability in the infarct zone (“ ≥40%” of peak tracer uptake). There were no significant between-group changes in ED or ES volumes. Ejection fraction increased nominally more in the patients with moderately preserved viability.
Table 3. REMODELING CHANGES BY BASELINE VIABILITY.
| SEVERELY REDUCED VIABILITY < 40% |
MODERATE RETAINED VIABILITY≥ 40% |
||||
|---|---|---|---|---|---|
| (N = 33 | (N = 79) | p-value | |||
| n | Mean±SD | n | Mean±SD | ||
| LV ED Volume (ml) | |||||
| Baseline | 29 | 156.5±44.6 | 76 | 114.0±37.0 | <0.001 |
| 1 yr | 29 | 148.1±47.8 | 76 | 113.4±37.7 | <0.001 |
| Change (1yr-Baseline) | 29 | −8.3±21.9 | 76 | 0.57±28.0 | 0.18 |
| p-value (within group) | 0.05 | 0.86 | |||
| LV ES Volume (ml) | |||||
| Baseline | 29 | 95.5±40.0 | 76 | 60.2±29.7 | <0.001 |
| 1 yr | 29 | 88.6±41.0 | 76 | 55.7±28.9 | <0.001 |
| Change (1yr-Baseline) | 29 | −6.9±20.3 | 76 | −4.5±20.6 | 0.60 |
| p-value (within group) | 0.08 | 0.06 | |||
| Ejection Fraction (%) | |||||
| Baseline | 29 | 41.3±11.5 | 76 | 49.2±10.2 | 0.001 |
| 1 yr | 29 | 42.7±13.3 | 76 | 53.6±10.6 | <0.001 |
| Change (1yr-Baseline) | 29 | 1.4±7.0 | 76 | 4.4±7.0 | 0.05 |
| p-value (within group) | 0.30 | <0.001 | |||
| Wall Motion Score | |||||
| Baseline | 30 | 24.0±6.9 | 76 | 15.3±7.3 | <0.001 |
| 1 yr | 30 | 18.8±9.2 | 76 | 10.1±8.1 | <0.001 |
| Change (1yr-Baseline) | 30 | 5.2±6.7 | 76 | −5.2±6.8 | 0.98 |
| p-value (within group) | <0.001 | <0.001 | |||
LV ED=left ventricular end-diastolic dimension,
LV ES =ventricular end-systolic dimension,
WM=wall motion
Influence of Treatment PCI vs. MED on Remodeling Over 1 Year
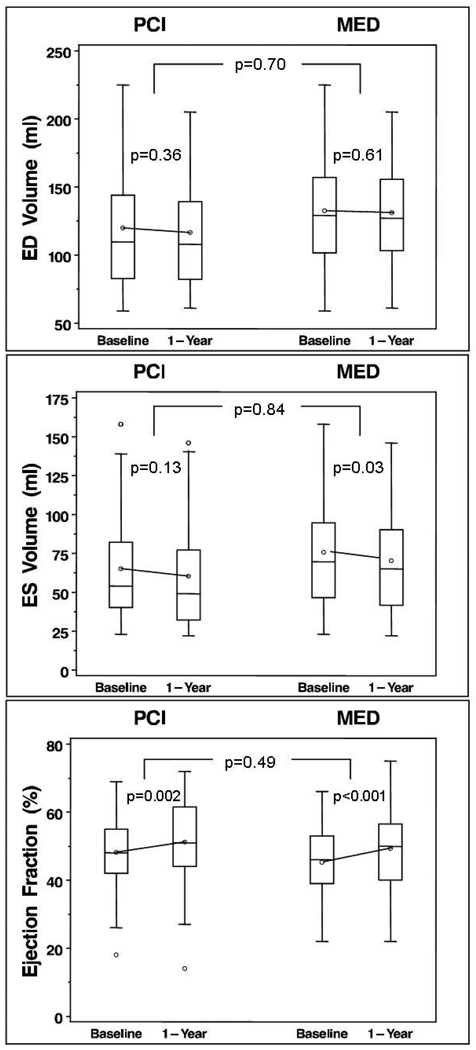
There were no significant differences in 1-year change in EDV, ESV, or EF when compared by treatment group PCI vs. MED (Figure 4 and Table 4). There was similar improvement in EF in both treatment groups (within-group changes both p<0.01). As-treated analysis accounting for 12 failed PCIs, 1 PCI not done, and 2 MED crossovers to PCI of IRA <30 days did not change these estimates.
