Abstract
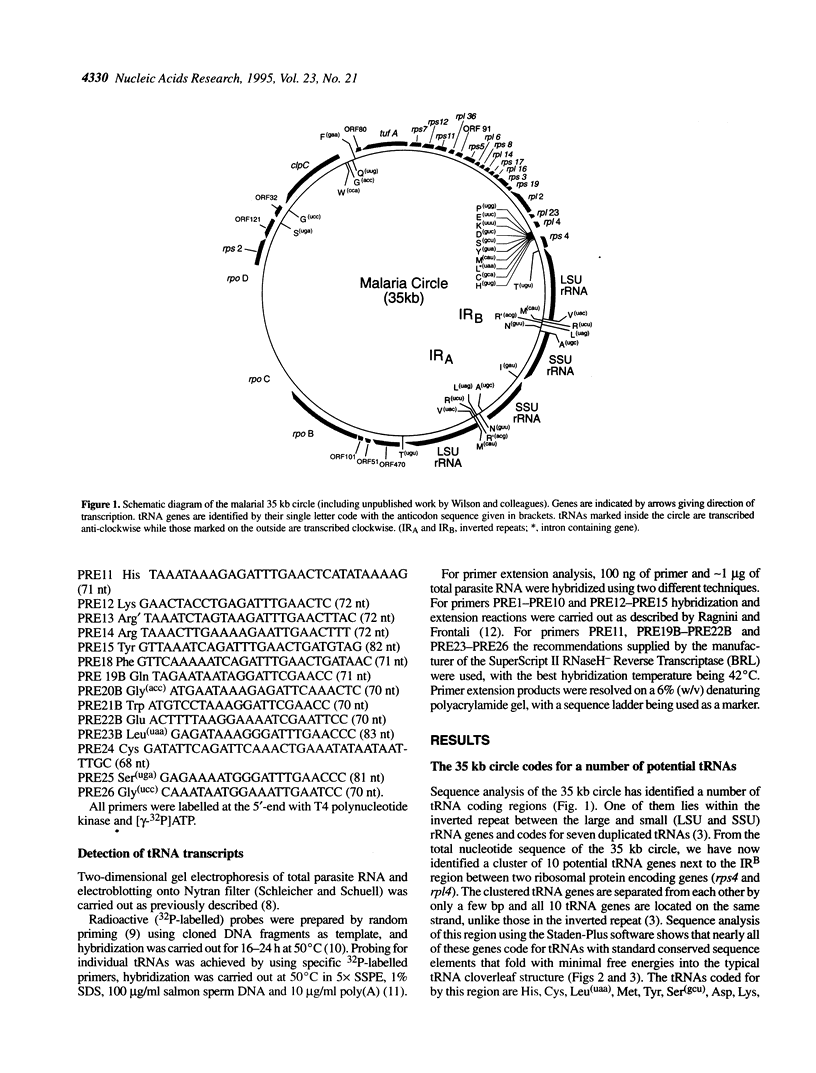
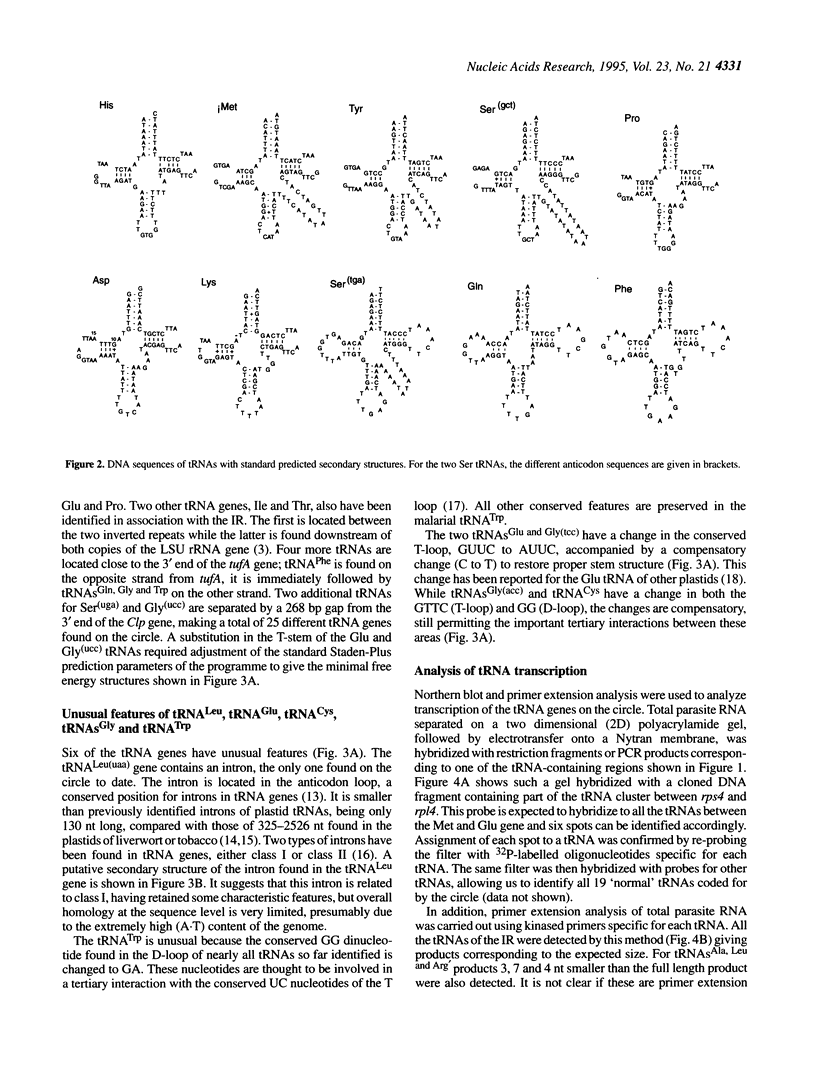
Besides their mitochondrial genome, malarial parasites contain a second organellar DNA. This 35 kb circular molecule has a number of features reminiscent of plastid DNAs. Sequence analysis shows that along with other genes the circle codes for 25 different tRNAs all of which are transcribed. Six of the tRNAs have some unusual features, and one has an intron, the only one found so far on the circle. Comparison of codon and anticodon usage indicates that the 25 tRNAs are sufficient to decode all the protein genes present on the circle. The maintenance of such a parsimonious but complete translation system is further evidence for the functionality of the circle.
