Abstract
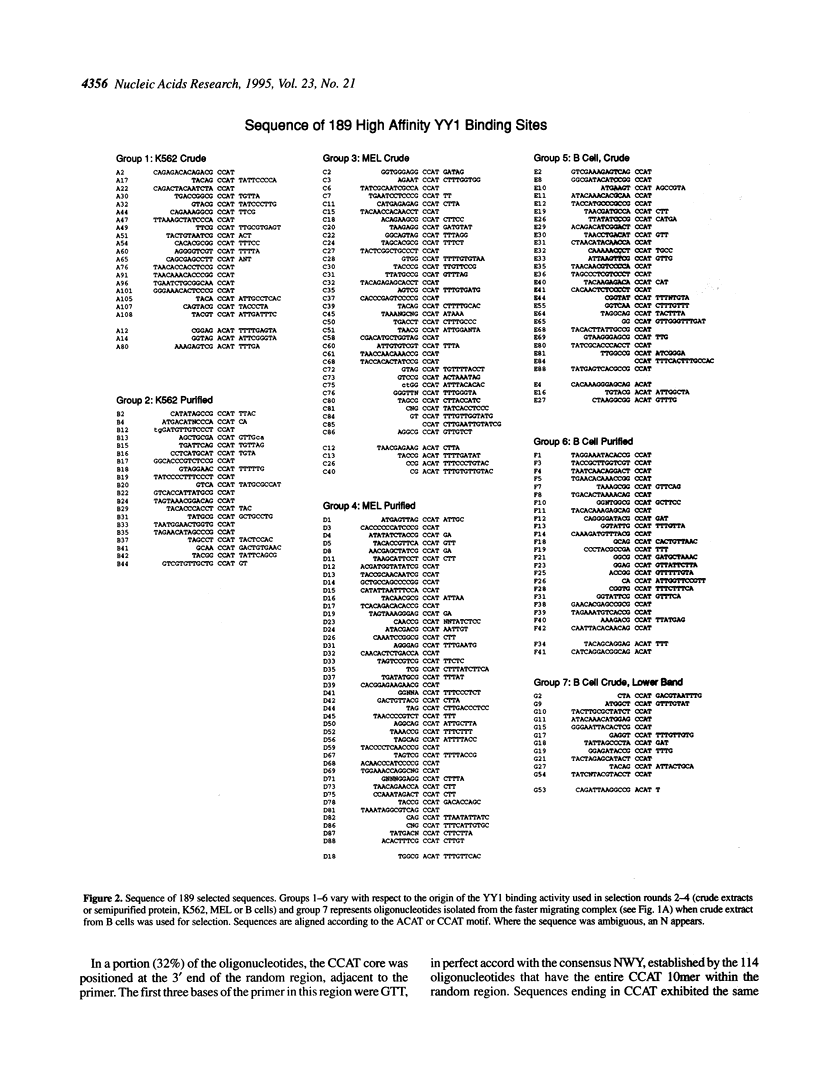
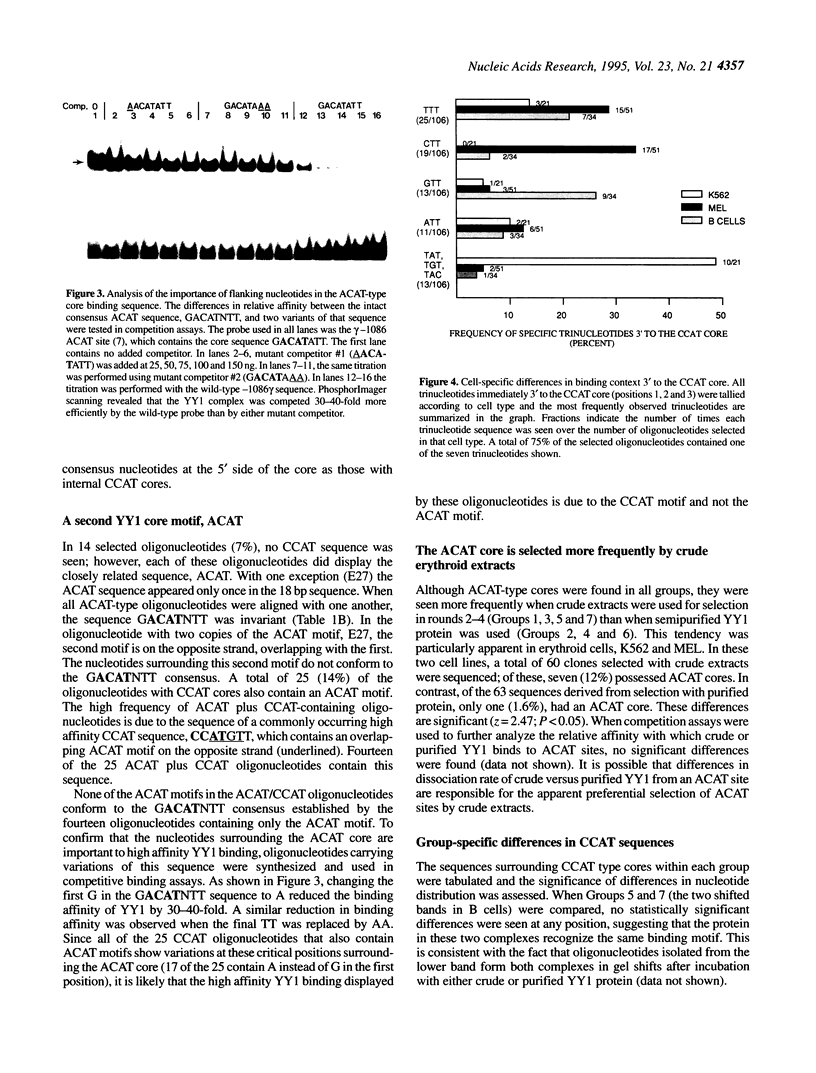
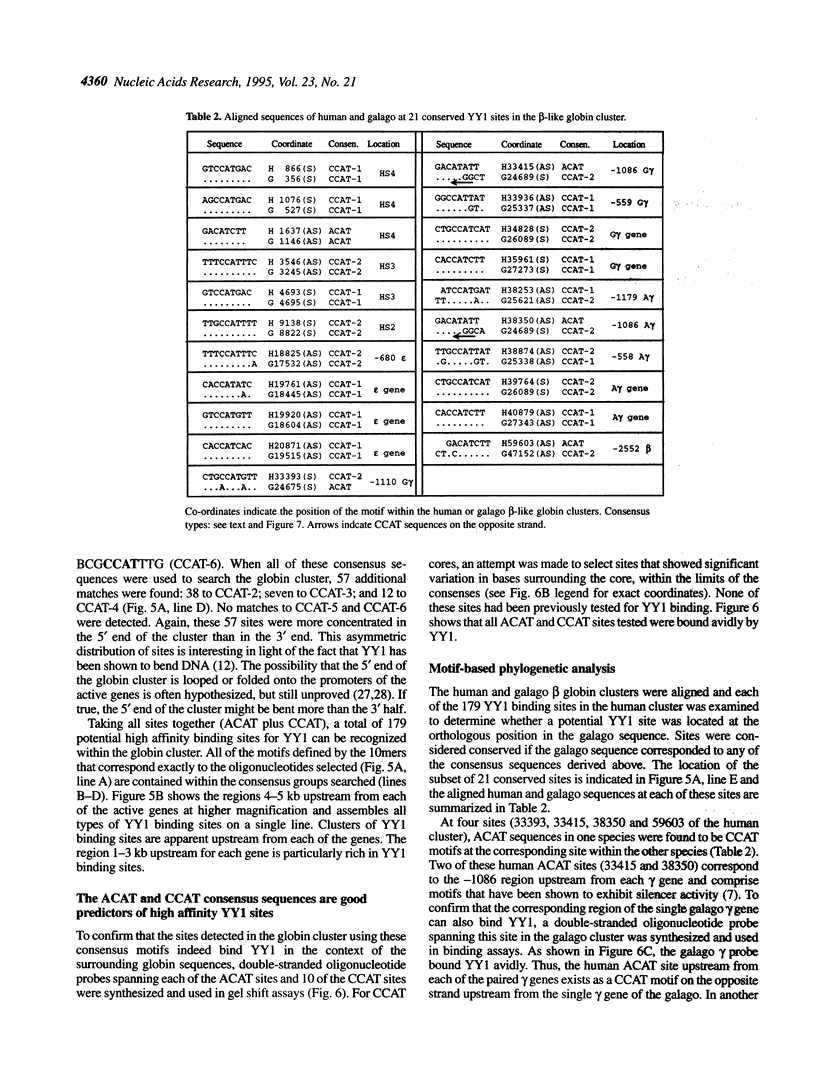
PCR-assisted binding site selection was used to define the sequence characteristics of high affinity YY1 binding sites. Compilation of the sequences of 189 selected oligonucleotides containing high affinity YY1 binding sites revealed two types of core sequence: ACAT and CCAT. ACAT cores were surrounded by other invariant nucleotides, forming the consensus GACATNTT. A search of the 73 kb human beta-like globin cluster with this consensus revealed eight matching motifs, six of which were located within 1-3 kb upstream of the gamma and beta genes. CCAT-type cores were more variable in surrounding sequence context; the consensus VDCCATNWY was found to fit 89% of the selected CCAT-containing oligonucleotides. A search of the human beta globin cluster with CCAT consensus sequences revealed 171 potential YY1 binding sites. Several of these were tested directly in gel shift assays and confirmed as high affinity YY1 binding sites. Finally, a strategy called motif-based phylogenetic analysis was employed to determine which of the 179 total sites are evolutionarily conserved. This analysis permits the detection of functionally conserved binding sites despite sequence differences present between the two species. The 21 conserved sites identified will serve as important starting points in further dissection of the possible role of YY1 in globin gene regulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauknecht T., Angel P., Royer H. D., zur Hausen H. Identification of a negative regulatory domain in the human papillomavirus type 18 promoter: interaction with the transcriptional repressor YY1. EMBO J. 1992 Dec;11(12):4607–4617. doi: 10.1002/j.1460-2075.1992.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K. G., Jedlicka P., Templeton N. S., Liotta L., Ozato K. Characterization of hUCRBP (YY1, NF-E1, delta): a transcription factor that binds the regulatory regions of many viral and cellular genes. Gene. 1994 Dec 15;150(2):259–266. doi: 10.1016/0378-1119(94)90435-9. [DOI] [PubMed] [Google Scholar]