Figure 4.
One year changes in end-diastolic (ED) and end-systolic (ES) volumes and ejection fraction, in patients who were randomized to the OAT PCI strategy compared to those randomized to optimal medical therapy (MED). There were no significant between-group changes in any of the parameters.
Table 4. REMODELING CHANGES BY TREATMENT GROUP.
| PCI§ | MED‖ | p-value (between groups) |
|||
|---|---|---|---|---|---|
| (N = 55) | (N = 57) | ||||
| n | Mean±SD | n | Mean±SD | ||
| LV ED Volume (ml) | |||||
| Baseline | 53 | 118.9±44.1 | 52 | 132.7±42.7 | 0.11 |
| 1 yr | 53 | 115.2±43.5 | 52 | 131.0±42.3 | 0.06 |
| Change (1yr-Baseline) | 53 | −3.7±29.4 | 52 | −1.7±23.6 | 0.70 |
| p-value (within group) | 0.36 | 0.61 | |||
| LV ES Volume (ml) | |||||
| Baseline | 53 | 64.3±35.6 | 52 | 75.7±36.5 | 0.11 |
| 1 yr | 53 | 59.5±35.3 | 52 | 70.1±35.6 | 0.13 |
| Change (1yr-Baseline) | 53 | −4.8±22.3 | 52 | −5.6±18.5 | 0.84 |
| p-value (within group) | 0.13 | 0.03 | |||
| Ejection Fraction (%) | |||||
| Baseline | 53 | 48.7±11.0 | 52 | 45.2±11.0 | 0.11 |
| 1 yr | 53 | 51.8±13.0 | 52 | 49.3±11.8 | 0.31 |
| Change (1yr-Baseline) | 53 | 3.1±7.0 | 52 | 4.1±7.2 | 0.49 |
| p-value (within group) | 0.002 | <0.001 | |||
| Wall Motion Score | |||||
| Baseline | 53 | 16.8±8.5 | 53 | 18.7±7.9 | 0.26 |
| 1 yr | 53 | 11.6±9.7 | 53 | 13.6±8.7 | 0.27 |
| Change (1yr-Baseline) | 53 | −5.3±7.5 | 53 | −5.1±5.8 | 0.89 |
| p-value (within group) | <0.001 | <0.001 | |||
LV ED=left ventricular end-diastolic dimension,
LV ES =ventricular end-systolic dimension
WM=wall motion,
PCI=percutaneous coronary intervention,
MED=medical therapy alone
Interaction of Treatment and Baseline Viability on Remodeling Over 1 Year
No significant interactions were found for any of the measured remodeling changes, i.e. treatment effect for PCI vs. MED did not differ by baseline viability (Table 5). There was more improvement in EF among those with moderate compared to severely reduced viability in both PCI and MED groups. As-treated analyses did not change these estimates, and there were no interactions found with baseline viability analyzed as a continuous function.
Table 5. INTERACTION – REMODELING CHANGES BY BASELINE VIABILITY & TREATMENT GROUP.
