Summary
Dendritic exocytosis is required for a broad array of neuronal functions including retrograde signaling, neurotransmitter release, synaptic plasticity, and establishment of neuronal morphology. While the details of synaptic vesicle exocytosis from presynaptic terminals have been intensely studied for decades, the mechanisms of dendritic exocytosis are only now emerging. Here we review the molecules and mechanisms of dendritic exocytosis, and discuss how exocytosis from dendrites influences neuronal function and circuit plasticity.
Keywords: dendrite, dendritic spine, postsynaptic, AMPA receptor, trafficking, exocytosis, glutamate, neuronal polarity
Introduction
Fusion of intracellular membrane stores with the plasma membrane (PM) governs the molecular composition of the cell surface, influences cellular morphology and allows for the release of soluble factors (Gundelfinger et al., 2003; Horton and Ehlers, 2003; Lippincott-Schwartz, 2004; Sudhof, 2004). While constitutive exocytosis maintains the surface composition of integral PM proteins and lipids, many forms of exocytosis are regulated by molecular or electrical stimuli. The most intensely studied form of regulated exocytosis is neurotransmitter release triggered by electrical depolarization of presynaptic terminals (Sollner et al., 1993a; Sudhof, 2004). The exquisite sensitivity with which synaptic vesicle fusion can be measured and the robust biochemical preparations of synaptosomes, which harbor the requisite molecular machinery for vesicle fusion, have made this system the benchmark of regulated exocytosis (Blasi et al., 1993; Fried and Blaustein, 1976; Link et al., 1992; Nicholls and Sihra, 1986).
The study of neurotransmitter release has led to the discovery and functional characterization of many key molecules required for exocytosis, including the soluble N-ethyl maleimide (NEM)-sensitive factor attachment protein receptor protein family (SNAREs), which are involved not only in neurotransmitter release, but in nearly all forms of eukaryotic membrane fusion (Box 1) (Jahn and Scheller, 2006; Martens and McMahon, 2008; Sollner et al., 1993a; Sollner et al., 1993b). While there is an overwhelming abundance of literature on synaptic vesicle fusion in presynaptic terminals, much less is known about postsynaptic exocytosis, although it is increasingly recognized that exocytosis occurs from all dendrites and that dendritic membrane trafficking regulates diverse neuronal functions. This review focuses on the emerging mechanisms, machinery, and organelles of dendritic exocytosis with an emphasis on functional and morphological plasticity, retrograde signaling, and dendritic neurotransmitter release.
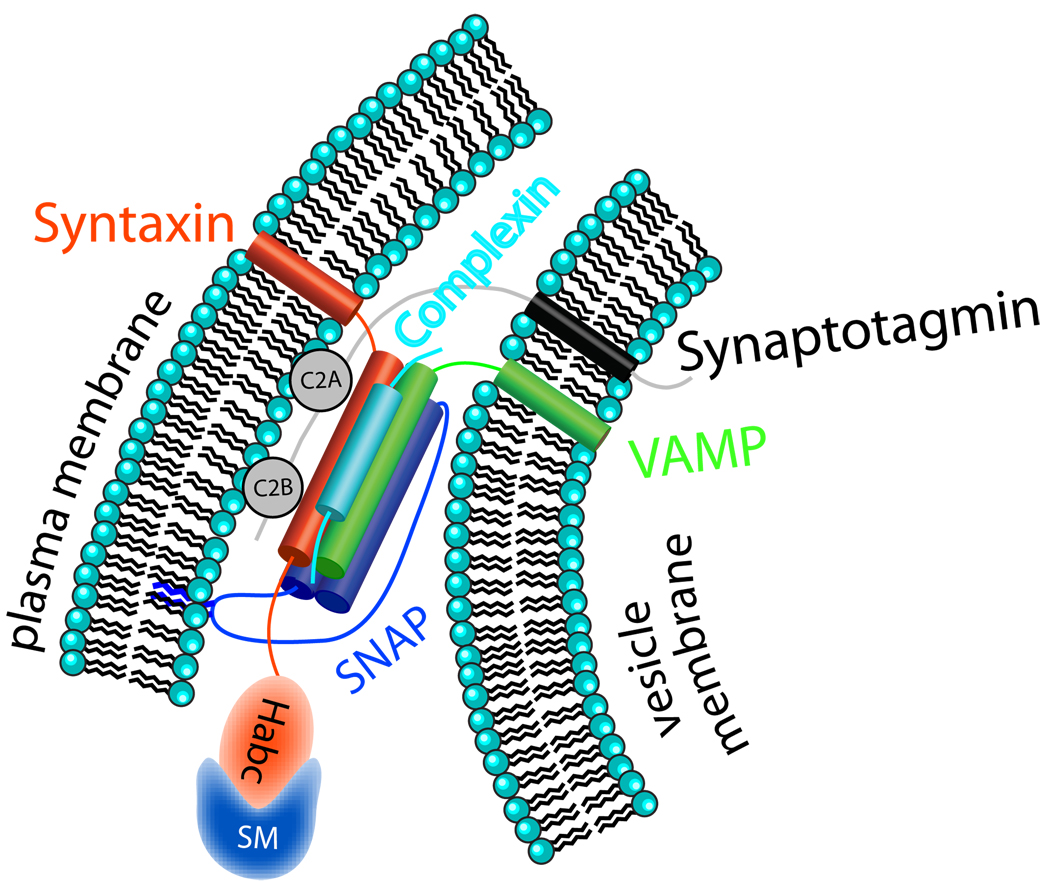
Box 1 Core SNARE Proteins in Pre- and Postsynaptic Exocytosis.
The NSF-sensitive attachment protein receptor (SNARE) family plays a role in a wide variety of membrane fusion mechanisms in diverse cell types (Figure). Communication across chemical synapses occurs by fusion of neurotransmitter vesicles mediated by the target membrane SNARE (t-SNARE) syntaxin 1 and the vesicular SNAREs (v-SNAREs) VAMP2/synaptobrevin and SNAP-25 (Martens and McMahon, 2008). SNARE domains from these three proteins form the tetrahelical core SNARE complex that drives membrane fusion. Synaptotagmins 1 and 2 act as Ca2+ sensors that initiate exocytosis upon Ca2+ entry into the terminal (Geppert et al., 1994; Sun et al., 2007). The Sec/Munc (SM) protein family member Munc18-1 binds to the N-terminal Habc domain of syntaxin and is required for neurotransmitter secretion (Verhage et al., 2000). Complexins I and II are thought to compete with synaptotagmins for SNARE bundle binding, possibly maintaining or clamping docked vesicles in a metastable state (Giraudo et al., 2009; Maximov et al., 2009; McMahon et al., 1995; Tang et al., 2006). Upon Ca2+ binding, synaptotagmin displaces complexin to trigger membrane fusion (Tang et al., 2006).
Box 1 Table.
Organelles for Dendritic Exocytosis
Electron micrographs of dendrites reveal a dense network of intracellular membranes, comprising all stages of the secretory pathway including endoplasmic reticulum (ER), Golgi membranes, endosomes and in some cell types, dense core vesicles situated throughout the dendritic arbor (Figure 1) (Cooney et al., 2002; Horton et al., 2005; Palay and Palade, 1955; Park et al., 2006; Pow and Morris, 1989; Spacek and Harris, 1997). Thus, dendrites possess the requisite cellular machinery for local, constitutive trafficking of lipids and newly synthesized membrane proteins through the canonical secretory pathway. However, non-canonical membrane trafficking pathways may also be utilized by neurons. For example, the highly convoluted ER extends throughout the somatodendritic compartment, and in some cases into dendritic spines (Spacek and Harris, 1997). A specialized smooth ER (SER)-derived organelle known as the spine apparatus (SA) is found in a large fraction of spines (Gray and Guillery, 1963). Small vesicular structures are often observed at the tip of the SA, raising the possibility that exocytic vesicles are derived directly from spine ER structures. Indeed, SER membrane can be found in close contact with the PM, suggesting direct fusion of ER membrane with the dendritic PM (Spacek and Harris, 1997). While this route to the PM has received little direct evidence beyond static EM micrographs, it would represent a unique, non-canonical trafficking route to the plasma membrane that bypasses Golgi membranes and may help explain how membrane proteins could be locally trafficked in dendrites lacking Golgi outposts. Interestingly the SA is missing in mice lacking synaptopodin, an actin-associated protein of unknown function that normally localizes to this organelle. Mice lacking synaptopodin have impaired hippocampal long-term potentiation (LTP) and spatial learning deficits, underscoring the importance of this SER-derived organelle (Deller et al., 2003).
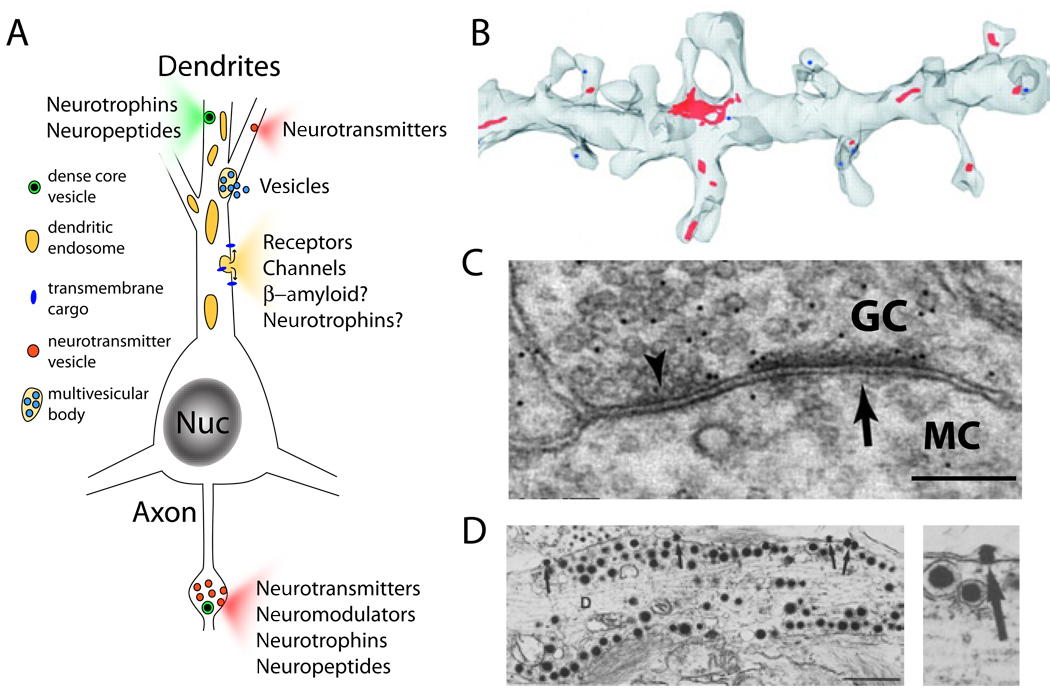
Figure 1. Dendritic Organelles for Exocytosis.
(A) Schematic of a neuron showing exocytosis from dendrites and axons. Dendritic secretory cargo includes soluble factors such as neurotransmitters, neuropeptides, and neurotrophins as well as transmembrane proteins including neurotransmitter receptors and ion channels.
(B) Serial section reconstruction of electron micrographs from hippocampal CA1 pyramidal neurons showing recycling endosomes in red. Note the presence of recycling endosomes in several of the dendritic spines. Reprinted with permission from Cooney et al. (2002).
(C) Electron micrograph of a dendrodendritic synapse between a granule cell (GC) and a mitral cell (MC) in the olfactory bulb. The inhibitory synaptic specialization is labeled with an arrowhead while the excitatory specialization is labeled with an arrow. Scale bar, 200 nm. Reprinted with permission from Lagier et al (2007).
(D) Dense core vesicles from presumptive vasopressin/oxytocinergic magnocellular neurons in the supraoptic nucleus of the hypothalamus. Note the presence of docked dense core vesicles at the dendritic plasma membrane and the presence of vesicle contents in the extracellular space (arrow, right panel). Scale bar, 1 µm. Reprinted with permission from from Pow and Morris (1989).
