Abstract
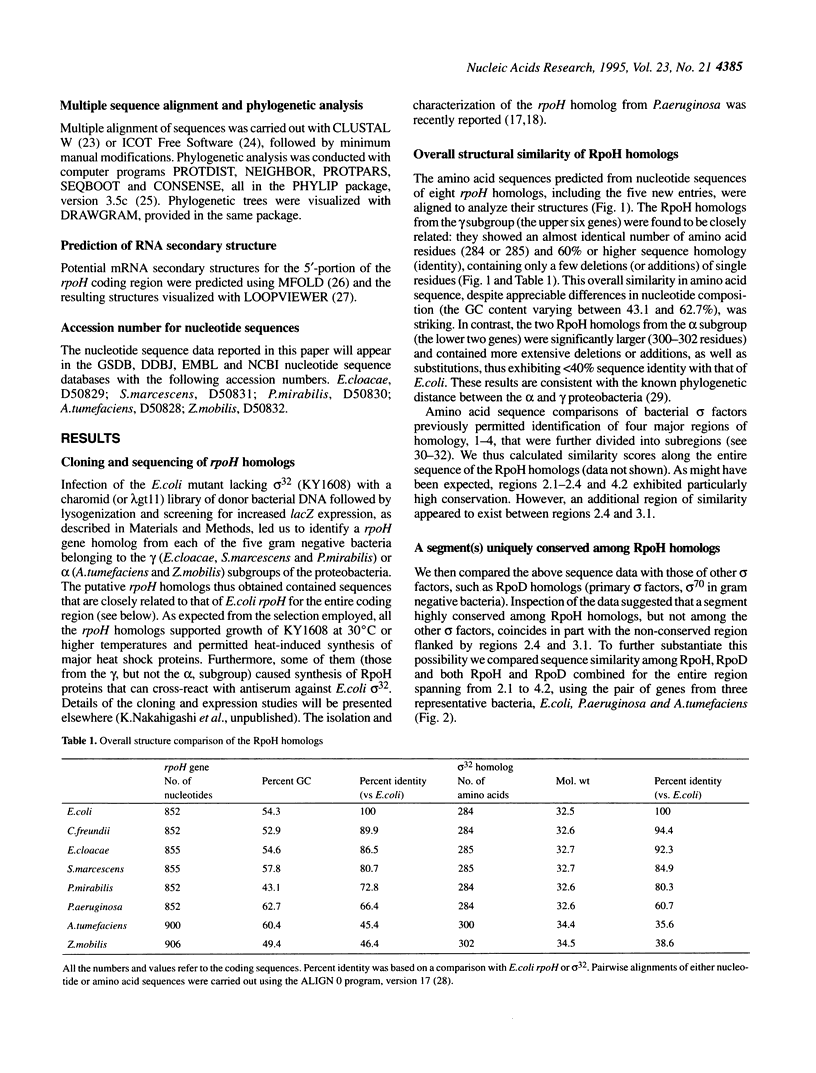
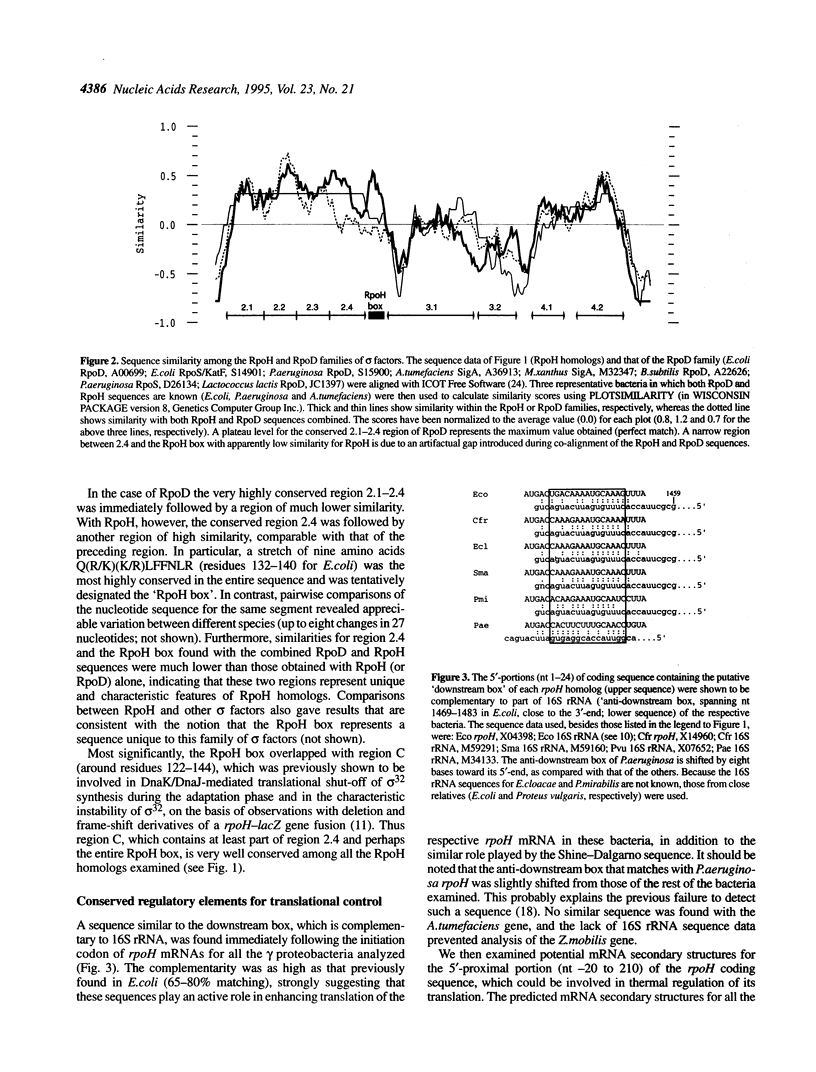
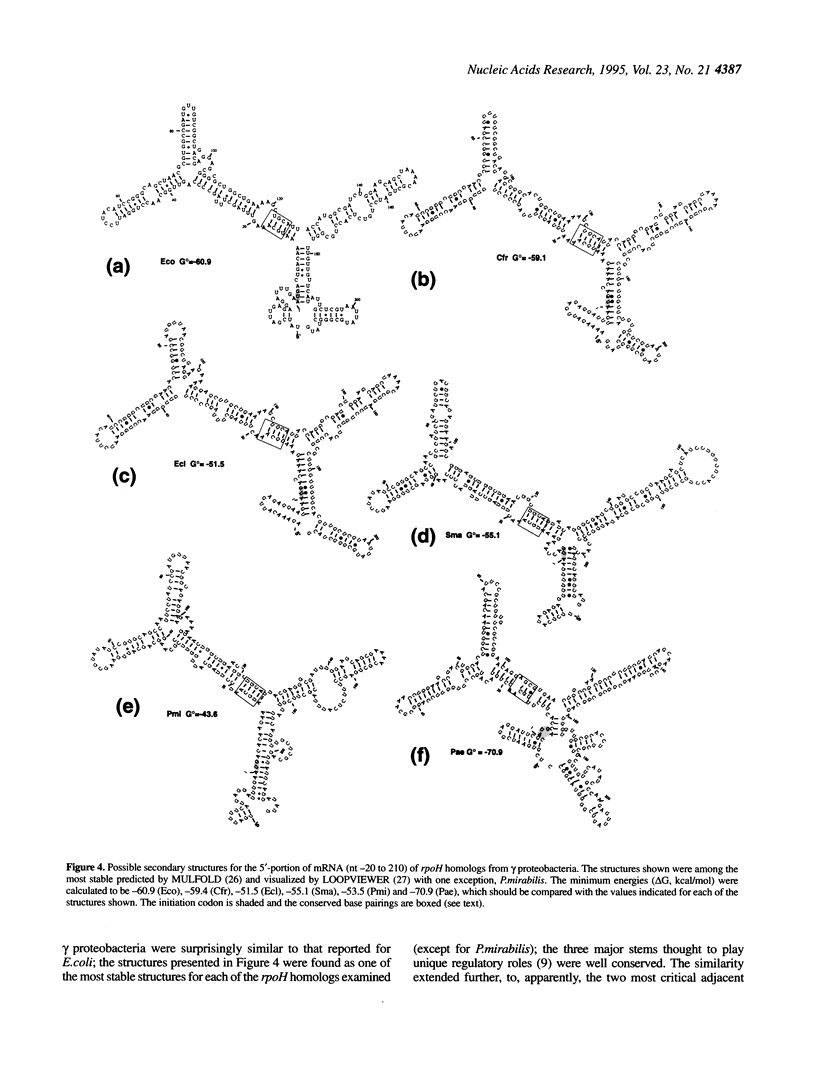
The rpoH genes encoding homologs of Escherichia coli sigma 32 (heat shock sigma factor) were isolated and sequenced from five gram negative proteobacteria (gamma or alpha subgroup): Enterobacter cloacae (gamma), Serratia marcescens (gamma), Proteus mirabilis (gamma), Agrobacterium tumefaciens (alpha) and Zymomonas mobilis (alpha). Comparison of these and three known genes from E.coli (gamma), Citrobacter freundii (gamma) and Pseudomonas aeruginosa (gamma) revealed marked similarities that should reflect conserved function and regulation of sigma 32 in the heat shock response. Both the sequence complementary to part of 16S rRNA (the 'downstream box') and a predicted mRNA secondary structure similar to those involved in translational control of sigma 32 in E.coli were found for the rpoH genes from the gamma, but not the alpha, subgroup, despite considerable divergence in nucleotide sequence. Moreover, a stretch of nine amino acid residues Q(R/K)(K/R)LFFNLR, designated the 'RpoH box', was absolutely conserved among all sigma 32 homologs, but absent in other sigma factors; this sequence overlapped with the segment of polypeptide thought to be involved in DnaK/DnaJ chaperone-mediated negative control of synthesis and stability of sigma 32. In addition, a putative sigma E (sigma 24)-specific promoter was found in front of all rpoH genes from the gamma, but not