| SEVERELY REDUCED VIABILITY < 40% |
MODERATE RETAINED VIABILITY ≥ 40% |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCI§ | MED‖ | PCI | MED | ||||||||
| (N = 13) | (N = 20) | (N = 42) | (N = 37) | Interaction | |||||||
| n | Mean±SD | n | Mean±SD | p-value | n | Mean±SD | n | Mean±SD | p-value | p-value | |
| LV ED Volume (ml) | |||||||||||
| 1 | 3 | ||||||||||
| Baseline | 12 | 153.2±52.2 | 7 | 158.8±39.9 | 0.75 | 41 | 108.9±36.4 | 5 | 120.0±37.4 | 0.19 | 0.75 |
| 1 | 3 | ||||||||||
| 1 yr | 12 | 142.6±54.1 | 7 | 152.1±44.2 | 0.61 | 41 | 107.2±36.9 | 5 | 120.7±37.9 | 0.12 | 0.82 |
| 1 | 3 | ||||||||||
| Change (1 yr-Baseline) | 12 | −10.7±26.1 | 7 | −6.7±19.2 | 0.64 | 41 | −1.7±30.3 | 5 | 0.74±25.3 | 0.71 | 0.90 |
| p-value (within group) | 0.18 | 0.17 | 0.72 | 0.86 | |||||||
| LV ES Volume (ml) | |||||||||||
| 1 | 3 | ||||||||||
| Baseline | 12 | 88.4±44.8 | 7 | 100.5±36.7 | 0.43 | 41 | 57.2±29.5 | 5 | 59.1±30.0 | 0.35 | 0.70 |
| 1 | 3 | ||||||||||
| 1 yr | 12 | 82.7±48.0 | 7 | 92.8±36.2 | 0.52 | 41 | 52.7±27.8 | 5 | 59.1±30.0 | 0.34 | 0.80 |
| 1 | 3 | ||||||||||
| Change (1 yr-Baseline) | 12 | −5.8±22.1 | 7 | −7.7±19.5 | 0.80 | 41 | 4.5±22.7 | 5 | 4.5±18.2 | 0.99 | 0.84 |
| p-value (within group) | 0.39 | 0.12 | 0.21 | 0.15 | |||||||
| Ejection Fraction (%) | |||||||||||
| 1 | 3 | ||||||||||
| Baseline | 12 | 44.8±12.6 | 7 | 38.9±10.3 | 0.18 | 41 | 57.2±29.5 | 5 | 48.3±10.1 | 0.52 | 0.35 |
| 1 | 3 | ||||||||||
| 1 yr | 12 | 45.9±16.8 | 7 | 40.4±10.2 | 0.28 | 41 | 52.7±27.8 | 5 | 53.6±10.0 | 0.96 | 0.27 |
| 1 | 3 | ||||||||||
| Change (1 yr-Baseline) | 12 | 1.2±8.6 | 7 | 1.5±5.9 | 0.89 | 41 | 4.5±22.7 | 5 | 5.3±7.6 | 0.32 | 0.68 |
| p-value (within group) | 0.65 | 0.30 | 0.21 | <0.001 | |||||||
| Wall Motion Score | |||||||||||
| 1 | 3 | ||||||||||
| Baseline | 12 | 23.2±7.8 | 7 | 24.6±6.5 | 0.60 | 41 | 49.9±10.4 | 5 | 15.6±6.8 | 0.71 | 0.81 |
| 1 | 3 | ||||||||||
| 1 yr | 12 | 17.7±10.4 | 7 | 19.6±8.6 | 0.59 | 41 | 53.5±11.3 | 5 | 10.5±7.2 | 0.71 | 0.75 |
| 1 | 3 | ||||||||||
| Change (1 yr-Baseline) | 12 | −5.5±7.7 | 7 | −5.0±6.1 | 0.84 | 41 | 3.7±6.5 | 5 | −5.1±5.8 | 0.96 | 0.89 |
| p-value (within group) | 0.003 | <0.001 | <0.001 | ||||||||
LV ED=left ventricular end-diastolic dimension,
LV ES =ventricular end-systolic dimension
WM=wall motion,
PCI=percutaneous coronary intervention,
MED=medical therapy alone,
Multivariable Analyses
In multivariable regression models predicting 1 year change in ESV, EDV, and EF separately, treatment assignment and baseline infarct size were not predictors after adjusting for all variables attaining significance levels for univariate association with the corresponding changes in outcome. However, increasing baseline viability as a continuous variable was a significant predictor for increasing change in EF following adjustment for clinical and angiographic characteristics, but not for change in EDV or ESV. Treatment with PCI was not a significant predictor in any of the multivariable models for remodeling changes in ESV, EDV, or EF (Table 6).