In addition to ER and Golgi-derived membranes, endosomes are abundant in dendritic arbors from diverse neuronal subtypes (Cooney et al., 2002) (Figure 1B). Endosomes are intracellular membranous compartments up to several microns in size that accept internalized vesicles from the plasma membrane and ultimately sort membrane-associated cargo for recycling back to the plasma membrane or for lysosomal degradation. The endosomal network comprises early/sorting endosomes (ESEs), recycling endosomes (REs), late endosomes (LEs) and lysosomes (Maxfield and McGraw, 2004). Newly formed vesicles originating form the plasma membrane fuse with one another and with ESEs, which have tubulovesicular morphology. ESEs then progress to LEs over the course of minutes as they become more acidic and gain hydrolase activity leading to degradation of remaining cargo (Maxfield and McGraw, 2004). Prior to the LE transition, cargoes destined for recycling exit ESEs and fuse directly with the plasma membrane or with REs (Dunn et al., 1989; Mayor et al., 1993). In dendrites, REs are present within or at the base of ~70% of spines (Cooney et al., 2002; Park et al., 2004), suggesting that localized endosomal trafficking takes place throughout dendrites and that a majority of synapses are associated with at least one nearby endosomal compartment (Figure 1B). Functional evidence for endosome involvement in trafficking synaptic molecules has come from studies on α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-type glutamate receptors (Beattie et al., 2000; Carroll et al., 1999; Ehlers, 2000; Lin et al., 2000). Direct activation of these receptors with AMPA leads to rapid internalization and degradation while brief activation of N-methyl D-aspartate (NMDA)-type glutamate receptors causes internalization and subsequent reinsertion of AMPA receptors into the dendritic PM (Ehlers, 2000). These data highlight the involvement of the dendritic endosomal network in AMPA receptor trafficking and point to REs as potential dendritic storehouses of synaptic proteins. While dendritic REs are ubiquitous in neurons throughout the brain, many specialized neuronal subtypes house dendritic vesicular structures resembling presynaptic neurotransmitter vesicles or dense core vesicles (Figures 1C and D).
Membrane-bound endosomal compartments that contain intact vesicles, called multivesicular bodies (MVBs), are also found in dendrites (Cooney et al., 2002; Saito et al., 1997; Spacek and Harris, 1997). While most cargo trafficked to MVBs is thought to be ultimately degraded by fusion with lysosomes, studies have also shown that the outer limiting membrane of MVBs can fuse with the plasma membrane releasing intact vesicles, or exosomes, to the extracellar space where they can be taken up by neighboring cells (Heijnen et al., 1999; Simons and Raposo, 2009; Trams et al., 1981). In diverse cell types MVB fusion is an emerging mechanism for intercellular transport of integral membrane proteins, soluble proteins and nucleic acids (Simons and Raposo, 2009). MVBs have been observed in dendrites and in presynaptic terminals where they can fuse with the plasma membrane to release intact vesicles, possibly as a mechanism for trans-synaptic transfer of signaling molecules (Cooney et al., 2002; Lachenal et al., 2010; Von Bartheld and Altick, 2011). While experimental evidence points to a role in presynaptic fusion of MVBs in shuttling WNT signaling molecules across the Drosophila neuromuscular junction (Korkut et al., 2009), the functional significance of dendritic MVB fusion remains unknown.
Neurotransmitter and Peptide Release from Dendrites
Early models of information flow through neuronal circuitry were based on the highly polarized morphology of individual neurons (Cajal, 1911; Golgi, 1873). Most neurons have elaborately branched dendrites and a single axon that courses from microns to tens of centimeters away from the cell body. This architecture led Cajal to the hypothesis that information travels uni-directionally from dendrites to axons, ultimately culminating in neurotransmitter vesicle fusion at axonal terminals. Although generally correct, later work has demonstrated many exceptions to this rule. Ultrastructural studies from a number of brain regions have revealed secretory vesicles in dendrites that contain glutamate, GABA, dopamine, and neuroactive peptides. In many cases, these vesicles closely resemble presynaptic vesicles in shape, size, and their tendency to cluster close to presumed sites of fusion (Famiglietti, 1970; Hirata, 1964; Lagier et al., 2007; Price and Powell, 1970a, b; Rall et al., 1966; Shanks and Powell, 1981).
Dendrodendritic synapses
Ultrastructural analysis of olfactory bulb, thalamus, and cortex revealed the presence of dense regions of uniform vesicles reminiscent of presynaptic neurotransmitter vesicles in dendrites (Famiglietti, 1970; Hirata, 1964; Lagier et al., 2007; Price and Powell, 1970a, b; Rall et al., 1966; Shanks and Powell, 1981). These sites are often in contact with other dendrites which themselves contain apposing vesicle-rich regions, suggesting that these connections are reciprocal (Figure 1C). Subsequent electrophysiological analyses have confirmed the functional release of both excitatory and inhibitory neurotransmitters from dendrodendritic synapses (Isaacson and Strowbridge, 1998; Jahr and Nicoll, 1980, 1982; Phillips et al., 1963).
Although dendrodendritic synapses have been observed in many neuronal subtypes in different brain regions, we will concentrate our discussion on the prototypical reciprocal synapse between granule and mitral cell dendrites in the olfactory bulb. Olfactory bulb granule cells were originally described by Camillo Golgi as an anomalous neuronal subtype that did not fall into his long or short axon categories. In fact, most granule cells do not appear to have an axon at all, but instead consisted of “protoplasmic elongations” that span several adjacent regions of dense neuropil in close contact with dendrites of mitral cells (Cajal, 1911; Golgi, 1875; Woolf et al., 1991b). It was not until the advent of electron microscopy and intracellular recording techniques that it was appreciated that granule cells, even without an axon, contain structures resembling synaptic vesicles and that they could exert a robust, long lasting inhibitory effect on contacting mitral cells upon depolarization (Green et al., 1962; Jahr and Nicoll, 1980; Phillips et al., 1963; Price and Powell, 1970a, b).
A combination of modeling, ultrastructural analysis, and electrophysiology has led to current models where depolarization of mitral cell dendrites triggers release of glutamate onto granule cell dendrites. Mitral cell glutamate release in turn triggers feedback release of GABA from sites within large granule cell spines onto mitral cell dendrites, inhibiting the activated mitral cell (Figure 2) (Isaacson and Strowbridge, 1998; Phillips et al., 1963; Rall et al., 1966; Schoppa et al., 1998). Even though granule cells lack axons, they do express voltage gated sodium channels and can fire action potentials that can back-propagate into dendrites (Chen et al., 2002; Jahr and Nicoll, 1982; Wellis and Scott, 1990). Thus, granule cell activation is thought to trigger widespread feedback inhibition onto mitral cells stimulated by sensory input, as well as feedforward inhibition of unstimulated mitral cells that are coupled to activated granule cells (Rall and Shepherd, 1968). On the other hand, action potentials are not required for granule cell GABA release since feedback inhibition of mitral cells still occurs even in the presence of tetrodotoxin (TTX) (Jahr and Nicoll, 1982). These data suggest that under weakly stimulating conditions subthreshold for action potential firing, mitral cells participate in local feedback inhibition only onto activated olfactory circuits via local dendritic depolarization (Egger et al., 2003; Isaacson and Strowbridge, 1998; Jahr and Nicoll, 1980; Woolf et al., 1991a).
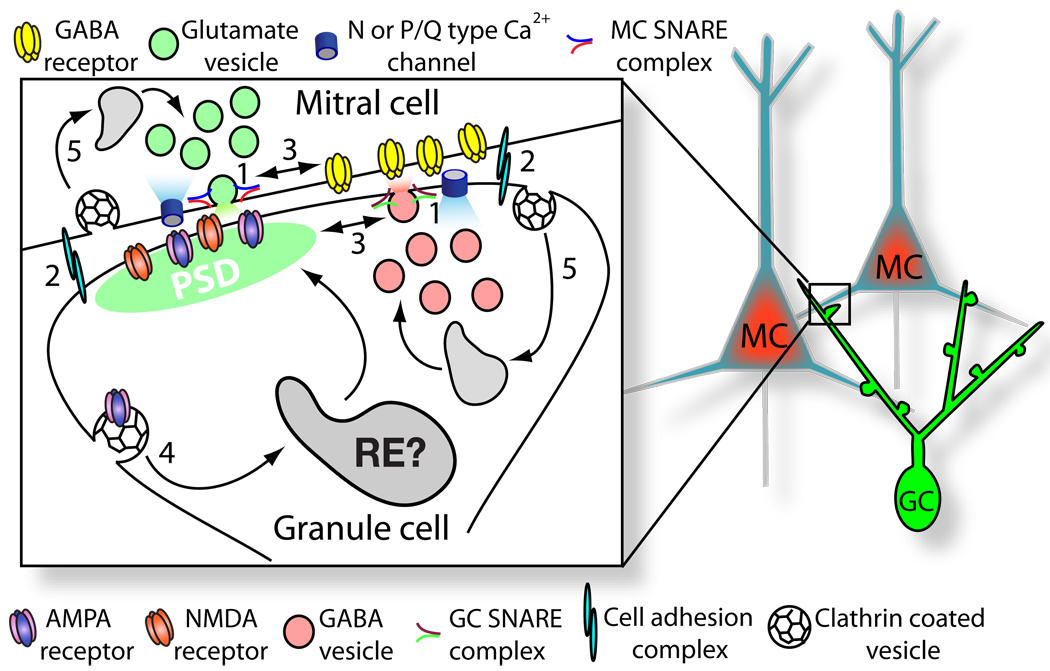
Figure 2. Schematic of a Dendrodendritic Synapse.
Schematic of a reciprocal synapse between a mitral cell (MC) and a granule cell (GC) in the main olfactory bulb. GABA-containing vesicles (pink) in granule cell spines are mobilized by glutamatergic input from mitral cells activating granule cell NMDA and AMPA receptors and Ca2+ entry through voltage-dependent Ca2+ channels. Reciprocal granule cell GABA release results in feedback inhibition of mitral cells. The numbers represent fundamental questions regarding the components of dendrodendritic synapses: (1) What are the SNARE proteins that drive GABA and glutamate vesicle fusion? (2) What cell adhesion molecules bridge dendrodendritic synapses? (3) Are there molecular tethers that keep postsynaptic receptors in proximity to vesicle release zones? (4) Are neurotransmitter receptors recycled at dendrodendritic synapses? (5) Does compensatory endocytosis occur near vesicle release sites to replenish neurotransmitter vesicles?
The reciprocal inhibition from granule to mitral cells is thought to play a role in shaping the output of the olfactory bulb, thus sculpting the representation of olfactory stimuli transmitted to piriform cortex and other higher brain centers (Shepherd et al., 2007). As in axons, dendritic release of GABA from granule cells requires intracellular Ca2+ (Isaacson, 2001; Isaacson and Strowbridge, 1998). To test the role of dendritic Ca2+ influx and neurotransmitter release in behavior, Abraham et al. (2010) recently augmented granule cell GABA release by conditionally disrupting the GluR2 AMPA receptor subunit specifically in granule cells. Consistent with the role of GluR2 in conferring Ca2+ impermeability to AMPA receptors (Burnashev et al., 1992; Hollmann et al., 1991; Verdoorn et al., 1991), removing granule cell GluR2 resulted in increased Ca2+ influx upon excitation from mitral cells, which in turn triggered more robust GABA release thus increasing mitral cell inhibition. At the behavioral level, this manipulation accelerated response latencies in odor discrimination tasks. Conversely, deleting the obligate NMDA receptor subunit NR1 in granule cells resulted in decreased GABA release and less robust mitral cell inhibition. Behaviorally, this reduction in dendritic GABA release slowed odor discrimination. Together demonstrating the importance of dendritic exocytosis in shaping olfactory sensory processing (Abraham et al., 2010).