- Becker K. G., Swergold G. D., Ozato K., Thayer R. E. Binding of the ubiquitous nuclear transcription factor YY1 to a cis regulatory sequence in the human LINE-1 transposable element. Hum Mol Genet. 1993 Oct;2(10):1697–1702. doi: 10.1093/hmg/2.10.1697. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D. Developmental regulation of human beta-globin gene transcription: a switch of loyalties? Trends Genet. 1993 Sep;9(9):304–309. doi: 10.1016/0168-9525(93)90248-g. [DOI] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P., Pruzina S., Antoniou M., Grosveld F. Each hypersensitive site of the human beta-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev. 1993 Jan;7(1):106–113. doi: 10.1101/gad.7.1.106. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Heilstedt-Williamson H., Gray T. A., Tarlé S. A., Shelton D. A., Tagle D. A., Slightom J. L., Goodman M., Collins F. S. Phylogenetic footprinting reveals a nuclear protein which binds to silencer sequences in the human gamma and epsilon globin genes. Mol Cell Biol. 1992 Nov;12(11):4919–4929. doi: 10.1128/mcb.12.11.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Gray T. A., Riordan M. F., Sartor C. I., Collins F. S. Nuclear proteins that bind the human gamma-globin gene promoter: alterations in binding produced by point mutations associated with hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1988 Dec;8(12):5310–5322. doi: 10.1128/mcb.8.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Shelton D. A., Bailey W. J., Slightom J. L., Goodman M. Phylogenetic footprinting reveals unexpected complexity in trans factor binding upstream from the epsilon-globin gene. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6018–6022. doi: 10.1073/pnas.90.13.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A. D., Rojas K., Mewar R., Moshinsky D., Overhauser J. Somatic cell hybrid deletion map of human chromosome 18. Genomics. 1992 May;13(1):1–6. doi: 10.1016/0888-7543(92)90193-v. [DOI] [PubMed] [Google Scholar]