TABLE 6.
Univariate and Multivariable predictors of Remodeling Changes (1 yr-BL*)
| LV ED†VOLUME | N | Parameter Estimate | p |
|---|---|---|---|
| Univariate Predictors | |||
| PCI‡ Treatment | 105 | 2.02 | 0.70 |
| BL ED Volume (continuous) | 105 | −0.19 | 0.002 |
| BL Viability (continuous) | 105 | 0.44 | 0.22 |
| BL Infarct Size (continuous) | 105 | −0.15 | 0.35 |
| Prior CHF§ | 105 | 40.49 | 0.03 |
| Current Smoker | 105 | −12.62 | 0.02 |
| Systolic BP‖< 140 mmHg | 105 | −15.75 | 0.05 |
| Killip Class II–IV Index MI | 105 | −18.21 | 0.004 |
| Mitral Regurgitation 1–4 | 66 | 12.39 | 0.02 |
| IRA LAD# | 105 | −17.99 | 0.001 |
| Fasting Glucose / 10 | 102 | 1.31 | 0.04 |
| Multivariable Model* | N | Parameter Estimate | p |
| PCI Treatment | 102 | −4.98 | 0.28 |
| BL ED Volume (continuous) | 102 | −0.15 | 0.01 |
| Current Smoker | 102 | −14.95 | 0.002 |
| Fasting Glucose / 10 | 102 | 1.76 | 0.002 |
| IRA LAD | 102 | −14.81 | 0.006 |
| LV ES** VOLUME | N | Parameter Estimate | p |
| Univariate Predictors | |||
| PCI Treatment | 105 | −0.80 | 0.84 |
| BL ES Volume (continuous) | 105 | −0.18 | 0.001 |
| BL Viability (continuous) | 105 | 0.21 | 0.44 |
| BL Infarct Size (continuous) | 105 | −0.13 | 0.27 |
| Killip Class II–IV Index MI | 105 | −11.39 | 0.02 |
| IRA LAD | 105 | −13.06 | 0.002 |
| Multivariable Model* | N | Parameter Estimate | p |
| PCI Treatment | 105 | −1.25 | 0.75 |
| BL ES Volume (continuous) | 105 | −0.18 | 0.001 |
| EJECTION FRACTION | N | Parameter Estimate | p |
| Univariate Predictors | |||
| PCI Treatment | 105 | 0.96 | 0.49 |
| BL EF†† (continuous) | 105 | −0.08 | 0.19 |
| BL Viability (continuous) | 105 | 0.18 | 0.05 |
| BL Infarct Size (continuous) | 105 | −0.03 | 0.54 |
| Female | 105 | 4.52 | 0.008 |
| Multivariable Model* | N | Parameter Estimate | p |
| PCI Treatment | 105 | −1.20 | 0.36 |
| BL EF (continuous) | 105 | −0.20 | 0.004 |
| BL Viability (continuous) | 105 | 0.29 | 0.005 |
| Female | 105 | 5.06 | 0.003 |
Univariate predictors include those with p<0.10 and forced BL variables. ALL MV models: p=0.01 to remain. Mitral Regurgitation NOT in initial MV models. With Mitral Regurgitation in model, no significant predictors found. EDV At p=.05 to remain in model, predictors also included Prior CHF.ESV: At p=.05 to remain in model, predictors also included current smoking, fasting glucose, LAD IRA, and diastolic BP. EF: Same results with p=.05.
BL=baseline,
LV ED=left ventricular end-diastolic dimension,
PCI=percutaneous coronary intervention,
CHF=congestive heart failure,
BP=blood pressure,
IRA LAD=infarct-related artery left anterior descending coronary artery,
LV ES=ventricular end-systolic dimension,
EF=ejection fraction
DISCUSSION
The results of this study suggest that in stable patients studied in the subacute phase post-MI with a totally occluded IRA, there is no influence of baseline IZ viability on the extent of LV remodeling at one year. However, there was an improvement in EF over one year in patients with moderately-retained viability. There was no benefit of PCI on 1-year change in EF or volumes, and no interaction between the degree of retained viability and the extent of remodeling in patients randomized to PCI vs. optimal medical therapy alone. Thus, we did not find an important influence of IZ viability on remodeling, with or without revascularization, in the setting of contemporary background therapy.
