Abstract
Acyclic nucleoside analogues with carboxamido- or nitro-substituted heterocyclic bases have been evaluated for their possible use as universal bases in oligodeoxynucleotides. The acyclic moiety endows the constructs with enough flexibility to allow good base stacking. The 5-nitroindazole analogue afforded the most stable duplexes among the acyclic derivatives with the least spread in Tm versus the four natural bases. In spite of the acyclic moiety, stabilities are comparable with those of duplexes incorporating the recently described 5-nitroindole nucleoside analogue, but considerably exceed those for the 3-nitropyrrole analogue.
Full text
PDF

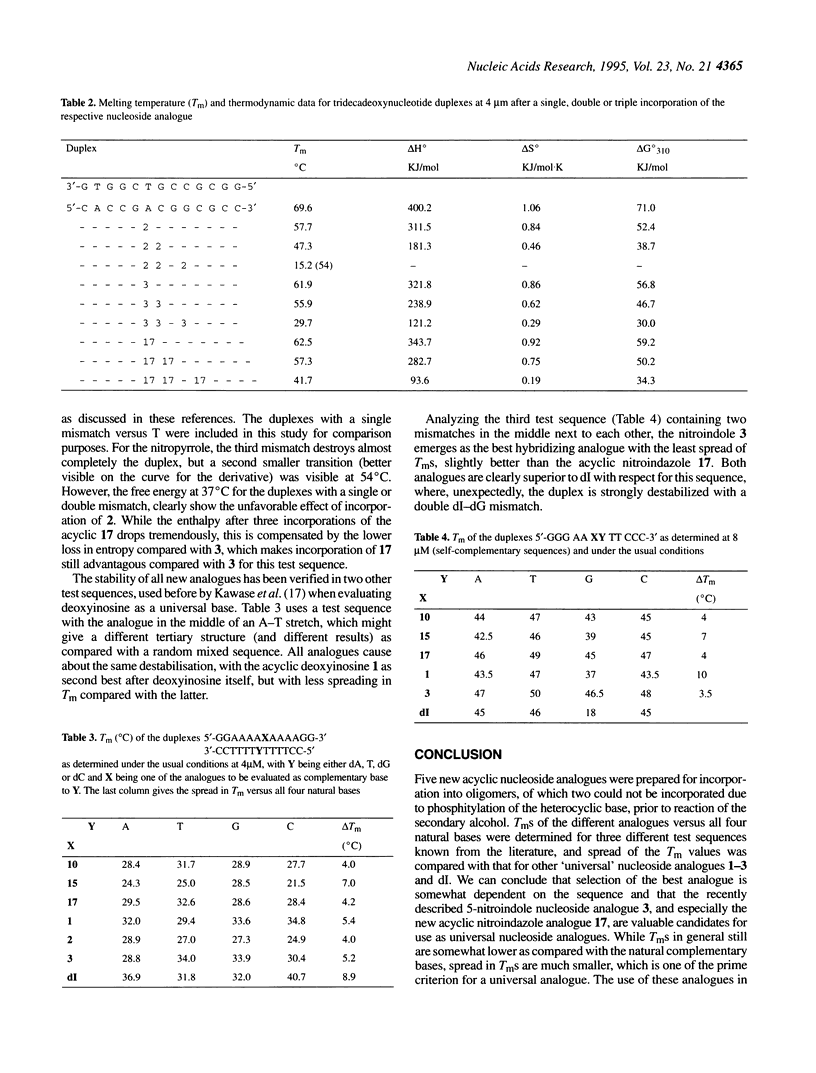
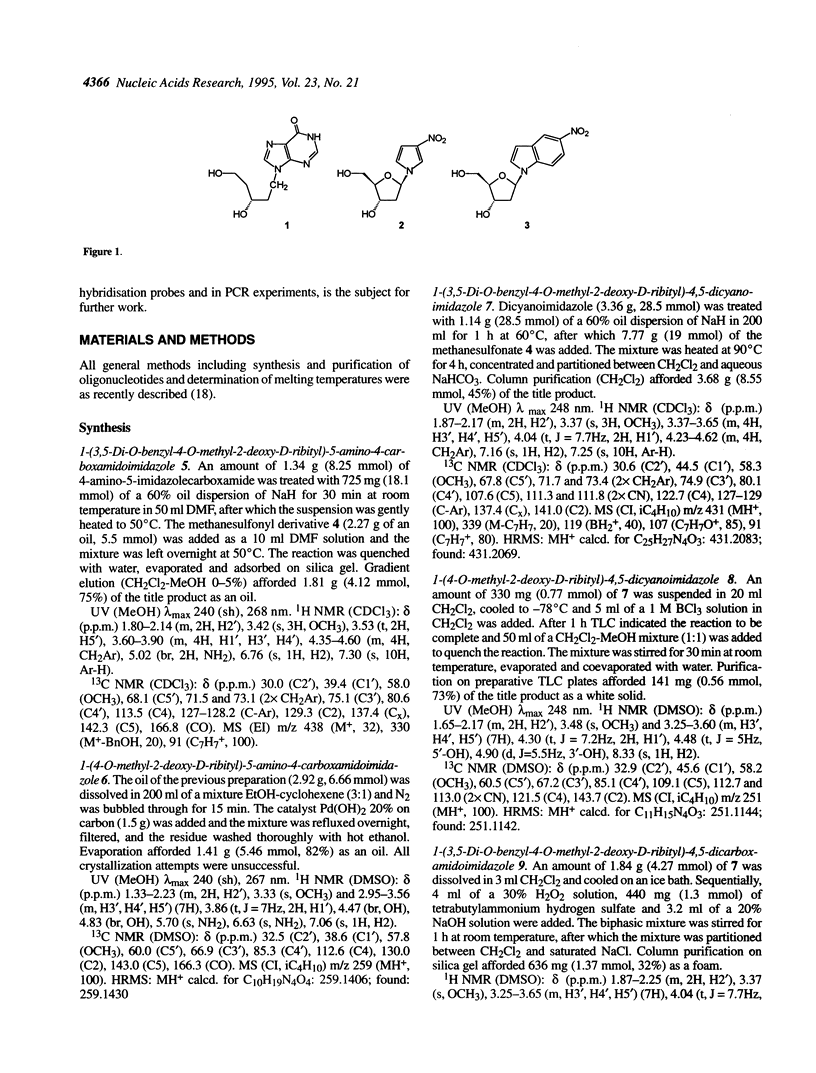
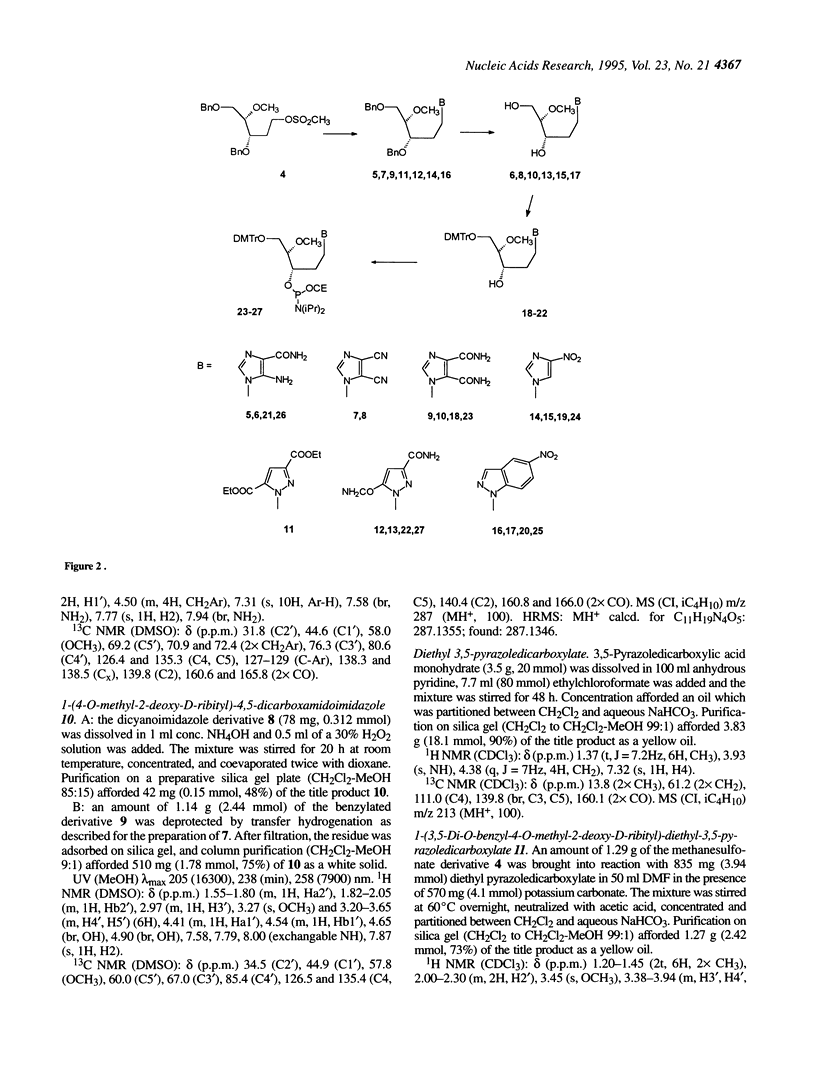



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Vo C. D., Le D. B., Gryan G. P., Ercolani L., Wang A. H. 5-Fluorodeoxyuridine as an alternative to the synthesis of mixed hybridization probes for the detection of specific gene sequences. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1735–1739. doi: 10.1073/pnas.85.