Full text
PDF
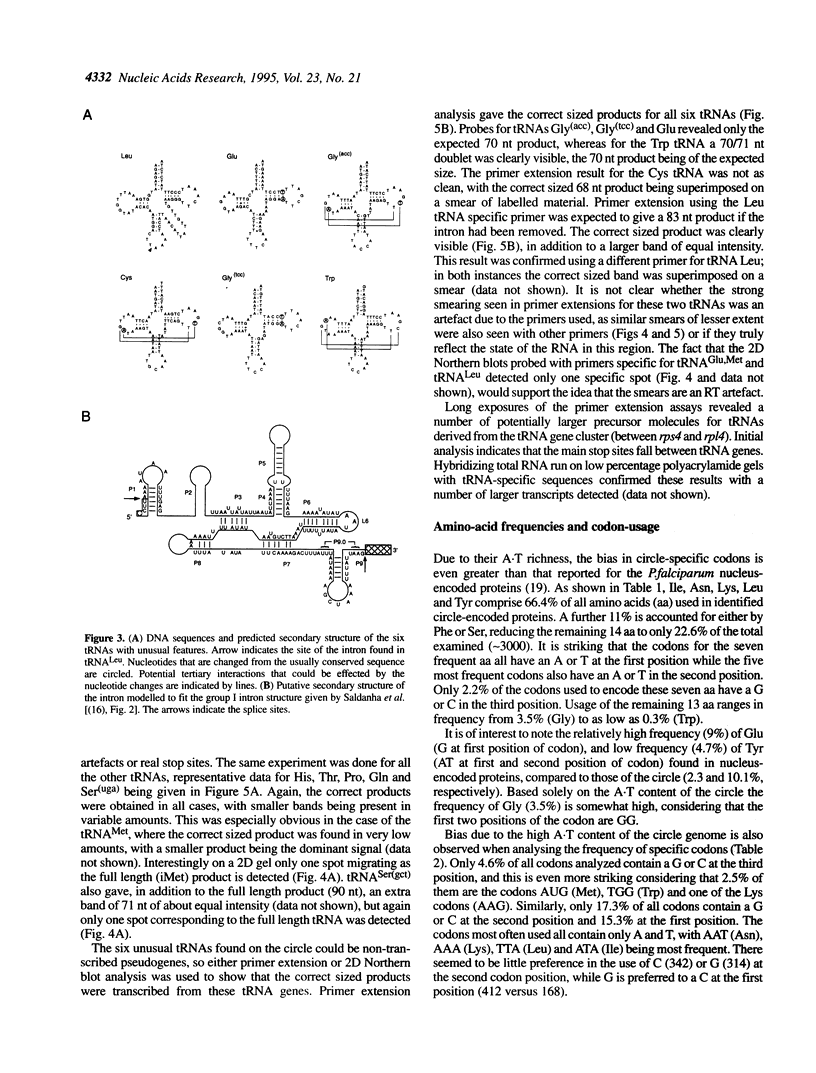




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Claesson C., Lustig F., Borén T., Simonsson C., Barciszewska M., Lagerkvist U. Glycine codon discrimination and the nucleotide in position 32 of the anticodon loop. J Mol Biol. 1995 Mar 24;247(2):191–196. doi: 10.1006/jmbi.1994.0132. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Elliott M. S., Trewyn R. W. Inosine biosynthesis in transfer RNA by an enzymatic insertion of hypoxanthine. J Biol Chem. 1984 Feb 25;259(4):2407–2410. [PubMed] [Google Scholar]
- Evrard J. L., Kuntz M., Straus N. A., Weil J. H. A class-I intron in a cyanelle tRNA gene from Cyanophora paradoxa: phylogenetic relationship between cyanelles and plant chloroplasts. Gene. 1988 Nov 15;71(1):115–122. doi: 10.1016/0378-1119(88)90083-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Feagin J. E., Moore D. J., Spencer D. F., Gray M. W., Williamson D. H., Wilson R. J. Organisation and expression of small subunit ribosomal RNA genes encoded by a 35-kilobase circular DNA in Plasmodium falciparum. Mol Biochem Parasitol. 1991 Sep;48(1):77–88. doi: 10.1016/0166-6851(91)90166-4. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Goldman N., Barnett P., Moore P. W., Rangachari K., Strath M., Whyte A., Williamson D. H., Wilson R. J. Phylogenetic analysis of the rpoB gene from the plastid-like DNA of Plasmodium falciparum. Mol Biochem Parasitol. 1994 Aug;66(2):221–231. doi: 10.1016/0166-6851(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Preiser P., Rangachari K., Moore D., Feagin J. E., Williamson D. H., Wilson R. J. Nine duplicated tRNA genes on the plastid-like DNA of the malaria parasite Plasmodium falciparum. Gene. 1994 Jul 8;144(2):307–308. doi: 10.1016/0378-1119(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Hempelmann E., Ling I., Wilson R. J. S-antigens and isozymes in strains of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1981;75(6):855–858. doi: 10.1016/0035-9203(81)90431-4. [DOI] [PubMed] [Google Scholar]
- Lagerkvist U. "Two out of three": an alternative method for codon reading. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1759–1762. doi: 10.1073/pnas.75.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerkvist U. Unorthodox codon reading and the evolution of the genetic code. Cell. 1981 Feb;23(2):305–306. doi: 10.1016/0092-8674(81)90124-0. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Nicholas H. B., Jr Differences between transfer RNA molecules. J Mol Biol. 1987 Apr 20;194(4):635–642. doi: 10.1016/0022-2836(87)90240-3. [DOI] [PubMed] [Google Scholar]
- McClain W. H. Transfer RNA identity. FASEB J. 1993 Jan;7(1):72–78. doi: 10.1096/fasebj.7.1.8422977. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Lagerkvist U. Codon-acticodon recognition in the valine codon family. J Biol Chem. 1977 Jan 25;252(2):471–478. [PubMed] [Google Scholar]
- Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988 Nov 10;336(6195):179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- Musto H., Rodriguez-Maseda H., Bernardi G. Compositional properties of nuclear genes from Plasmodium falciparum. Gene. 1995 Jan 11;152(1):127–132. doi: 10.1016/0378-1119(94)00708-z. [DOI] [PubMed] [Google Scholar]
- Ozeki H., Ohyama K., Inokuchi H., Fukuzawa H., Kohchi T., Sano T., Nakahigashi K., Umesono K. Genetic system of chloroplasts. Cold Spring Harb Symp Quant Biol. 1987;52:791–804. doi: 10.1101/sqb.1987.052.01.088. [DOI] [PubMed] [Google Scholar]
- Ragnini A., Frontali L. Ordered processing of the polygenic transcripts from a mitochondrial tRNA gene cluster in K. lactis. Curr Genet. 1994 Apr;25(4):342–349. doi: 10.1007/BF00351488. [DOI] [PubMed] [Google Scholar]
- Saldanha R., Mohr G., Belfort M., Lambowitz A. M. Group I and group II introns. FASEB J. 1993 Jan;7(1):15–24. doi: 10.1096/fasebj.7.1.8422962. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 1989 Jul 25;17(14):5427–5445. doi: 10.1093/nar/17.14.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A computer program to search for tRNA genes. Nucleic Acids Res. 1980 Feb 25;8(4):817–825. [PMC free article] [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Ueda T., Yokogawa T., Nishikawa K., Watanabe K. Characterization of serine and leucine tRNAs in an asporogenic yeast Candida cylindracea and evolutionary implications of genes for tRNA(Ser)CAG responsible for translation of a non-universal genetic code. Nucleic Acids Res. 1994 Jan 25;22(2):115–123. doi: 10.1093/nar/22.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tyobeka E. M., Masemola A. M. Protein synthesis in HL-60 cells treated with DMSO and hypoxanthine. FEBS Lett. 1992 Aug 17;308(2):165–169. doi: 10.1016/0014-5793(92)81267-p. [DOI] [PubMed] [Google Scholar]
- Wakita K., Watanabe Y., Yokogawa T., Kumazawa Y., Nakamura S., Ueda T., Watanabe K., Nishikawa K. Higher-order structure of bovine mitochondrial tRNA(Phe) lacking the 'conserved' GG and T psi CG sequences as inferred by enzymatic and chemical probing. Nucleic Acids Res. 1994 Feb 11;22(3):347–353. doi: 10.1093/nar/22.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
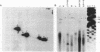
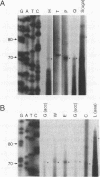
- Williamson D. H., Gardner M. J., Preiser P., Moore D. J., Rangachari K., Wilson R. J. The evolutionary origin of the 35 kb circular DNA of Plasmodium falciparum: new evidence supports a possible rhodophyte ancestry. Mol Gen Genet. 1994 Apr;243(2):249–252. doi: 10.1007/BF00280323. [DOI] [PubMed] [Google Scholar]