While dendrodendritic synapses have been characterized anatomically and electrophysiologically, very little is known about the molecular composition of these synapses. How similar are dendrodendritic synapses to typical axo-dendritic synapses? Immunolabeling EM studies have revealed the presence of canonical glutamatergic postsynaptic scaffolding molecules PSD-93 and PSD-95 at granule/mitral cell dendrodendritic synapses, suggesting that these synapses resemble typical axo-dendritic synapses (Sassoe-Pognetto et al., 2003). Additionally, both AMPA receptors and NMDA receptors are found on granule cells at sites apposed to mitral cell dendritic vesicle release zones (Sassoe-Pognetto et al., 2003). Interestingly, NMDA receptor activation is sufficient to activate dendritic GABA release from granule cells (Chen et al., 2002; Halabisky et al., 2000; Schoppa et al., 1998). However, Ca2+ influx through NMDA receptors may not be directly coupled to vesicle release. Rather, Ca2+ influx through voltage gated N- or P/Q-type Ca2+ channels triggered by depolarizing NMDA receptor currents has been shown to mediate vesicle fusion (Isaacson, 2001). Similar to presynaptic axon terminals, Ca2+ influx into granule cells appears to be tightly coupled to vesicle fusion. Introduction of the slow Ca2+ chelator EGTA has no effect on GABA release from granule cells (Isaacson, 2001). This observation mirrors the differential effects of slow (EGTA) versus fast (BAPTA) Ca2+ chelators on vesicle release from presynaptic terminals, indicating that sites of presynaptic Ca2+ entry are tightly coupled to the vesicle release machinery (Adler et al., 1991).
From a cell biological perspective, the organization of dendrodendritic synapses raises the question of how specialized vesicle release and postsynaptic sub-domains are established and maintained in close proximity to each other in the same cell. Many fundamental questions regarding the formation and maintenance of these synapses remain unanswered (Figure 2). At presynaptic terminals, endocytosis is required for regenerating synaptic vesicles (Heuser and Reese, 1973; Robitaille and Tremblay, 1987). Is there a similar specialization near dendrodendritic active zones? In hippocampal neurons, an endocytic zone adjacent to the PSD is required for retaining and recycling glutamate receptors at individual synapses, disruption of which results in a loss of synaptic glutamate receptors (Blanpied and Ehlers, 2004; Blanpied et al., 2002; Lu et al., 2007; Petrini et al., 2009; Racz et al., 2004). Perhaps a similar endocytic domain is responsible for maintaining neurotransmitter receptors or regenerating dendritic vesicles at dendrodendritic synapses. If this is the case, how is segregation maintained between the recycling membrane pools containing neurotransmitter and the recycling membrane pools containing receptors? If the axon terminal and dendritic release machinery are the same, how do mitral cells subvert the normal polarized trafficking itinerary of axonal molecules required for neurotransmitter release and redirect them to dendrites? Are there components that bridge postsynaptic densities and vesicle release sites that are required to maintain these subdomains in close but non-overlapping proximity? What are the cell adhesion molecules that bridge the dendrodendritic synapse? The dendrodendritic synapse represents a remarkable exception to the normal rules of neuronal cell polarity, thus the answers to these questions will provide insight not only into the details of olfactory circuitry, but also general principles of how cellular subdomains are specified and maintained.
Dendritic dopamine release
Dopamine has been observed in a variety of dendritic organelles including large dense core vesicles, small synaptic vesicles, and tubulovesicular structures resembling smooth ER in dopaminergic neurons (Bjorklund and Lindvall, 1975; Nirenberg et al., 1996). Depletion of dendritic dopamine by reserpine treatment, a vesicular monamine transporter (VMAT) inhibitor, was the first evidence that dopamine in dendrites was a secreted, biologically active pool of neurotransmitter (Bjorklund and Lindvall, 1975; Nirenberg et al., 1996). Perhaps the best characterized neurons that exhibit dendritic dopamine release are dopamine neurons of the substantia nigra (SN), which have long striatal axonal projections. The anatomical separation of dendrites and axon terminals in the nigrostriatal circuit makes it possible to measure dendritic dopamine release with little contamination from axonal release. Interestingly, as with mitral cells of the olfactory bulb, SN dopamine neurons have long, sparsely branched dendrites, which would better support high-amplitude backpropagating spikes over long distances and may represent a common morphological principle for neurons that efficiently release neurotransmitters from their dendrites.
The first direct evidence for dendritic dopamine release came from experiments in brain slices directly measuring release of 3H-dopamine in SN upon potassium-evoked cell depolarization (Geffen et al., 1976). These results were confirmed in vivo using push-pull canula, microdialysis, and voltammetry in the SN and ventral tegmental area (VTA) (Cheramy et al., 1981; Jaffe et al., 1998; Kalivas and Duffy, 1988). Dopamine is also released from dendrites in more reduced preparations including dissociated culture (Fortin et al., 2006). Interestingly, when VMAT2 is exogenously expressed in dissociated hippocampal neurons, which don’t normally secrete dopamine from their dendrites or axons, robust dendritic dopamine release is observed (Li et al., 2005). This experiment reveals that existing activity-dependent secretory pathways in non-dopaminergic cells can be co-opted for dopamine release simply by expressing VMAT2, raising the question of what additional release factors might be required for dendritic release from SN dopamine neurons.
The mechanism of dendritic dopamine release has been controversial. One study suggested that dopamine release was not mediated by vesicular fusion, but by reversal of the dopamine transporter (DAT) upon activation of glutamatergic inputs (Falkenburger et al., 2001). However, other studies have shown that DAT inhibitors do not block dopamine release whereas Clostridial neurotoxins that cleave VAMP/synaptobrevin SNARE proteins (Box 1) block exocytosis of dendritic dopamine, confirming a role for vesicular fusion in dendritic dopamine release (Bergquist et al., 2002; Fortin et al., 2006; John et al., 2006). As further evidence for a vesicular mechanism, quantal release events can be recorded from the somatodendritic region of dopamine neurons using carbon fiber amperometry (Jaffe et al., 1998).
Although dendritic dopamine appears to be released by vesicle fusion, the mechanisms are distinct from axonal dopamine release. Both dendritic and axonal dopamine release are dependent on Ca2+, but dendritic release is sustained in extracellular Ca2+ levels below those required for axonal release, suggesting different Ca2+ sensors for dendritic and axonal exocytosis (Fortin et al., 2006). The voltage-gated Ca2+ channel coupled to dendritic dopamine release appears to be different from the axonal channel, perhaps explaining the differential release properties of dendrites and axons. Unlike axonal exocytosis, dendritic dopamine release is not blocked by P/Q- or N-type Ca2+ channel blockers (Bergquist et al., 1998; Bergquist and Nissbrandt, 2003; Chen et al., 2006; Chen and Rice, 2001). Although Ca2+ is clearly required for dendritic dopamine release, little is known about the Ca2+ sensor(s) that couple Ca2+ influx to dopamine vesicle fusion (Chen and Rice, 2001). Staining for the canonical axonal Ca2+ sensors synaptotagmin 1 and 2 revealed little signal in the somatodendritic compartment of SN dopamine neurons (Witkovsky et al., 2009). Staining for other typical axonal release molecules, including syntaxin1a/b, VAMP1, and synaptophysin, is also weak, suggesting a distinct ensemble of dendritic release factors. Indeed, VAMP2, SNAP25, and the plasma membrane t-SNARE syntaxin 3 are present throughout the somatodendritic compartment (Fortin et al., 2006; Witkovsky et al., 2009). However, the only functional evidence that any of these proteins are involved in dendritic dopamine release comes from experiments demonstrating sensitivity to botulinum toxin A, which cleaves SNAP-25 (Fortin et al., 2006). In addition to being a mechanism for release of neurotransmitters, peptides or other soluble factors, secretory granule fusion may serve as a mechanism for regulated delivery of specific transmembrane proteins to the dendritic plasma membrane. For example in axons, opioid receptors are localized to LDCVs containing neuropeptides and are co-inserted into the plasma membrane upon peptide release by LDCV fusion (Bao et al., 2003). Further studies will be required to determine whether co-insertion/secretion also serves as a mechanism for dendritic trafficking of membrane proteins.
Neuropeptide release from dendrites
Neuroactive peptides and peptide hormones are also released from dendrites. Dendritic vasopressin and oxytocin release from magnocellular neurons of the supraoptic nucleus have been studied intensely as this system offers a unique anatomical arrangement allowing independent measure of axonal and dendritic peptide release. These neurons span the blood brain barrier with their dendrites situated in and receiving input from the CNS, and their axons projecting into the peripheral hypophyseal portal circulation. Thus, axonal exocytosis from these neurons results in peripheral release of neuropeptide which, in the case of oxytocin, mediates reproductive physiology including milk ejection and uterine contractions while dendritically released oxytocin remains in the CNS where it can modify various social behaviors. At the ultrastructural level, the dendrites of these neurons are filled with large dense core vesicles (LDCVs), which are often in close association with the plasma membrane (Figure 1D). Pow and Morris (1989) directly observed LDCV fusion intermediate “omega structures” in these cells over 20 years ago. As with other forms of regulated dendritic exocytosis, fusion of LDCVs is regulated by Ca2+, although the mechanisms of Ca2+ entry are not firmly established. Interestingly, NMDA receptor activation alone in the absence of cell firing appears to be sufficient to drive somatodendritic release of oxytocin from dorsomedial supraoptic nucleus neurons (de Kock et al., 2004).
The activity requirements for dendritic release of oxytocin are distinct from those required for axonal release. While synaptic vesicles in axonal terminals fuse with high reliability upon cell spiking, dendritic LDCV fusion requires sustained Ca2+ elevation, and the actual fusion events are not tightly time locked to cell firing (Xia et al., 2009). Ca2+ release from intracellular stores by thapsigargin treatment or by oxytocin is sufficient to induce LDCV exocytosis and promotes priming of the releasable pool of LDCVs in dendrites. However, these treatments have no effect on axonal release (Ludwig et al., 2002; Tobin et al., 2004). Pretreatment with either oxytocin or thapsigargin enhances subsequent activity-triggered release of oxytocin supporting a feed forward mechanism of oxytocin release through binding of the Gq-coupled oxytocin receptor and subsequent activation of phospholipase-C and Ca2+ release from ER stores. This feed-forward enhancement lasts for tens of minutes suggesting that it is a form of plasticity that transiently lowers the threshold for peptide release (Tobin et al., 2004).
Dynorphin peptide exocytosis is another example where axonal and dendritic release is differentially regulated. Dynorphin is secreted from hippocampal granule cell dendrites and acts retrogradely through presynaptic κ-opioid receptors to inhibit neurotransmitter vesicle release from perforant path terminals (Drake et al., 1994). Dynorphin-mediated depression at perforant path synapses is blocked by both N-type and L-type Ca2+ receptor antagonists, but only N-type inhibitors block axonal release of dynorphin, demonstrating a distinct role for L-type Ca2+ channels in dendritic exocytosis (Simmons et al., 1995). The differential activity requirements for dendritic versus axonal release is a common theme in various subtypes of peptide-secreting neurons and suggests the presence of distinct dendritic release machinery that can respond to Ca2+ from different sources to trigger vesicle release. However, the release machinery, including the complete cast of SNARE proteins and Ca2+ sensors in dendrites and axons of peptidergic neurons remain to be identified.
Dendritic Exocytosis and Neuronal Morphology
Neurons are polarized cells with typically one axon housing the molecular machinery for neurotransmitter release and several dendrites containing receptors and signaling molecules necessary to respond to neurotransmitter. How neuronal polarity is established and how molecules are sorted, delivered and retained in neuronal subdomains remain central questions in cellular neurobiology (Barnes and Polleux, 2009). From the first steps of neurite outgrowth to maturity, the total membrane surface area of neurons can increase by several orders of magnitude, requiring massive amounts of membrane synthesis and mobilization to growing dendritic and axonal processes. Disruption of the endoplasmic reticulum (ER) – Golgi secretory pathway in developing neurons using pharmacologic or genetic methods prevents dendritic outgrowth in both mammals and insects (Horton et al., 2005; Ye et al., 2007), illustrating a conserved role for membrane addition in shaping developing neurons. However, when tetanus toxin (TeNT), a protease that cleaves most VAMP proteins, is added to dissociated cultures, dendritic arbor development is largely unaffected even after weeks of exposure to TeNT (Harms and Craig, 2005). Furthermore, neuronal morphology was normal in animals lacking VAMP2 or SNAP25 (Schoch et al., 2001; Washbourne et al., 2002). These observations may be reconciled by data demonstrating that a toxin insensitive VAMP family member, Ti-VAMP/VAMP7, is involved in neurite outgrowth in differentiating PC12 cells (Burgo et al., 2009; Martinez-Arca et al., 2001). Whether Ti-VAMP/VAMP7 plays a role specifically in axon or dendritic outgrowth, or whether a SNARE-independent pathway exists for neuronal development, remain open questions.
