Abstract
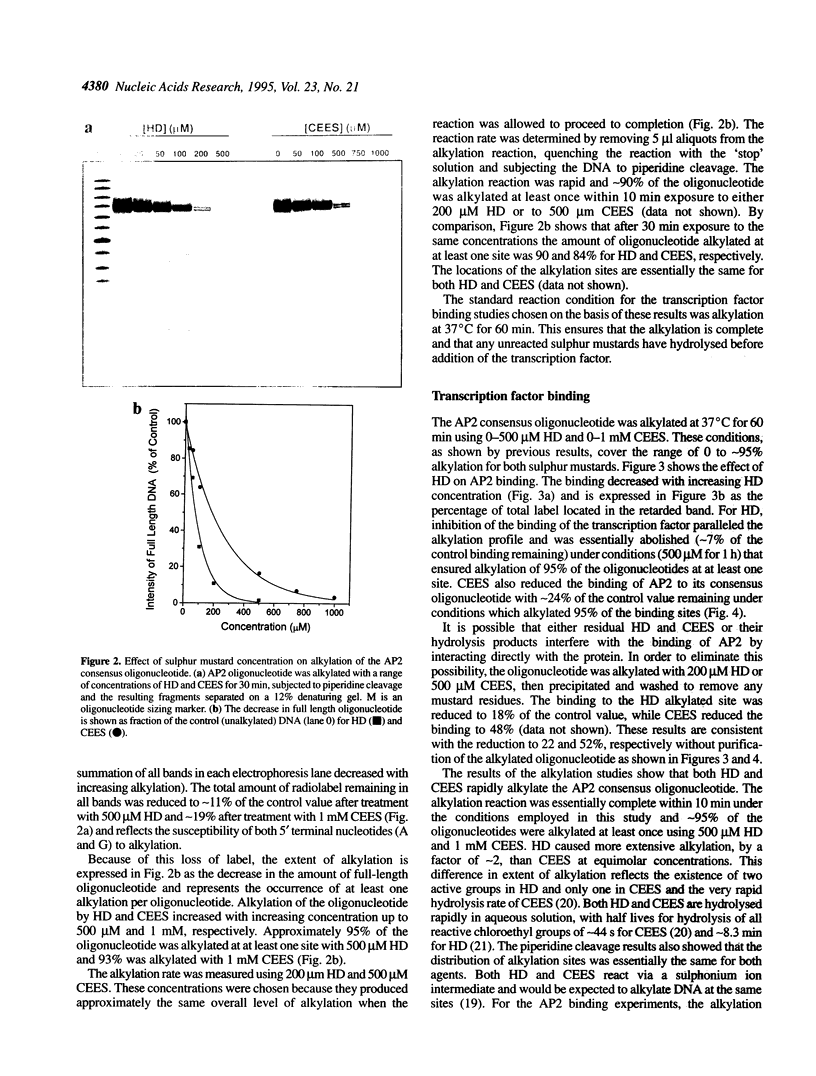
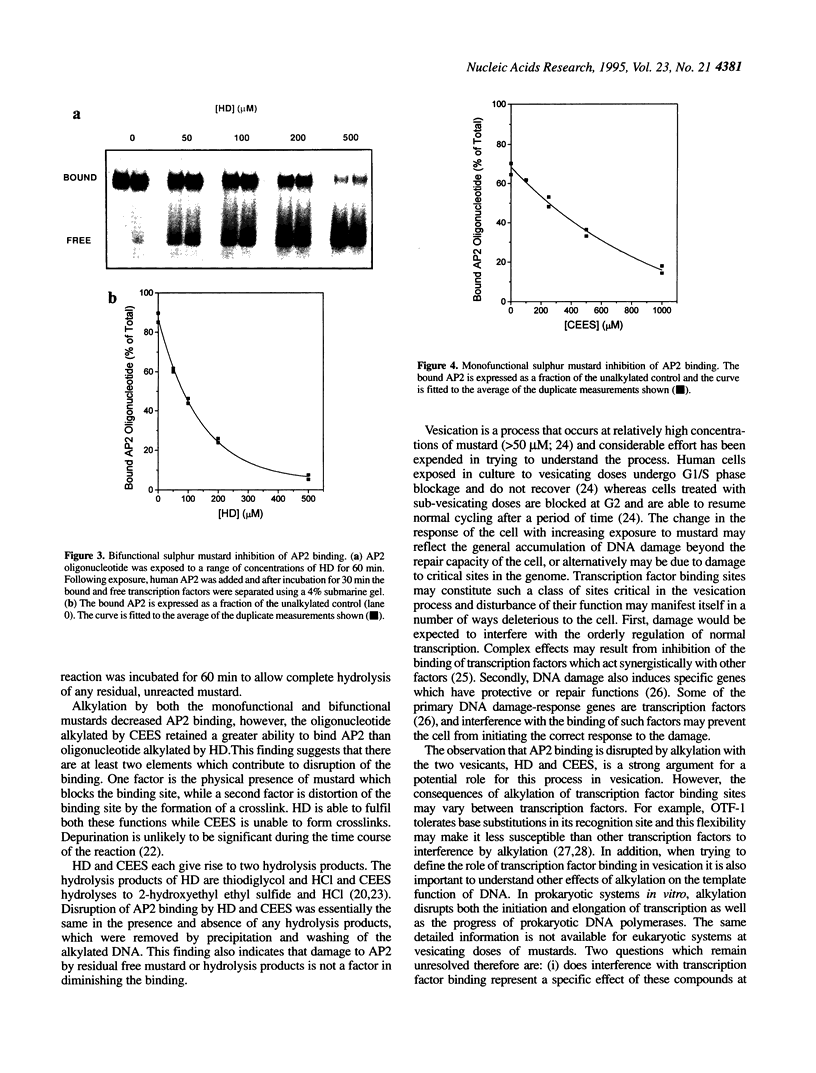
The bifunctional sulphur mustard (bis-(2-chloroethyl)sulphide, HD) and its monofunctional analogue (2-chloroethyl ethyl sulphide, CEES) are both vesicants. In this study, both mustards were shown to rapidly alkylate the AP2 consensus binding sequence incorporated in a 26mer oligonucleotide. The reaction was essentially complete within 10 min under the conditions employed in this study and -95% of the oligonucleotides were alkylated at least once using 500 microM HD and 1 mM CEES. Progressive alkylation of the consensus sequence was parallelled by a decrease in transcription factor binding. Under reaction conditions which alkylated approximately 95% of the oligonucleotides at least once, the binding of cloned human AP2 was reduced by 93 and 76% by HD and CEES, respectively, compared with control values. The interference with binding is a result of alkylation of the DNA and not damage to the transcription factor by mustard or its hydrolysis products. Interference with transcription factor binding would be expected to have a profound influence on the ability of the cell to function normally and to respond to DNA damage and may contribute significantly to the skin damage produced by these compounds.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendall A. J., Sturm R. A., Danoy P. A., Molloy P. L. Broad binding-site specificity and affinity properties of octamer 1 and brain octamer-binding proteins. Eur J Biochem. 1993 Nov 1;217(3):799–811. doi: 10.1111/j.1432-1033.1993.tb18308.x. [DOI] [PubMed] [Google Scholar]
- Bonfanti M., Broggini M., Prontera C., D'Incalci M. O6-methylguanine inhibits the binding of transcription factors to DNA. Nucleic Acids Res. 1991 Oct 25;19(20):5739–5742. doi: 10.1093/nar/19.20.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggini M., D'Incalci M. Modulation of transcription factor--DNA interactions by anticancer drugs. Anticancer Drug Des. 1994 Aug;9(4):373–387. [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mustard gas with nucleic acids in vitro and in vivo. Biochem J. 1960 Dec;77(3):478–484. doi: 10.1042/bj0770478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. A., Herr W. Mechanisms for flexibility in DNA sequence recognition and VP16-induced complex formation by the Oct-1 POU domain. Mol Cell Biol. 1995 Apr;15(4):2090–2100. doi: 10.1128/mcb.15.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri S., Prontera C., Broggini M., D'Incalci M. Differential inhibition of the DNA binding of transcription factors NF kappa B and OTF-1 by nitrogen mustard and quinacrine mustard: transcriptional implications. Carcinogenesis. 1993 Sep;14(9):1963–1967. doi: 10.1093/carcin/14.9.1963. [DOI] [PubMed] [Google Scholar]