The Open Artery Trial (TOAT) randomized 66 asymptomatic, stable patients with an anterior MI and proximal occlusion of the LAD to medical therapy or late stenting-approximately 1 month post-MI. The results documented greater ESV and EDV at 1-year follow-up in the PCI group when compared to medically treated patients17. In contrast to the main study findings, in a substudy of TOAT where viability was assessed utilizing cardiac magnetic resonance imaging, a significant relationship between viability and improvement in ESV and EF was seen in patients undergoing PCI, but not in medically-treated patients18.
At baseline, patients in OAT-NUC had a high rate of prescription of therapies documented to mitigate post-MI LV remodeling when compared to prior trials19. Importantly, OAT-NUC was a substudy of a randomized trial, with protocol-driven patient follow-up and optimization of medical care. This approach likely led to a reduction in the ability of non-pharmacologic factors to alter remodeling – a phenomenon previously reported20. Factors other than infarct zone viability that may contribute to post-MI remodeling, such as enhanced sympathetic drive and beta-adrenergic receptor down-regulation21, could not be readily assessed in this study. In addition, prior studies and meta-analyses have shown a more profound impact of retained viability and revascularization on remodeling and clinical outcomes in those patients with the greatest degree of LV functional impairment5,22,23. It is plausible that the patient population in OAT-NUC with relatively preserved baseline LV systolic function may have differed significantly from those in prior studies. The possibility also exists that the inability to detect an effect of IZ viability or PCI on subsequent EDV and ESV could have been due to the relatively brief duration of follow-up in OAT-NUC. The time course of recovery of viable myocardium can extend beyond 1 year20, with some clinical trials documenting improvement in LV systolic function or clinical outcomes after > 4 years 19,24–26.
Several authors have suggested that IZ viability and delayed revascularization have their greatest impact in those patients with the most severe or extensive ischemia 19,27. As OAT excluded patients with rest angina and /or severe inducible ischemia, the patient population in OAT-NUC differed from patients in prior studies in this regard. In addition, it is not clear from prior studies what proportion of patients actually had total occlusion of the IRA, which was a critical inclusion criterion of OAT 5,20, 22,23,28.
The Total Occlusion Study of Canada (TOSCA)-2 was a mechanistic substudy of OAT which documented that coronary stenting resulted in significantly higher rates of IRA patency at 1-year when compared to those patients solely treated medically29. Similar to OAT-NUC, both stented and medically-treated patients improved EF at 1-year follow-up LV angiography with no between-group difference. In a subset of 42% of TOSCA 2 patients with paired volumes measured one year apart, there was an apparent modest benefit of PCI on EDV index 29, although there was no significant between-group difference when compared with the medically-treated group. Multivariable analyses controlling for baseline LV volumes suggested that randomization to PCI therapy was an independent predictor of a small absolute increase in EDV index. Whether this finding would have been verified in the whole OAT cohort is unknown. This latter finding was not observed in the OAT NUC data. Several variables were found to be independently related to measures of remodeling in multivariable analyses (Table 6). Potential mechanisms underlying these noted associations are speculative, but would be of interest for generating testable hypotheses if confirmed in independent data sets.
Strengths of OAT-NUC include a central core lab, which performed systematic, standardized, blinded readings. The population represents an important subset of survivors of MI with the presented data providing the mechanistic underpinnings to explain the outcome in the main trial 1. There are also important limitations to the present data set. The population sample represents a nonconsecutive series of patients enrolled in the main OAT study who consented to participate in this substudy. Due to the limited number of enrolled patients in OAT-NUC (as well as the insufficient recruitment relative to initial projections) there was not enough power to detect small differences in remodeling between the comparison groups if indeed the study hypothesis is correct. Finally, it is conceivable that between-group differences may have become apparent had the patients been followed for a longer period of time. However, there was no signal of an apparent effect on remodeling in this population sample. The findings presented here are consistent with the absence of effect of PCI on clinical outcomes including heart failure hospitalization over 3.2 years average follow-up in the main OAT population1.