Once established, neuronal polarity and morphology are maintained for months or years in spite of rapid turnover of cell membrane lipids and proteins. Demonstrating the importance of ongoing membrane trafficking in maintaining neuronal morphology, Horton et al. (2005), blocked the secretory pathway by disrupting trafficking at the level of the Golgi apparatus in mature neurons. This manipulation triggered a dramatic simplification of the dendritic arbor and a ~30% loss in total dendrite length after 24 hours, indicating that forward trafficking through the secretory pathway to the PM is required for maintenance of dendritic morphology. Consistent with results from Drosophila sensory neurons (Ye et al., 2007), axonal morphology of cortical and hippocampal neurons was not affected by blocking secretory trafficking (Horton et al., 2005), indicating that ongoing membrane trafficking through the canonical secretory pathway is selective for dendritic growth and stability, perhaps due to a switch in the directionality of polarized post-Golgi traffic and exocytosis from axons to dendrites (de Anda et al., 2005).
While the overall architecture of mature neurons is stable, dendrites from cortical neurons exhibit activity-dependent morphological plasticity, particularly during development. This is illustrated by experiments demonstrating the influence of sensory experience on cortical ocular dominance columns and whisker barrel columns. In both cases, dendrites from layer IV stellate neurons in regions bordering sensory deprived receptive fields orient themselves away from the deprived field, demonstrating the role of ongoing dendrite remodeling in shaping neuronal connectivity in response to experience (Datwani et al., 2002; Kossel et al., 1995). While future experiments will be necessary to determine how neuronal activity is coupled to experience-dependent changes in cellular morphology, it is likely that sensory input ultimately impinges upon factors influencing cytoskeletal rearrangement and exocytic trafficking to sculpt dendritic architecture important for circuit connectivity and sensory plasticity.
Neuronal Transcytosis
Once neuronal morphology and polarity is established, it must be maintained by sorting dendritic and axonal factors to their respective subcellular domains. In some cases, post-Golgi carriers deliver lipids and transmembrane components directly to either dendrites or axons. However, in many cases intracellular vesicles harboring presynaptic proteins, including VAMP2, synaptophysin, TrkA, and L1/NgCaM, are first exocytosed to the dendritic PM followed by endocytosis and subsequent transport to axons (Ascano et al., 2009; Sampo et al., 2003; Wisco et al., 2003; Yap et al., 2008). This circuitous mode of trafficking, termed transcytosis, was first discovered in capillaries where it was observed that circulating macromolecules could traverse the vascular epithelia to the interstitium (Pappenheimer et al., 1951). Another well-studied example of transcytosis is immunoglobulin A secretion from epithelial cells (Rojas and Apodaca, 2002). Later it was discovered that not only soluble factors, but also integral membrane proteins can be transferred from one end of polarized epithelial cells to the other via transcytosis (Bartles et al., 1987). In neurons, VAMP2 was among the first axonal proteins shown to undergo transcytosis on its journey to axonal terminals. Disrupting the VAMP2 endocytosis signal leaves it stranded at the somatodendritic PM, demonstrating directly that VAMP2 is initially trafficked to the somatodendritic PM and that a subsequent endocytosis step is required to redirect it to axons (Sampo et al., 2003).
Trafficking of axonal molecules to the dendritic PM raises the intriguing possibility that these molecules are not simply passive bystanders on their way to the axon, but may actually perform postsynaptic functions even though their steady-state levels are higher in axons. For example, does VAMP2 reside on vesicles harboring postsynaptic factors such as neurotransmitter receptors? Does VAMP2 participate in exocytosis to the dendritic PM or is it merely hitchhiking on vesicles directed to the dendritic PM by a different VAMP? Intriguingly, NEEP21, a factor residing on dendritic recycling endosomes involved in AMPA receptor trafficking (Steiner et al., 2005), also influences the appropriate targeting of L1/NgCaM to axons, suggesting that L1/NgCaM may be temporarily co-transported in AMPA receptor-containing endosomes (Steiner et al., 2002; Yap et al., 2008). Although more experiments are needed to define the roles of “presynaptic” molecules in dendrites, transcytosis could be an economical way for neurons to utilize the same factors for both pre- and postsynaptic vesicle trafficking.
Dendritic Exocytosis of Amyloid β
Neuronal release of amyloid beta (Aβ) is implicated in the pathophysiology of Alzheimer’s disease (AD) (Haass and Selkoe, 2007; Palop and Mucke, 2010). Pathogenic Aβ is released from both presynaptic terminals and dendrites (Cirrito et al., 2005; Kamenetz et al., 2003; Wei et al., 2010). Interestingly, endocytosis of the amyoid precursor protein (APP) is required for proteolytic conversion to pathogenic Aβ (Cirrito et al., 2008; Dash and Moore, 1993; Marquez-Sterling et al., 1997), and APP is found in the somatodendritic compartment of neurons (Allinquant et al., 1994), suggesting that at least a fraction of APP is first delivered to the somatodendritic plasma membrane, and subsequently endocytosed and either processed into Aβ and retained in dendrites, or processed while it is trafficked to axon terminals through a transcytotic mechanism. While most studies have focused on Aβ release from presynaptic terminals, a recent study demonstrated that Aβ is also released from dendrites and that dendritic Aβ release decreases the number of excitatory synapses not only on cells overexpressing APP, but also in neighboring cells up to 10 µm away (Wei et al., 2010). Furthermore, drugs that block action potentials (TTX) or NMDA receptors (AP5) rescued the reduction in spine number indicating that neuronal firing and NMDA receptor activity are required for the Aβ-induced synapse loss. Secretion of Aβ was reduced by TTX, indicating that neuronal firing is required for Aβ release. Several questions remain: what is the identity of the subcellular vesicles harboring Aβ prior to release? Is there any overlap with known secreted factors? Is presynaptic Aβ derived from APP trafficked first to dendrites, and then endocytosed, processed, and trafficked to terminals along with other presynaptic molecules that undergo transcytosis? Finally, what are the molecules that mediate the ultimate fusion and release of Aβ both pre- and postsynaptically? A careful dissection of Aβ release mechanisms will yield potential therapeutic targets that could limit pathogenic Aβ accumulation and will offer clues as to whether this pathway is altered in disease states. In this regard, it is interesting to note that the neuronal sortilin-like receptor 1 (SORL1, also known as SORLA and LR11) directs trafficking of APP into recycling pathways and is genetically associated with late-onset AD (Andersen et al., 2005; Rogaeva et al., 2007).
Postsynaptic Neurotrophin Exocytosis
Retrograde signaling from dendrites to presynaptic terminals has been implicated in synapse growth, function, and plasticity (Regehr et al., 2009). Among these retrograde factors are growth factors and neurotrophins including brain-derived neurotrophic factor (BDNF). Secreted BDNF binds to and activates TrkB receptors, which control a variety of cellular functions including gene regulation, synaptic transmission, and morphological plasticity (Lessmann et al., 1994; Lohof et al., 1993; Tanaka et al., 2008). Although it is widely accepted that BDNF is released from axon terminals (Altar et al., 1997; Conner et al., 1997; von Bartheld et al., 1996), several recent studies also suggest activity-triggered BDNF release from dendrites. In cultured hippocampal neurons, exogenously expressed BDNF fused to GFP localizes to punctate vesicular structures throughout the dendritic arbor (Dean et al., 2009; Hartmann et al., 2001; Kolarow et al., 2007; Kuczewski et al., 2008; Matsuda et al., 2009). Evidence for release of vesicular BDNF comes from experiments examining the intensity of BDNF puncta in dendrites following electrical stimulation or high K+ induced depolarization. BDNF-GFP puncta disappear within seconds following stimulation, suggesting vesicular exocytosis and release into the extracellular media (Hartmann et al., 2001).
Dendritic BDNF-GFP vesicle fusion requires Ca2+/calmodulin-dependent protein kinase type IIα (CaMKIIα), which is also required in postsynaptic neurons for induction of LTP, raising the possibility that BDNF release shares mechanisms with prototypical Hebbian synaptic plasticity (Kolarow et al., 2007). Activity-triggered BDNF release also requires dendritic depolarization by back-propagating action potentials, and voltage dependent Ca2+ channels have been implicated as the source of Ca2+ for BDNF release (Kuczewski et al., 2008). Although neuronal firing is required for dendritic BDNF release, the activity requirements appear to be distinct from axonal terminal release of BDNF (Matsuda et al., 2009). Low frequency cell spiking resulted in axonal vesicles partially fusing with the axolemma followed by quick retrieval and little BDNF release. However, under the same conditions, dendritic BDNF vesicles appeared to fully fuse with the PM, releasing their full complement of BDNF. Only after prolonged bursts of activity would BDNF vesicles in axon terminals fully fuse and release BDNF, consistent with terminal release of BDNF during epileptiform activity (Matsuda et al., 2009). However, the bulk of these studies relied on expression of exogenous BDNF-GFP. Whether endogenous BDNF follows the same trafficking rules remain to be determined.
Tanaka et al. (2008) showed that BDNF signaling through TrkB is involved in morphological changes that occur following glutamate uncaging over individual dendritic spines. Interestingly, glutamate uncaging immediately followed by postsynaptic cell spiking triggered a robust increase in spine volume that was much larger than glutamate uncaging alone. Spike-dependent spine growth persisted for 10–15 min following uncaging and required protein synthesis. Inhibitors of BDNF/TrkB signaling, including a blocking antibody and the Trk kinase inhibitor K252a, blocked spike-dependent spine growth, supporting a model where spiking elicits synthesis and secretion of BDNF, which acts in an autocrine manner to influence morphological plasticity (Tanaka et al., 2008).
The identity of the intracellular vesicular structures harboring BDNF have not been well defined. Dense core vesicles (DCVs) are thought to house a majority of BDNF at presynaptic terminals, but DCVs are rare in the dendrites of many central neurons thought to release BDNF. For example, numerous studies have used hippocampal pyramidal neurons as a model for dendritic BDNF release, yet ultrastructural analysis reveals only sparse if any DCVs in the dendrites of these neurons (Cooney et al., 2002; Hartmann et al., 2001; Kolarow et al., 2007; Kuczewski et al., 2008). Alternatively, BDNF could be directly mobilized to the PM in Golgi carriers or through a Golgi-to-endosome pathway, but more experiments are required to unravel the subcellular trafficking itinerary of dendritically-released neurotrophins.
Retrograde neurotrophic signaling has also been observed at the Drosophila neuromuscular junction (NMJ). Muscle derived factors are known to coordinate NMJ growth during development. For example, the BMP homologue glass bottom boat (Gbb) is secreted from muscle and binds to the presynaptic BMP receptor wishful thinking (Wit), which is known to initiate a BMP signaling cascade culminating in nuclear accumulation of P-Mad (McCabe et al., 2004; McCabe et al., 2003). Mutant animals lacking Gbb have smaller NMJs, disorganized presynaptic terminals, and lack nuclear accumulation of P-MAD, phenotypes that overlap with Wit-null animals. Evidence that Gbb acts in a retrograde manner comes from rescuing Gbb-null animals with a muscle-specific promoter, which results in restored synapse size, bouton number, and levels of nuclear P-MAD in motoneurons (McCabe et al., 2003).