- Fidder A., Moes G. W., Scheffer A. G., van der Schans G. P., Baan R. A., de Jong L. P., Benschop H. P. Synthesis, characterization, and quantitation of the major adducts formed between sulfur mustard and DNA of calf thymus and human blood. Chem Res Toxicol. 1994 Mar-Apr;7(2):199–204. doi: 10.1021/tx00038a013. [DOI] [PubMed] [Google Scholar]
- Gray P. J., Cullinane C., Phillips D. R. In vitro transcription analysis of DNA alkylation by nitrogen mustard. Biochemistry. 1991 Aug 13;30(32):8036–8040. doi: 10.1021/bi00246a022. [DOI] [PubMed] [Google Scholar]
- Herschlag D., Johnson F. B. Synergism in transcriptional activation: a kinetic view. Genes Dev. 1993 Feb;7(2):173–179. doi: 10.1101/gad.7.2.173. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlum D. B., Austin-Ritchie P., Hagopian M., Niu T. Q., Yu D. Detection of sulfur mustard-induced DNA modifications. Chem Biol Interact. 1994 Apr;91(1):39–49. doi: 10.1016/0009-2797(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Ludlum D. B., Kent S., Mehta J. R. Formation of O6-ethylthioethylguanine in DNA by reaction with the sulfur mustard, chloroethyl sulfide, and its apparent lack of repair by O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 1986 Jul;7(7):1203–1206. doi: 10.1093/carcin/7.7.1203. [DOI] [PubMed] [Google Scholar]
- Masta A., Gray P. J., Phillips D. R. Molecular basis of nitrogen mustard effects on transcription processes: role of depurination. Nucleic Acids Res. 1994 Sep 25;22(19):3880–3886. doi: 10.1093/nar/22.19.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res. 1986 Apr 11;14(7):2971–2987. doi: 10.1093/nar/14.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papirmeister B., Gross C. L., Meier H. L., Petrali J. P., Johnson J. B. Molecular basis for mustard-induced vesication. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S134–S149. [PubMed] [Google Scholar]
- Vogt R. F., Jr, Dannenberg A. M., Jr, Schofield B. H., Hynes N. A., Papirmeister B. Pathogenesis of skin lesions caused by sulfur mustard. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 2):S71–S83. doi: 10.1016/0272-0590(84)90139-8. [DOI] [PubMed] [Google Scholar]
- Wingender E. Transcription regulating proteins and their recognition sequences. Crit Rev Eukaryot Gene Expr. 1990;1(1):11–48. [PubMed] [Google Scholar]