Hence, the data from the OAT-NUC study suggest that in the contemporary era of comprehensive post-infarction medical therapy, IZ viability does not influence LV remodeling nor the remodeling response to PCI, as opposed to medical therapy alone. The data also suggest that residual IZ viability fails to identify a subgroup of clinically stable post-MI patients with total occlusion of the IRA who will accrue a remodeling benefit over a one year observation as a consequence of revascularization during the subacute phase of MI.
Acknowledgments
FUNDING SOURCES
OAT was supported by National Heart, Lung, and Blood Institute Awards U01HL062509 and U01HL062511. OAT-NUC was funded by NIH Grant R01 HL075456 - Myocardial Viability & Remodeling in the Occluded Artery Trial. Supplemental funding was received by Dr. Camille A. Pearte, MD between 2006–2008 by a Research Supplement (Grant Number R01 HL 075456-04S1) to Promote Diversity in Health-Related Research PA-05-015. Supplemental grant funds and product donations equivalent to 6% of total study costs were received from: Eli Lilly, Millennium Pharmaceuticals and Schering Plough, Guidant, Cordis/ Johnson and Johnson, Medtronic, Merck and Bristol Myers Squibb Medical Imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no conflicts of interest to disclose related to this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH. We request the journal to acknowledge that the Author retains the right to provide a copy of the final manuscript to the NIH upon acceptance for Journal publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by Journal.
References
- 1.Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolognese L, Cerisano G, Buonamici P, et al. Influence of infarct-zone viability on left ventricular remodeling after acute myocardial infarction. Circulation. 1997;96:3353–3359. doi: 10.1161/01.cir.96.10.3353. [DOI] [PubMed] [Google Scholar]
- 3.Nijland F, Kamp O, Verhorst PM, et al. Myocardial viability: impact on left ventricular dilatation after acute myocardial infarction. Heart. 2002;87:17–22. doi: 10.1136/heart.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabia PJ, Powers ER, Ragosta M, et al. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–1831. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 5.Senior R, Lahiri A, Kaul S. Effect of revascularization on left ventricular remodeling in patients with heart failure from severe chronic ischemic left ventricular dysfunction. Am J Cardiol. 2001;88:624–629. doi: 10.1016/s0002-9149(01)01803-3. [DOI] [PubMed] [Google Scholar]
- 6.Hochman JS, Lamas GA, Knatterud GL, et al. Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150:627–642. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Faber TL, Cooke CD, Folks RD, et al. Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med. 1999;40:650–659. [PubMed] [Google Scholar]
- 8.Germano G, Kiat H, Kavanagh PB, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138–2147. [PubMed] [Google Scholar]
- 9.Christian TF, Gibbons RJ. Myocardial perfusion imaging in myocardial infarction and unstable angina. Cardiology Clinics. 1994;12:247–260. [PubMed] [Google Scholar]
- 10.Gibbons RJ, Miller TD, Christian TF. Infarct size measured by single photon emission computed tomographic imaging with (99m)Tc-sestamibi: A measure of the efficacy of therapy in acute myocardial infarction. Circulation. 2000;101:101–108. doi: 10.1161/01.cir.101.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Miller TD, Christian TF, Hopfenspirger MR, et al. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation. 1995;92:334–341. doi: 10.1161/01.cir.92.3.334. [DOI] [PubMed] [Google Scholar]
- 12.Udelson JE, Coleman PS, Metherall J, et al. Predicting recovery of severe regional ventricular dysfunction. Comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation. 1994;89:2552–2561. doi: 10.1161/01.cir.89.6.2552. [DOI] [PubMed] [Google Scholar]