Retrograde signaling has also been found to have robust effects on presynaptic vesicle release probability at the Drosophila NMJ. Blocking postsynaptic glutamate receptors by genetic deletion of GluRIIA initiates a compensatory increase in vesicle release probability that precisely offsets decreased postsynaptic responsiveness to glutamate (Petersen et al., 1997). Surprisingly, this retrograde signaling pathway could also be activated within minutes by acute introduction of the pharmacological glutamate receptor inhibitor philanthotoxin to NMJ preparations (Frank et al., 2006). This experiment demonstrates that pre- and postsynaptic compartments are in constant communication with one another and that changes in muscle responsiveness can be quickly compensated through modulating the probability of presynaptic vesicle release. It is not yet known whether this form of homeostatic plasticity requires vesicular fusion in muscle or whether a membrane permeable signal is generated that can freely diffuse from muscle to axon terminals.
A different form of retrograde plasticity at the Drosophila NMJ involves postsynaptic vesicular fusion. Following strong stimulation, the frequency of presynaptic spontaneous vesicle release increases for minutes (Yoshihara et al., 2005). Ca2+ is required for this effect and it is blocked at restrictive temperatures by postsynaptic expression of the temperature sensitive dynamin mutant shibirets1, which is required for compensatory endocytosis following vesicle fusion. These data suggest that ongoing endocytosis in muscle is required for this presynaptic effect, perhaps by generation of postsynaptic endocytic vesicles. Direct visualization of postsynaptic vesicles revealed that exocytosis does indeed occur near apposing presynaptic active zones (Yoshihara et al., 2005). Although the physiological significance of this form of plasticity is not fully delineated, these data suggest that vesicles in muscle fuse and release a soluble signal that traverses the synaptic cleft and signals to the presynaptic release machinery.
Postsynaptic Exocytosis and Synaptic Plasticity
Some of the first evidence that synaptic plasticity requires postsynaptic exocytosis came from experiments where various factors that inhibit SNARE-mediated membrane fusion were infused into postsynaptic neurons via a recording pipette (Lledo et al., 1998). Each of these factors, which included N-ethylmaleimide, botulinum toxin B, and a short peptide designed to interfere with the binding of NSF to SNAP, blocked long term potentiation (LTP) triggered by stimulating nearby Schaffer collateral axons. This early observation led to a model where intra-dendritic vesicles harboring AMPA-type glutamate receptors, fuse with the plasma membrane upon LTP induction. Synaptic strength increases as newly inserted AMPA receptors become incorporated into synapses (Newpher and Ehlers, 2008). In addition to functional plasticity, several studies have shown that postsynaptic exocytosis is critical for morphological plasticity at glutamatergic synapses (Kopec et al., 2006; Kopec et al., 2007; Park et al., 2004; Park et al., 2006; Yang et al., 2008). Dendritic spines, the micron-sized protrusions contacted by axonal terminals at excitatory synapses, stably increase their volume by ~2-fold following NMDA receptor activation (Matsuzaki et al., 2004). Infusion of botulinum toxin B, which cleaves VAMP family SNARE proteins, or expression of dominant negative SNARE proteins in postsynaptic neurons blocks stimulus-induced spine growth (Kopec et al., 2007; Park et al., 2006; Yang et al., 2008), indicating that morphological plasticity requires membrane fusion.
More recent experiments have sought to define the identity of the intracellular membrane stores, location of activity-triggered exocytosis, the cargo responsible for synapse potentiation, and the SNARE molecules involved in postsynaptic vesicle fusion. Serial reconstruction electron microscopy studies demonstrated the presence of membrane-bound structures, including recycling endosomes, throughout dendrites and in a large fraction of dendritic spines (Figure 1B) (Cooney et al., 2002). This observation, along with experiments demonstrating that AMPA receptors are endocytosed and reinserted upon synaptic activation, suggested that dendritic recycling endosomes are the internal membrane stores mobilized to the plasma membrane in response to LTP-inducing stimuli (Beattie et al., 2000; Carroll et al., 1999; Ehlers, 2000; Luscher et al., 1999). More direct evidence for RE involvement came from studies demonstrating that LTP was impaired when RE function was blocked using dominant negative versions of syntaxin 13 (Stx13) and the Eps15 homology domain protein Rme1/EHD1, thus establishing a direct link between REs and synaptic plasticity (Park et al., 2004). However, the exact location, kinetics, and activity requirements for postsynaptic exocytosis underlying synaptic plasticity remain controversial.
In initial efforts to determine where glutamate receptors are exocytosed, Adesnik et al. (2005), used a cell-impermeable photoreactive AMPA receptor inhibitor. By irreversibly inhibiting AMPA receptors on the cell surface, the exchange rate of surface AMPA receptors could be measured by recording synaptic or extrasynaptic AMPA currents originating from different regions of the neuron through a combination of electrical stimulation or glutamate uncaging. Surprisingly, exchange of synaptic AMPA receptors took place only after several hours, a timescale much slower than previously thought. In contrast, AMPA receptor currents measured at the cell body by glutamate uncaging recovered within minutes, suggesting more rapid cycling of receptors at the neuronal soma under basal conditions (Adesnik et al., 2005).
Efforts to directly visualize postsynaptic exocytosis following plasticity induction relied on the use of several different optical probes. The first optical demonstration of activity-triggered exocytosis in dendrites relied on the lipophilic styryl dye FM1-43, which partitions into the plasma membrane, and eventually into internal membrane stores upon endocytosis (Maletic-Savatic and Malinow, 1998). While FM dyes have mainly been used to monitor presynaptic vesicle fusion, long-term exposure of cultured neurons to FM1-43 resulted in dye uptake into postsynaptic compartments. This postsynaptic signal destains within minutes upon neuronal stimulation indicating postsynaptic vesicle fusion (Maletic-Savatic and Malinow, 1998). More recent optical probes have been largely based on superecliptic pHluorin (SEP), a pH-sensitive GFP variant, which is brightly fluorescent at neutral pH but is quenched in acidic endosomal lumen (Miesenbock et al., 1998). By analogy to presynaptic terminals, where synaptopHluorin (VAMP2-SEP) has been widely used to visualize glutamate vesicle release, SEP-labeled AMPA-type glutamate receptors have been used in a number of studies to visualize postsynaptic exocytosis (Araki et al., 2010; Jaskolski et al., 2009; Kennedy et al., 2010; Kopec et al., 2006; Kopec et al., 2007; Lin et al., 2009; Makino and Malinow, 2009; Patterson et al., 2010; Yudowski et al., 2007). There are four different AMPA receptor sub-types (GluA1-4) with synapses in adult hippocampal pyramidal cells containing heterotetramers composed of mainly GluA1/2 or GluA2/3 subtypes. Current models suggest that GluA2/3 receptors are constitutively trafficked to synapses while GluA1-containing AMPA receptors are trafficked to synapses in response to synaptic activity (Araki et al., 2010; Kopec et al., 2006; Passafaro et al., 2001; Shi et al., 2001). However reevaluation of this model may be necessary in light of a recent study that used a conditional knockout strategy to show that under basal conditions most AMPA receptor current is mediated by GluA1-containing receptors (Lu et al., 2009). Whether plasticity is altered in neurons in which various AMPA receptor subunits have been conditionally deleted remains to be seen.
Because GluA1 is thought to be trafficked to synapses during plasticity, most studies have used SEP-GluA1 as a reporter for activity-dependent AMPA receptor insertion (Kopec et al., 2006; Kopec et al., 2007; Lin et al., 2009; Makino and Malinow, 2009; Patterson et al., 2010; Petrini et al., 2009; Yudowski et al., 2007). One elegant study using fluorescence recovery after photobleaching (FRAP) demonstrated that constitutive exocytosis of SEP-GluA1 occurs in dendrites on the timescale of minutes (Petrini et al., 2009). In this study, the SEP-GluA1 signal was bleached over a large dendritic region. Newly exocytosed signal was isolated by repeatedly bleaching a small region at the boundary of the bleached region creating an optical barrier to prevent contamination of the recovery signal from laterally diffusing SEP-GluA1 from unbleached regions. Under these conditions, approximately twenty percent of the total signal recovered in 20 min indicating constitutive cycling of receptors and providing an optical correlate complementary to prior electrophysiology studies demonstrating that blocking postsynaptic exocytosis leads to a gradual rundown in synaptic AMPA receptor-mediated currents (Luscher et al., 1999).
SEP-GluA1 has also been used to study exocytosis following various forms of neuronal stimulation. Following exposure of neurons to 0 Mg2+/glycine, the frequency of SEP-GluA1 insertion events increases, implying that internal membrane-bound stores of GluA1 are mobilized by NMDA receptor activation (Yudowski et al., 2007). Conversely, including glutamate receptor blockers and TTX decreases the frequency of GluA1 exocytic events (Lin et al., 2009). SEP-GluA1 has also been used as a functional reporter to identify molecules involved in AMPA receptor insertion. For example, Lin et al., 2009 used an optical approach to demonstrate that 4.1N, which interacts directly with GluA1, is involved in GluA1 insertion. The interaction between 4.1N and GluA1 depends on phosphorylation at two serine residues (S816, S818) by PKC on the C-terminal tail of GluA1 by PKC. Mutation of these sites to alanine prevented GluA1/4.1N interaction and impaired GluA1 from reaching the cell surface. Loss and gain of function by shRNA and overexpression blocked and enhanced GluA1 insertion respectively. These data suggest that PKC regulates the GluA1/4.1N interaction, which is required for trafficking of GluA1 to the plasma membrane. In this and other studies (Lin et al., 2009; Makino and Malinow, 2009; Yudowski et al., 2007), SEP-GluA1 exocytic events were observed throughout the somatodendritic compartment, but not in dendritic spines. Although SEP-GluA1 inserted into the dendritic shaft can diffuse into nearby spines (Yudowski et al., 2007), receptor levels quickly re-equilibrate to near basal levels within seconds suggesting that newly inserted GluA1 is either not efficiently trapped in spines, the number of trapped receptors are too few to quantify, or that GluA1 is quickly endocytosed upon spine entry and is trapped in internal spine endosomes. This is a counterintuitive result considering that enrichment of surface GluA1-containing AMPA receptors at synapses is thought to be a principle mechanism for LTP (Hayashi et al., 2000; Shi et al., 1999).
More selective activity manipulations, including 2-photon glutamate uncaging at individual dendritic spines also revealed that SEP-GluA1 is inserted in the dendritic shaft near and within activated spines (Makino and Malinow, 2009; Patterson et al., 2010). Whole cell voltage clamp recordings performed while uncaging glutamate over a spine or the adjacent shaft showed that the amplitude of uncaging-induced excitatory postsynaptic currents (uEPSCs) increases first in spines and then in the adjacent dendritic shaft following LTP induction (Makino and Malinow, 2009). These findings indicate that AMPA receptor content, conductance, or both increase in spines before an increase is seen in the shaft, consistent with insertion of glutamate receptors directly in the activated spine. Alternatively, fast diffusion of dendritic AMPA receptors to activated spines could take place, followed by gradual replenishment of AMPA receptors by dendritic exocytosis. Unexpectedly, the relative amplitudes of dendritic uEPSCs were as large or larger than spine uEPSCs and remained elevated over baseline levels for at least 10 min following LTP induction, a surprising result given that AMPA receptors are thought to be enriched at synapses (Tarusawa et al., 2009). This finding suggests a higher sustained extrasynaptic concentration of dendritic AMPA receptors than previously appreciated. One corollary of the sustained increase in extrasynaptic AMPA currents following single spine LTP is that newly inserted dendritic AMPA receptors have limited mobility following exocytosis (Makino and Malinow, 2009). One alternative scenario is that exocytosis of a membrane-associated “synaptic tag” marks potentiated synapses for incorporation of AMPA receptors derived from the existing pool of surface receptors via lateral diffusion. Although the identity of such synaptic tags and the complement of molecules co-transported with AMPA receptors are unknown, such a model could explain how postsynaptic exocytosis contributes to LTP through recruiting an existing pool of surface AMPA receptors.