- Ko L. J., Engel J. D. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993 Jul;13(7):4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letovsky J., Dynan W. S. Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence. Nucleic Acids Res. 1989 Apr 11;17(7):2639–2653. doi: 10.1093/nar/17.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. Y., Rodriguez M., Liao W. S. YY1 represses rat serum amyloid A1 gene transcription and is antagonized by NF-kappa B during acute-phase response. Mol Cell Biol. 1994 Sep;14(9):6253–6263. doi: 10.1128/mcb.14.9.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis D. M., Somasundaran M., Green M. R. Human transcription factor YY1 represses human immunodeficiency virus type 1 transcription and virion production. J Virol. 1994 Feb;68(2):905–910. doi: 10.1128/jvi.68.2.905-910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M., Dong X. P., Beyer-Finkler E., Stubenrauch F., Fuchs P. G., Pfister H. The E6/E7 promoter of extrachromosomal HPV16 DNA in cervical cancers escapes from cellular repression by mutation of target sequences for YY1. EMBO J. 1994 Mar 15;13(6):1460–1466. doi: 10.1002/j.1460-2075.1994.tb06400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier V. S., Groner B. The nuclear factor YY1 participates in repression of the beta-casein gene promoter in mammary epithelial cells and is counteracted by mammary gland factor during lactogenic hormone induction. Mol Cell Biol. 1994 Jan;14(1):128–137. doi: 10.1128/mcb.14.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merika M., Orkin S. H. DNA-binding specificity of GATA family transcription factors. Mol Cell Biol. 1993 Jul;13(7):3999–4010. doi: 10.1128/mcb.13.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S., Gilman M. Z. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes Dev. 1993 Dec;7(12B):2497–2509. doi: 10.1101/gad.7.12b.2497. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B., Merezhinskaya N., Diffley J. F., Noguchi C. T. Protein-DNA interactions in the epsilon-globin gene silencer. J Biol Chem. 1993 Feb 15;268(5):3430–3437. [PubMed] [Google Scholar]