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix C., Devreese B., Rozenski J., van Aerschot A., De Bruyn A., Van Beeumen J., Herdewijn P. Incorporation of 2'-amido-nucleosides in oligodeoxynucleotides and oligoribonucleotides as a model for 2'-linked conjugates. Nucleic Acids Res. 1995 Jan 11;23(1):51–57. doi: 10.1093/nar/23.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase Y., Iwai S., Inoue H., Miura K., Ohtsuka E. Studies on nucleic acid interactions. I. Stabilities of mini-duplexes (dG2A4XA4G2-dC2T4YT4C2) and self-complementary d(GGGAAXYTTCCC) containing deoxyinosine and other mismatched bases. Nucleic Acids Res. 1986 Oct 10;14(19):7727–7736. doi: 10.1093/nar/14.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loakes D., Brown D. M. 5-Nitroindole as an universal base analogue. Nucleic Acids Res. 1994 Oct 11;22(20):4039–4043. doi: 10.1093/nar/22.20.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millican T. A., Mock G. A., Chauncey M. A., Patel T. P., Eaton M. A., Gunning J., Cutbush S. D., Neidle S., Mann J. Synthesis and biophysical studies of short oligodeoxynucleotides with novel modifications: a possible approach to the problem of mixed base oligodeoxynucleotide synthesis. Nucleic Acids Res. 1984 Oct 11;12(19):7435–7453. doi: 10.1093/nar/12.19.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R., Andrews P. C., Zhang P., Bergstrom D. E. A universal nucleoside for use at ambiguous sites in DNA primers. Nature. 1994 Jun 9;369(6480):492–493. doi: 10.1038/369492a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Suwinski J., Szczepankiewicz W., Widel M. Nitroimidazoles, XIV: Synthesis of 4-nitroimidazoles with 1-substituents containing acid, ester or phenol functions, and radiosensitizing efficiency of some of these compounds. Arch Pharm (Weinheim) 1992 Jun;325(6):317–324. doi: 10.1002/ardp.19923250602. [DOI] [PubMed] [Google Scholar]
- Vandendriessche F., Snoeck R., Janssen G., Hoogmartens J., Van Aerschot A., De Clercq E., Herdewijn P. Synthesis and antiviral activity of acyclic nucleosides with a 3(S),5-dihydroxypentyl or 4(R)-methoxy-3(S),5-dihydroxypentyl side chain. J Med Chem. 1992 Apr 17;35(8):1458–1465. doi: 10.1021/jm00086a015. [DOI] [PubMed] [Google Scholar]