Further addressing this point, a recent study demonstrated that exocytosis of AMPA receptor-containing endosomes occurs within spines, immediately adjacent to the PSD (Kennedy et al., 2010). This study used an optical reporter for recycling endosome fusion based on transferrin receptor, a classic marker for recycling endosomes, to demonstrate that recycling endosomes present within spines fuse in all-or-none events with the spine plasma membrane (Figure 3). In contrast to most previous studies, these experiments demonstrate directly that SEP-GluA1 housed in TfR-positive endosomes can be inserted into the spine plasma membrane and is retained in spines for at least several minutes following exocytosis. In contrast, co-exocytosed TfR from the same endosome quickly diffuses out of the spine head within seconds, demonstrating selective AMPA receptor retention at or near synapses (Borgdorff and Choquet, 2002; Ehlers et al., 2007; Kennedy et al., 2010; Tardin et al., 2003). In a more recent study, spine exocytosis and trapping of newly inserted SEP-GluA1 was observed in response to glutamate uncaging at single synapses, indicating that spine exocytosis may play an important role in synaptic plasticity induced by both global and spatially restricted synaptic stimulation (Patterson et al., 2010). Surprisingly, exocytosis did not depend on CaMKIIα, whose activity is known to be required for NMDA receptor-dependent synaptic potentiation. Instead, postsynaptic exocytosis was mediated by the small GTPase Ras, which had been previously demonstrated to play a role in AMPA receptor mobilization during synaptic potentiation (Zhu et al., 2002).
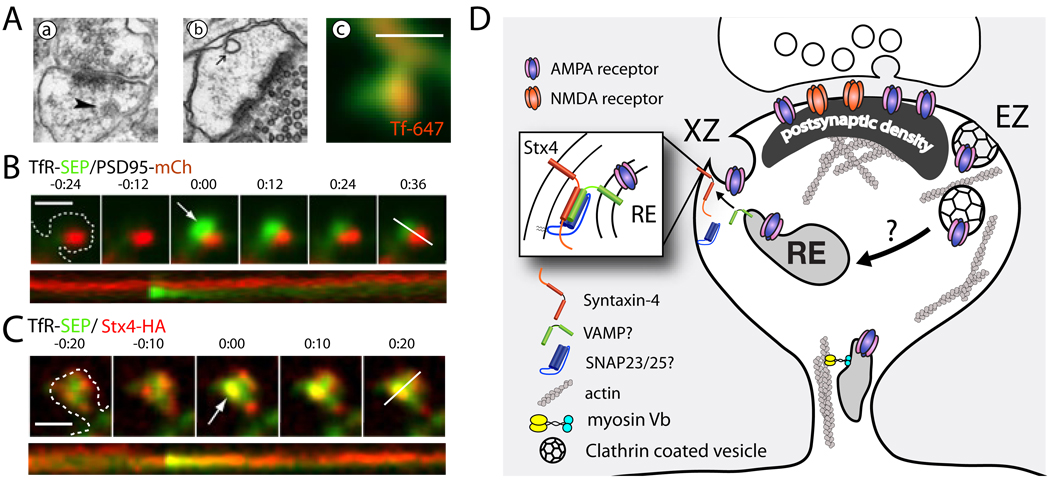
Figure 3. Postsynaptic Exocytosis and Synaptic Plasticity.
(A) Membrane cycling in spines. (a) Electron micrograph of a dendritic spine with a coated pit (arrowhead) indicating spine endocytosis. Reprinted with permission from Racz et al. (2004). (b) Uncoated vesicle within a spine head. Reprinted with permission from Cooney et al. (2002). (c) Spine endosomes can be loaded with exogenous transferrin (red), a marker for recycling endosomes indicating spine endosomes participate in ongoing membrane cycling. Scale bar, 1 µm. Reprinted from Kennedy et al. (2010) with permission.
(B) Exocytosis of the recycling endosome marker transferrin receptor fused to the pH-sensitive GFP variant superecliptic pHluorin (TfR-SEP, green) occurs in dendritic spines adjacent to the postsynaptic density labeled with PSD-95-mCherry. Kymograph of TfR-SEP (green) and PSD-95-mCh (red) channels is shown in the lower panel. Scale bar, 1 µm. Timestamp min:sec. Reprinted with permission from Kennedy et al. (2010).
(C) Exocytosis from recycling endosomes (TfR-SEP, green) occurs in dendritic spines at domains enriched for the plasma membrane t-SNARE Stx4 (red). Kymograph of TfR-SEP (green) and surface labeled Stx4 (red) is shown in the lower panel. Scale bar, 1 µm. Timestamp min:sec. Modified with permission from Kennedy et al. (2010).
(D) Schematic of postsynaptic membrane trafficking underlying synaptic plasticity. Highlighted is the exoctyic domain (XD) containing Stx4 where recycling cargo fuses with the spine membrane, and the endocytic zone (EZ) where clathrin-mediated endocytosis occurs. Stx4, syntaxin-4; RE, recycling endosome; PSD, postsynaptic density.
To summarize, studies using SEP-GluA1 as an optical reporter in dissociated cultures or in cultured slices have demonstrated activity-triggered insertion of GluA1 in dendrites following either local (Makino and Malinow, 2009) or global (Lin et al., 2009; Yudowski et al., 2007) synaptic activity, but currently only two studies have observed exocytosis directly within dendritic spines using SEP-GluA1 in response to local synaptic activity (Patterson et al., 2010) or TfR-SEP in response to global synaptic activity (Kennedy et al., 2010). An explanation for this discrepancy may be that expressed SEP-GluA1 traffics to only a small fraction of endosomes within spines (in contrast to endogenous GluA1, which is found in the majority of spine endosomes) making observation of spine exocytic events difficult when using SEP-GluA1 as a probe (Kennedy et al., 2010). This difference between expressed and endogenous receptor localization may be due to trafficking differences between expressed homomeric vs. native heteromeric receptors, or to incomplete stochiometry between expressed AMPA receptors and their accessory subunits (e.g. TARPS).
Molecules Mediating Activity-Triggered Postsynaptic Exocytosis
It has long been known that postsynaptic SNARE proteins are responsible for membrane fusion (Lledo et al., 1998), but the molecules mediating postsynaptic membrane fusion are only beginning to emerge. SNARE proteins, including the syntaxin, SNAP-23/25, and synaptobrevin/VAMP protein families, link intracellular vesicles to their target membranes and drive membrane fusion (Box 1) (Jahn and Scheller, 2006; Martens and McMahon, 2008). Of the ~15 members of the syntaxin family in mammalian cells (Teng et al., 2001), only four (Stx1-4) localize to the plasma membrane. While Stx1 localizes to presynaptic terminals, the role of other syntaxins in neurons is not well understood. A recent study demonstrated that of all the PM syntaxins, only Stx4 is enriched in spines (Kennedy et al., 2010), perhaps due to its unique ability to directly bind actin (Jewell et al., 2008). Imaging RE exocytosis in spines revealed that exocytosis occurs at spine microdomains enriched for syntaxin-4 (Stx4) clusters (Figures 3C and 3D) (Kennedy et al., 2010). Functional disruption of Stx4 blocks spine RE fusion and impairs LTP, indicating that Stx4 defines an exocytic domain in dendritic spines for synaptic plasticity.
Interestingly, Stx4 also plays a role in other forms of regulated exocytosis in diverse cell types. For example, Stx4 is involved in glucose-triggered insulin secretion from pancreatic β cells, IgE-dependent granule release from mast cells, and insulin-stimulated glucose receptor trafficking from adipose cells, highlighting a conserved role for Stx4 in different forms of regulated secretion (Mollinedo et al., 2006; Olson et al., 1997; Paumet et al., 2000; Saito et al., 2003; Spurlin and Thurmond, 2006; Volchuk et al., 1996; Yang et al., 2001). It is interesting to note the role of Stx4 in insulin-triggered glucose receptor exocytosis in adipocytes and muscle (Olson et al., 1997; Volchuk et al., 1996; Yang et al., 2001) since Passafaro et al. (2001) demonstrated that exposing neurons to insulin results in increased surface GluA1. Moreover, in developing Xenopus optic tectum, insulin receptor signaling regulates dendritic morphological plasticity and synapse number (Chiu et al., 2008). One possibility is that insulin mobilizes a selective pool of receptors, membrane, and synaptic molecules through a conserved signaling pathway involving Stx4 (Passafaro et al., 2001). The other SNARE proteins that partner with Stx4 to form the core SNARE complex for AMPA receptor trafficking during plasticity have yet to be determined. A VAMP family member is known to be involved based on experiments demonstrating that postsynaptic infusion of either botulinum toxin B or tetanus toxin blocks LTP (Lledo et al., 1998; Lu et al., 2001). However, because these toxins target many VAMP family members the identity of the VAMP family member(s) that controls postsynaptic exocytosis for LTP currently remains unknown.
A different SNARE protein, SNAP-25, participates in exocytosis of NMDA receptors in dendrites (Lan et al., 2001b; Lau et al., 2010). Lan et al., 2001b first demonstrated that activation of group I metabotropic glutamate receptors potentiates NMDA receptor surface experession in a Xenopus oocyte expression system. Botulinum toxin A, which specifically disrupts SNAP-25 blocked this effect, demonstrating a SNARE-dependent mechanism for regulated NMDA receptor trafficking. Lau et al., 2010 later demonstrated that SNAP-25 is a direct substrate of PKC, and that NMDA receptor insertion in response to PKC activation could be blocked by mutating a single serine residue (S187). In hippocampal neurons, introduction of PKM, a constitutively active isoform of PKC, potentiated NMDA currents and this effect was shown to be SNAP-25-dependent based on its sensitivity to botulinum toxin A. While many of these experiments were performed by bath application of NMDA, which does not differentiate between extrasynaptic and synaptic receptors, this study also used stimulation of mossy fibers in hippocampal slices to measure synaptic NMDA currents in CA3 pyramidal neurons (Lau et al., 2010) demonstrating that PKC activation induces NMDA receptor insertion and incorporation into synapses within minutes.
In a different study, Makino et al. (2009) demonstrated that GluA1 insertion triggered by global synaptic activity is sensitive to botulinum toxin A, implicating SNAP-25 in activity-dependent delivery of AMPA receptors to the plasma membrane. Spontaneous excitatory postsynaptic potentials measured in neurons from mice deficient for SNAP-25 display slightly decreased amplitude indicating that SNAP-25 may be involved in, but is not required for constitutive delivery of glutamate receptors to the plasma membrane (Bronk et al., 2007). The relative contribution of AMPA receptors vs. NMDA receptors in response to synaptic stimulation was not measured, but currents measured in response to NMDA application were normal in SNAP-25 deficient neurons indicating normal total surface levels of NMDA receptors (Washbourne et al., 2002).
A different SNAP family member, SNAP-23 has been shown to be enriched in dendritic spines and to localize at or near the PSD (Suh et al., 2010). Neurons from mice lacking SNAP-23 or RNAi knock-down of SNAP-23 reduced NMDA receptor surface expression and NMDA receptor currents, while loss of SNAP-25 had no effect. These findings provide strong evidence that SNAP-23 contributes to the exocytic machinery of dendrites, perhaps along with its known SNARE partner Stx4 (Paumet et al., 2000). SNAP-23 is also involved in the transport of NMDA receptors to the plasma membrane prior to synapse formation (Washbourne et al., 2004). Although the reasons for the differential involvement of SNAP-25 and SNAP-23 in NMDA receptor trafficking in different studies is unclear, it could reflect different stages in neuronal development, changes with chronic versus acute SNARE disruption, or multiple overlapping combinations of SNARE proteins that each contribute to NMDA receptor trafficking. In any case, these studies provide new clues in an unexplored area of glutamate receptor trafficking, which has largely focused on AMPA receptors. The physiological significance of PKC-dependent NMDA receptor exocytosis remains to be elucidated but one possibility is that PKC activation boosts synaptic NMDA receptor content, thus lowering the threshold for Hebbian forms of plasticity.