- Pious D., Krangel M. S., Dixon L. L., Parham P., Strominger J. L. HLA antigen structural gene mutants selected with an allospecific monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7832–7836. doi: 10.1073/pnas.79.24.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Clegg C. H., Grofti J., Roméo P. H., Stamatoyannopoulos G. GATA1 and YY1 are developmental repressors of the human epsilon-globin gene. EMBO J. 1995 Feb 15;14(4):801–809. doi: 10.1002/j.1460-2075.1995.tb07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Papayannopoulou T., Stamatoyannopoulos G., Enver T. Demonstration of a human epsilon-globin gene silencer with studies in transgenic mice. Blood. 1992 Feb 15;79(4):861–864. [PubMed] [Google Scholar]
- Riggs K. J., Saleque S., Wong K. K., Merrell K. T., Lee J. S., Shi Y., Calame K. Yin-yang 1 activates the c-myc promoter. Mol Cell Biol. 1993 Dec;13(12):7487–7495. doi: 10.1128/mcb.13.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K., Park K., Atchison M. L., Howe C. C. The intracisternal A-particle upstream element interacts with transcription factor YY1 to activate transcription: pleiotropic effects of YY1 on distinct DNA promoter elements. Mol Cell Biol. 1993 Nov;13(11):6621–6628. doi: 10.1128/mcb.13.11.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Shi Y., Shenk T. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature. 1991 Nov 21;354(6350):241–245. doi: 10.1038/354241a0. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Shrivastava A., Calame K. An analysis of genes regulated by the multi-functional transcriptional regulator Yin Yang-1. Nucleic Acids Res. 1994 Dec 11;22(24):5151–5155. doi: 10.1093/nar/22.24.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S. Identification of common molecular subsequences. J Mol Biol. 1981 Mar 25;147(1):195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]