- 13.Achtert AD, King MA, Dahlberg ST, et al. An investigation of the estimation of ejection fractions and cardiac volumes by a quantitative gated SPECT software package in simulated gated SPECT images. J Nucl Cardiol. 1998;5:144–152. doi: 10.1016/s1071-3581(98)90197-0. [DOI] [PubMed] [Google Scholar]
- 14.Iskandrian AE, Germano G, VanDecker W, et al. Validation of left ventricular volume measurements by gated SPECT 99mTc-labeled sestamibi imaging. J Nucl Cardiol. 1998;5:574–578. doi: 10.1016/s1071-3581(98)90111-8. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki T, Murase K, Tanaka H, et al. Assessment of left ventricular volume using ECG-gated SPECT with technetium-99m-MIBI and technetium-99m-tetrofosmin. J Nucl Med. 1997;38:53–57. [PubMed] [Google Scholar]
- 16.Barnett V, Lewis T. Outliers in Statistical Data. 3rd. New York: Wiley and Sons; 1993. pp. 78–81. [Google Scholar]
- 17.Yousef ZR, Redwood SR, Bucknall CA, et al. Late intervention after anterior myocardial infarction: effects on left ventricular size, function, quality of life, and exercise tolerance: results of the Open Artery Trial (TOAT Study) J Am Coll Cardiol. 2002;40:869–876. doi: 10.1016/s0735-1097(02)02058-2. [DOI] [PubMed] [Google Scholar]
- 18.Bellenger NG, Yousef Z, Rajappan K, et al. Infarct zone viability influences ventricular remodelling after late recanalisation of an occluded infarct related artery. Heart. 2005;91:478–483. doi: 10.1136/hrt.2004.034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbate A, Biondi-Zoccai GG, Appleton DL, et al. Survival and cardiac remodeling benefits in patients undergoing late percutaneous coronary intervention of the infarct-related artery: evidence from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:956–964. doi: 10.1016/j.jacc.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 20.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–114. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- 21.Spyrou N, Rosen SD, Fath-Ordoubadi F, et al. Myocardial Beta-Adrenoceptor Density One Month After Acute Myocardial Infarction Predicts Left Ventricular Volumes at Six Months. J Am Coll Cardiol. 2002;40:1216–1224. doi: 10.1016/s0735-1097(02)02162-9. [DOI] [PubMed] [Google Scholar]
- 22.Allman KC, Shaw LJ, Hachamovitch R, et al. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 23.Chareonthaitawee P, Gersh BJ, Araoz PA, et al. Revascularization in severe left ventricular dysfunction: the role of viability testing. J Am Coll Cardiol. 2005;46:567–574. doi: 10.1016/j.jacc.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA. 2007;297:1985–1991. doi: 10.1001/jama.297.18.1985. [DOI] [PubMed] [Google Scholar]
- 25.Horie H, Takahashi M, Minai K, et al. Long-term beneficial effect of late reperfusion for acute anterior myocardial infarction with percutaneous transluminal coronary angioplasty. Circulation. 1998;98:2377–2382. doi: 10.1161/01.cir.98.22.2377. [DOI] [PubMed] [Google Scholar]
- 26.Zeymer U, Uebis R, Vogt A, et al. Randomized comparison of percutaneous transluminal coronary angioplasty and medical therapy in stable survivors of acute myocardial infarction with single vessel disease: a study of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte. Circulation. 2003;108:1324–1328. doi: 10.1161/01.CIR.0000087605.09362.0E. [DOI] [PubMed] [Google Scholar]
- 27.Sabate M. Revascularization of the infarct-related artery: never too late to do well. J Am Coll Cardiol. 2008;51:965–967. doi: 10.1016/j.jacc.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Carluccio E, Biagioli P, Alunni G, et al. Patients with hibernating myocardium show altered left ventricular volumes and shape, which revert after revascularization: evidence that dyssynergy might directly induce cardiac remodeling. J Am Coll Cardiol. 2006;47:969–977. doi: 10.1016/j.jacc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 29.Dzavik V, Buller CE, Lamas GA, et al. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: the Total Occlusion Study of Canada (TOSCA)-2 trial. Circulation. 2006;114:2449–2457. doi: 10.1161/CIRCULATIONAHA.106.669432. [DOI] [PMC free article] [PubMed] [Google Scholar]