The exocyst family of proteins has also been implicated in postsynaptic membrane trafficking required for plasticity. The exocyst complex consists of eight members, including Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (Hsu et al., 2004; Lipschutz and Mostov, 2002). The exocyst was first discovered in yeast, where mutants of exocyst components disrupt polar secretion and cause accumulation of intracellular vesicles (Novick et al., 1980). Homologues of the exocyst complex are also found in the mammalian nervous system at subcellular domains of membrane addition, including growth cones, neurites, and filopodia (Hazuka et al., 1999; Vega and Hsu, 2001). Evidence for the involvement of the exocyst in AMPA receptor trafficking comes from experiments using dominant negative versions of the exocyst components Exo70 and Sec8 (Gerges et al., 2006). Disrupting Sec8 interfered with AMPA receptor targeting to synapses, but not total surface levels of AMPA receptors. Disrupting Exo70 interfered with trafficking of AMPA receptors to the cell surface and resulted in an accumulation of internal AMPA receptors in spines, providing evidence that Exo70 plays a role in late steps of AMPA receptor trafficking. A similar accumulation of endosomes was observed upon Stx4 disruption (Kennedy et al., 2010), suggesting that Exo70 and Stx4 may serve a coordinated function in dendritic spine exocytosis, although more experiments are needed to establish a direct link.
An important but unresolved question is the identity of the Ca2+ sensor(s) for activity-triggered postsynaptic exocytosis. Although it has long been appreciated that a rise in postsynaptic Ca2+ is necessary for LTP (Lynch et al., 1983) and sufficient to drive synaptic potentiation (Malenka et al., 1988), the full range of molecular sensors responsible is only recently emerging. A well-studied Ca2+ sensor required for LTP is CaMKIIα (Colbran and Brown, 2004; Lisman et al., 2002), which directly phosphorylates a number of postsynaptic proteins, including AMPA receptors (Barria et al., 1997). Exactly how Ca2+ and CaMKII are coupled to the mechanisms of membrane trafficking underlying LTP is not clear. The actin-based motor protein myosin Vb (MyoVb) is required for mobilizing AMPA receptor-containing endosomes for exocytosis following synaptic activity (Wang et al., 2008). The three dimensional conformation of MyoVb is sensitive to the level of Ca2+, with micromolar Ca2+ levels triggering a conformational change that exposes a binding motif for the endosomal adaptor protein Rab11-FIP2. Upon NMDA receptor activation and Ca2+ influx, MyoVb is recruited to REs and mobilizes them into actin-rich spines where they undergo exocytosis. Selective chemical-genetic inhibition demonstrated that acutely blocking MyoVb motor activity prevents LTP in hippocampal slices, suggesting that Ca2+ activates MyoVb to recruit endosomes to activated synapses. A different study suggests a role for myosinVa (MyoVa) in AMPA receptor mobilization to synapses following LTP (Correia et al., 2008). Expressing a dominant negative version of MyoVa or siRNA against MyoVa blocked LTP. However, synaptic plasticity deficits were not observed in mice lacking MyoVa (Schnell and Nicoll, 2001) indicating either compensation induced by lack of MyoVa during development or off-target effects of siRNA/dominant negative reagents used in Correia et al., 2008. Further experiments using conditional MyoVa alleles to disrupt MyoVa at later stages of development are needed to address this discrepancy.
In presynaptic terminals, synaptic vesicle fusion is triggered by influx of Ca2+, which directly binds C2 domains of synaptotagmin 1, thereby directly coupling elevated Ca2+ to SNARE-mediated exocytosis (Chapman, 2008). A recent study demonstrated that disrupting a different synaptotagmin family member, synaptotagmin 4 (Syt4), blocks retrograde signal-mediated plasticity at the Drosophila NMJ. Yoshihara et al. (2005) demonstrated that high frequency stimulation of muscle cells triggers an increase in the probability of presynaptic vesicle release. Animals null for Syt4 lack this form of retrograde signaling, which can be rescued by expressing Syt4 in muscle, suggesting that Ca2+ influx is coupled to postsynaptic vesicular trafficking. Interestingly, BDNF release from cultured mouse hippocampal neurons is also regulated by Syt4 (Dean et al., 2009). Syt4 localizes to BDNF-containing vesicles in dendrites. Expression of a pHluorin-tagged version of Syt4 allowed visualization of Syt4-containing vesicle fusion events, which increased upon depolarization. Moreover, neurons from Syt4 knockout mice displayed increased BDNF release compared to wild type neurons suggesting that Syt4 may actually play a negative role in postsynaptic exocytosis. Using an elegant co-culture method, this study also demonstrated that WT presynaptic terminals connected to Syt4-null neurons exhibit increased vesicle release probability, providing strong evidence that, as in Drosophila, Syt4 regulates retrograde signaling to modify presynaptic release probability (Dean et al., 2009; Yoshihara et al., 2005). Intriguingly, the quantal response amplitude was higher in Syt4-null neurons, indicating higher postsynaptic glutamate receptor content and raising the possibility that Syt4/BDNF positive vesicles also harbor AMPA receptors. Interestingly, although Syt4 plays a negative role in BDNF secretion in mammalian neurons, it appears to play a positive role in retrograde signaling at the Drosophila NMJ. It is notable that even though mammalian and Drosophila Syt4 are ~50% identical at the amino acid level, mammalian Syt4 does not show enhanced binding to phospholipids upon elevated Ca2+, while the Drosophila version does, providing a potential explanation for this difference (Wang and Chapman, 2010). Alternatively, in the absence of Syt4, a different, more efficient Ca2+ sensor could take its place, resulting in enhanced BDNF release and giving the appearance of a negative regulatory role for mammalian Syt4.
While Ca2+-influx through NMDA receptors is required to mobilize postsynaptic membrane fusion for LTP, it remains unknown whether Ca2+ acts directly at the level of postsynaptic membrane fusion. For example, postsynaptic exocytosis could be Ca2+-independent, but require upstream Ca2+-dependent signaling pathways. In many cell types, elevated cAMP levels are sufficient to drive exocytosis independent of Ca2+ through protein kinase A (PKA)-dependent pathways (Ammala et al., 1993; Hille et al., 1999; Knight et al., 1989). Interestingly, Ca2+ influx through activated NMDA receptors is known to trigger elevated cAMP levels and to activate PKA (Chetkovich et al., 1991; Frey et al., 1993), but whether the ultimate postsynaptic membrane fusion step necessary for expression of LTP requires a Ca2+ sensor such as synaptotagmin remains unknown.
Future Perspectives
Altering the composition of the postsynaptic plasma membrane is widely accepted as a principle mechanism of synaptic plasticity (Kerchner and Nicoll, 2008). While attention has focused on the insertion of AMPA receptors as a mechanism of plasticity at individual synapses, there are still many open questions regarding activity-triggered postsynaptic exocytosis. What cargo, besides AMPA receptors, is present in dendritic endosomes that could influence synaptic properties? While plasticity at individual synapses is mostly attributed to changes in glutamate receptor levels, recent experiments have demonstrated that dendritic segments tens of micrometers in length, containing multiple synapses, undergo activity-induced changes that locally increase or decrease excitability and alter their ability to propagate spatially concentrated synaptic input from a single dendritic branch to the soma (Frick et al., 2004; Losonczy et al., 2008). These forms of plasticity in dendritic excitability broaden traditional synaptocentric models of plasticity and implicate dendritic segments as novel loci for anatomical memory (Govindarajan et al., 2006).
The molecular mechanisms for dendritic branch plasticity are only emerging, but involve changes in the function and surface expression of ion channels including A-type K+ channels (Jung et al., 2008; Kim et al., 2007), voltage-gated Na+ channels such as Nav1.6 (Lorincz and Nusser, 2010), HCN channels mediating Ih current (Santoro et al., 2004), and others. In some cases, accessory molecules have been described that control channel trafficking (Lewis et al., 2009; Lin et al., 2010; Rhodes et al., 2004; Santoro et al., 2009; Shibata et al., 2003). It will be interesting to determine how vesicular trafficking regulates dendritic plasticity, whether ion channels that influence dendritic excitability are housed in the same classes of endosomes that are mobilized in response to activity, and whether dendritic endosomes migrate to dendritic segments with high synaptic activity. Finally, the complete cast of molecular components that enable dendritic exocytosis remain unknown. Using presynaptic vesicle fusion as a template, myriad SNARE proteins, SNARE protein regulators, Ca2+ sensors, and motor proteins involved in dendritic exocytosis almost certainly remain to be discovered. Uncovering such mechanisms promises to fuse cell biology with neuronal physiology to help define how dendrites compute and shape neural information.
Acknowledgements
We thank Ian Davison, Cyril Hanus, Juliet Hernandez, Angela Mabb, Tom Newpher, Chandra Tucker and Richard Weinberg for critical review of the manuscript. Work in the lab of MDE is supported by grants from the NIH and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NM, Egger V, Shimshek DR, Renden R, Fukunaga I, Sprengel R, Seeburg PH, Klugmann M, Margrie TW, Schaefer AT, et al. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron. 2010;65:399–411. doi: 10.1016/j.neuron.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allinquant B, Moya KL, Bouillot C, Prochiantz A. Amyloid precursor protein in cortical neurons: coexistence of two pools differentially distributed in axons and dendrites and association with cytoskeleton. J Neurosci. 1994;14:6842–6854. doi: 10.1523/JNEUROSCI.14-11-06842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Ammala C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993;363:356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Lin DT, Huganir RL. Plasma membrane insertion of the AMPA receptor GluA2 subunit is regulated by NSF binding and Q/R editing of the ion pore. Proc Natl Acad Sci U S A. 2010;107:11080–11085. doi: 10.1073/pnas.1006584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer BT, 3rd, Ozcelik T, Jahn R, Francke U, Sudhof TC. Structures and chromosomal localizations of two human genes encoding synaptobrevins 1 and 2. J Biol Chem. 1990;265:17267–17273. [PubMed] [Google Scholar]
- Ascano M, Richmond A, Borden P, Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J Neurosci. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, et al. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bartles JR, Feracci HM, Stieger B, Hubbard AL. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987;105:1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Jonason J, Pileblad E, Nissbrandt H. Effects of local administration of L-, N-, and P/Q-type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: a microdialysis study in rat. J Neurochem. 1998;70:1532–1540. doi: 10.1046/j.1471-4159.1998.70041532.x. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Niazi HS, Nissbrandt H. Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res. 2002;950:245–253. doi: 10.1016/s0006-8993(02)03047-0. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Nissbrandt H. Influence of R-type (Cav2.3) and t-type (Cav3.1-3.3) antagonists on nigral somatodendritic dopamine release measured by microdialysis. Neuroscience. 2003;120:757–764. doi: 10.1016/s0306-4522(03)00385-3. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- Burgo A, Sotirakis E, Simmler MC, Verraes A, Chamot C, Simpson JC, Lanzetti L, Proux-Gillardeaux V, Galli T. Role of Varp, a Rab21 exchange factor and TI-VAMP/VAMP7 partner, in neurite growth. EMBO Rep. 2009;10:1117–1124. doi: 10.1038/embor.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. Histologie du Syseme Nerveux de l'Homme et des Vertebres. 1911 [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- Chen BT, Moran KA, Avshalumov MV, Rice ME. Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca channels contrasted with strong regulation of axonal dopamine release. J Neurochem. 2006;96:645–655. doi: 10.1111/j.1471-4159.2005.03519.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Shen GY, Shepherd GM, Hines ML, Midtgaard J. Multiple modes of action potential initiation and propagation in mitral cell primary dendrite. J Neurophysiol. 2002;88:2755–2764. doi: 10.1152/jn.00057.2002. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Gray R, Johnston D, Sweatt JD. N-methyl-D-aspartate receptor activation increases cAMP levels and voltage-gated Ca2+ channel activity in area CA1 of hippocampus. Proc Natl Acad Sci U S A. 1991;88:6467–6471. doi: 10.1073/pnas.88.15.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN. Inhibitors of endocytosis, endosome fusion, and lysosomal processing inhibit the intracellular proteolysis of the amyloid precursor protein. Neurosci Lett. 1993;164:183–186. doi: 10.1016/0304-3940(93)90887-q. [DOI] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002;21:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Burnashev N, Lodder JC, Mansvelder HD, Brussaard AB. NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats. J Physiol. 2004;561:53–64. doi: 10.1113/jphysiol.2004.069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12:767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, et al. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger V, Svoboda K, Mainen ZF. Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells. J Neurosci. 2003;23:7551–7558. doi: 10.1523/JNEUROSCI.23-20-07551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM. Dendrodendritic inhibition through reversal of dopamine transport. Science. 2001;293:2465–2470. doi: 10.1126/science.1060645. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV., Jr Dendro-dendritic synapses in the lateral geniculate nucleus of the cat. Brain Res. 1970;20:181–191. doi: 10.1016/0006-8993(70)90287-8. [DOI] [PubMed] [Google Scholar]
- Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau LE. Basal somatodendritic dopamine release requires snare proteins. J Neurochem. 2006;96:1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Fried RC, Blaustein MP. Synaptic vesicle recycling in synaptosomes in vitro. Nature. 1976;261:255–256. doi: 10.1038/261255a0. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Garcia EP, Gatti E, Butler M, Burton J, De Camilli P. A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc Natl Acad Sci U S A. 1994;91:2003–2007. doi: 10.1073/pnas.91.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gerber SH, Rah JC, Min SW, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem. 2004;279:43870–43878. doi: 10.1074/jbc.M404982200. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C. Sulla struttura della grigia del cervello. Gaz Med Intalianna Lomb. 1873;6:244–246. [Google Scholar]
- Golgi C. Sulla fina struttura dei bulbi olfactorii. Riv Sper Freniatr Med Leg. 1875;1:66–78. [Google Scholar]
- Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- Gray EG, Guillery RW. A Note on the Dendritic Spine Apparatus. J Anat. 1963;97:389–392. [PMC free article] [PubMed] [Google Scholar]
- Green JD, Mancia M, von B. Recurrent inhibition in the olfactory bulb. I. Effects of antidromic stimulation of the lateral olfactory tract. J Neurophysiol. 1962;25:467–488. doi: 10.1152/jn.1962.25.4.467. [DOI] [PubMed] [Google Scholar]
- Gundelfinger ED, Kessels MM, Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat Rev Mol Cell Biol. 2003;4:127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hazuka CD, Foletti DL, Hsu SC, Kee Y, Hopf FW, Scheller RH. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Billiard J, Babcock DF, Nguyen T, Koh DS. Stimulation of exocytosis without a calcium signal. J Physiol. 1999;520(Pt 1):23–31. doi: 10.1111/j.1469-7793.1999.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y. Some Observations on the Fine Structure of the Synapses in the Olfactory Bulb of the Mouse, with Particular Reference to the Atypical Synaptic Configurations. Arch Histol Jpn. 1964;24:293–302. doi: 10.1679/aohc1950.24.293. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KAAMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, Dortland BR, Oorschot V, Govers R, Monti M, et al. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000283. e1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci U S A. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980;207:1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature. 1982;297:227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Jaskolski F, Mayo-Martin B, Jane D, Henley JM. Dynamin-dependent membrane drift recruits AMPA receptors to dendritic spines. J Biol Chem. 2009;284:12491–12503. doi: 10.1074/jbc.M808401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J Biol Chem. 2008;283:10716–10726. doi: 10.1074/jbc.M709876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Budygin EA, Mateo Y, Jones SR. Neurochemical characterization of the release and uptake of dopamine in ventral tegmental area and serotonin in substantia nigra of the mouse. J Neurochem. 2006;96:267–282. doi: 10.1111/j.1471-4159.2005.03557.x. [DOI] [PubMed] [Google Scholar]
- Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008;60:657–671. doi: 10.1016/j.neuron.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J Neurochem. 1988;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 Defines a Domain for Activity-Dependent Exocytosis in Dendritic Spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DE, von Grafenstein H, Athayde CM. Calcium-dependent and calcium-independent exocytosis. Trends Neurosci. 1989;12:451–458. doi: 10.1016/0166-2236(89)90095-7. [DOI] [PubMed] [Google Scholar]
- Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossel A, Lowel S, Bolz J. Relationships between dendritic fields and functional architecture in striate cortex of normal and visually deprived cats. J Neurosci. 1995;15:3913–3926. doi: 10.1523/JNEUROSCI.15-05-03913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa JL. Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci. 2008;28:7013–7023. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lagier S, Panzanelli P, Russo RE, Nissant A, Bathellier B, Sassoe-Pognetto M, Fritschy JM, Lledo PM. GABAergic inhibition at dendrodendritic synapses tunes gamma oscillations in the olfactory bulb. Proc Natl Acad Sci U S A. 2007;104:7259–7264. doi: 10.1073/pnas.0701846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001a;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Zheng X, Bennett MV, Zukin RS. Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J Neurosci. 2001b;21:6058–6068. doi: 10.1523/JNEUROSCI.21-16-06058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci. 2009;29:6250–6265. doi: 10.1523/JNEUROSCI.0856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–633. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lin L, Sun W, Wikenheiser AM, Kung F, Hoffman DA. KChIP4a regulates Kv4.2 channel trafficking through PKA phosphorylation. Mol Cell Neurosci. 2010;43:315–325. doi: 10.1016/j.mcn.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E, Edelmann L, Chou JH, Binz T, Yamasaki S, Eisel U, Baumert M, Sudhof TC, Niemann H, Jahn R. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189:1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. Dynamics of secretory membrane trafficking. Ann N Y Acad Sci. 2004;1038:115–124. doi: 10.1196/annals.1315.019. [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Mostov KE. Exocytosis: the many masters of the exocyst. Curr Biol. 2002;12:R212–R214. doi: 10.1016/s0960-9822(02)00753-4. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part I: trans-Golgi network-derived organelles undergo regulated exocytosis. J Neurosci. 1998;18:6803–6813. doi: 10.1523/JNEUROSCI.18-17-06803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH. Trafficking of cell-surface beta-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci. 1997;17:140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S, Coco S, Mainguy G, Schenk U, Alberts P, Bouille P, Mezzina M, Prochiantz A, Matteoli M, Louvard D, et al. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J Neurosci. 2001;21:3830–3838. doi: 10.1523/JNEUROSCI.21-11-03830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BD, Hom S, Aberle H, Fetter RD, Marques G, Haerry TE, Wan H, O'Connor MB, Goodman CS, Haghighi AP. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron. 2004;41:891–905. doi: 10.1016/s0896-6273(04)00073-x. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O'Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Calafat J, Janssen H, Martin-Martin B, Canchado J, Nabokina SM, Gajate C. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J Immunol. 2006;177:2831–2841. doi: 10.4049/jimmunol.177.5.2831. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Sihra TS. Synaptosomes possess an exocytotic pool of glutamate. Nature. 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, et al. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Palay SL, Palade GE. The fine structure of neurons. J Biophys Biochem Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Sudhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR, Renkin EM, Borrero LM. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG, Powell TP, Shepherd GM. Responses of Mitral Cells to Stimulation of the Lateral Olfactory Tract in the Rabbit. J Physiol. 1963;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP. The morphology of the granule cells of the olfactory bulb. J Cell Sci. 1970a;7:91–123. doi: 10.1242/jcs.7.1.91. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP. The synaptology of the granule cells of the olfactory bulb. J Cell Sci. 1970b;7:125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968;31:884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Rall W, Shepherd GM, Reese TS, Brightman MW. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966;14:44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, et al. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Tremblay JP. Incorporation of vesicular antigens into the presynaptic membrane during exocytosis at the frog neuromuscular junction: a light and electron microscopy immunochemical study. Neuroscience. 1987;21:619–629. doi: 10.1016/0306-4522(87)90147-3. [DOI] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–955. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Saito T, Okada S, Yamada E, Ohshima K, Shimizu H, Shimomura K, Sato M, Pessin JE, Mori M. Syntaxin 4 and Synip (syntaxin 4 interacting protein) regulate insulin secretion in the pancreatic beta HC-9 cell. J Biol Chem. 2003;278:36718–36725. doi: 10.1074/jbc.M305114200. [DOI] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron. 2009;62:802–813. doi: 10.1016/j.neuron.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Utvik JK, Camoletto P, Watanabe M, Stephenson FA, Bredt DS, Ottersen OP. Organization of postsynaptic density proteins and glutamate receptors in axodendritic and dendrodendritic synapses of the rat olfactory bulb. J Comp Neurol. 2003;463:237–248. doi: 10.1002/cne.10745. [DOI] [PubMed] [Google Scholar]
- Schnell E, Nicoll RA. Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J Neurophysiol. 2001;85:1498–1501. doi: 10.1152/jn.2001.85.4.1498. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci. 1998;18:6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer FE, Dresbach T, DeBello WM, O'Connor V, Augustine GJ, Betz H. Regulation of neurotransmitter release kinetics by NSF. Science. 1998;279:1203–1206. doi: 10.1126/science.279.5354.1203. [DOI] [PubMed] [Google Scholar]
- Shanks MF, Powell TP. An electron microscopic study of the termination of thalamocortical fibres in areas 3b, 1 and 2 of the somatic sensory cortex in the monkey. Brain Res. 1981;218:35–47. doi: 10.1016/0006-8993(81)90987-2. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–36454. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14:1265–1272. doi: 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic beta-cells. Mol Endocrinol. 2006;20:183–193. doi: 10.1210/me.2005-0157. [DOI] [PubMed] [Google Scholar]
- Steiner P, Alberi S, Kulangara K, Yersin A, Sarria JC, Regulier E, Kasas S, Dietler G, Muller D, Catsicas S, et al. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. 2005;24:2873–2884. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Sarria JC, Glauser L, Magnin S, Catsicas S, Hirling H. Modulation of receptor cycling by neuron-enriched endosomal protein of 21 kD. J Cell Biol. 2002;157:1197–1209. doi: 10.1083/jcb.200202022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Baumert M, Perin MS, Jahn R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989;2:1475–1481. doi: 10.1016/0896-6273(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci. 2010 doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnar E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009q;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng FY, Wang Y, Tang BL. The syntaxins. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-11-reviews3012. REVIEWS3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hurst G, Norrie L, Dal Rio FP, Bull PM, Ludwig M. Thapsigargin-induced mobilization of dendritic dense-cored vesicles in rat supraoptic neurons. Eur J Neurosci. 2004;19:2909–2912. doi: 10.1111/j.1460-9568.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci. 2001;21:3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Volchuk A, Wang Q, Ewart HS, Liu Z, He L, Bennett MK, Klip A. Syntaxin 4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell. 1996;7:1075–1082. doi: 10.1091/mbc.7.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bartheld CS, Altick AL. Multivesicular Bodies in Neurons: Distribution; Protein Content; and Trafficking Functions. Prog Neurobiol. 2011 doi: 10.1016/j.pneurobio.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Byers MR, Williams R, Bothwell M. Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature. 1996;379:830–833. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chapman ER. Rat and Drosophila synaptotagmin 4 have opposite effects during SNARE-catalyzed membrane fusion. J Biol Chem. 2010;285:30759–30766. doi: 10.1074/jbc.M110.137745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellis DP, Scott JW. Intracellular responses of identified rat olfactory bulb interneurons to electrical and odor stimulation. J Neurophysiol. 1990;64:932–947. doi: 10.1152/jn.1990.64.3.932. [DOI] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Folsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. J Cell Biol. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Patel JC, Lee CR, Rice ME. Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience. 2009;164:488–496. doi: 10.1016/j.neuroscience.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf TB, Shepherd GM, Greer CA. Local information processing in dendritic trees: subsets of spines in granule cells of the mammalian olfactory bulb. J Neurosci. 1991a;11:1837–1854. doi: 10.1523/JNEUROSCI.11-06-01837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf TB, Shepherd GM, Greer CA. Serial reconstructions of granule cell spines in the mammalian olfactory bulb. Synapse. 1991b;7:181–192. doi: 10.1002/syn.890070303. [DOI] [PubMed] [Google Scholar]
- Xia X, Lessmann V, Martin TF. Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J Cell Sci. 2009;122:75–82. doi: 10.1242/jcs.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Coker KJ, Kim JK, Mora S, Thurmond DC, Davis AC, Yang B, Williamson RA, Shulman GI, Pessin JE. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest. 2001;107:1311–1318. doi: 10.1172/JCI12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Wisco D, Kujala P, Lasiecka ZM, Cannon JT, Chang MC, Hirling H, Klumperman J, Winckler B. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J Cell Biol. 2008;180:827–842. doi: 10.1083/jcb.200707143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]