alpha, subgroup. These results suggest that the regulatory mechanisms, as well as the function, of the heat shock response known in E.coli are very well conserved among the gamma subgroup and partially conserved among the alpha proteobacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- An H., Scopes R. K., Rodriguez M., Keshav K. F., Ingram L. O. Gel electrophoretic analysis of Zymomonas mobilis glycolytic and fermentative enzymes: identification of alcohol dehydrogenase II as a stress protein. J Bacteriol. 1991 Oct;173(19):5975–5982. doi: 10.1128/jb.173.19.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. F., Yomano L. P., Ingram L. O. Cloning, sequencing and expression of stress genes from the ethanol-producing bacterium Zymomonas mobilis: the groESL operon. Gene. 1994 Oct 11;148(1):51–57. doi: 10.1016/0378-1119(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Benvenisti L., Koby S., Rutman A., Giladi H., Yura T., Oppenheim A. B. Cloning and primary sequence of the rpoH gene from Pseudomonas aeruginosa. Gene. 1995 Mar 21;155(1):73–76. doi: 10.1016/0378-1119(94)00829-h. [DOI] [PubMed] [Google Scholar]
- Fleischmann R. D., Adams M. D., White O., Clayton R. A., Kirkness E. F., Kerlavage A. R., Bult C. J., Tomb J. F., Dougherty B. A., Merrick J. M. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995 Jul 28;269(5223):496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Garvin L. D., Hardies S. C. Nucleotide sequence for the htpR gene from Citrobacter freundii. Nucleic Acids Res. 1989 Jun 26;17(12):4889–4889. doi: 10.1093/nar/17.12.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish W., States D. J. Identification of protein coding regions by database similarity search. Nat Genet. 1993 Mar;3(3):266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- Gomes S. L., Gober J. W., Shapiro L. Expression of the Caulobacter heat shock gene dnaK is developmentally controlled during growth at normal temperatures. J Bacteriol. 1990 Jun;172(6):3051–3059. doi: 10.1128/jb.172.6.3051-3059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Kamath-Loeb A. S., Gross C. A. Translational regulation of sigma 32 synthesis: requirement for an internal control element. J Bacteriol. 1991 Jun;173(12):3904–3906. doi: 10.1128/jb.173.12.3904-3906.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Vaughn V., Lau E. T., VanBogelen R. A., Erickson J. W., Neidhardt F. C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984 Aug;38(1):175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Lonetto M., Gribskov M., Gross C. A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992 Jun;174(12):3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis N. J., Winans S. C. Characterization of the Agrobacterium tumefaciens heat shock response: evidence for a sigma 32-like sigma factor. J Bacteriol. 1992 Feb;174(3):991–997. doi: 10.1128/jb.174.3.991-997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis N. J., Winans S. C. The Agrobacterium tumefaciens vir gene transcriptional activator virG is transcriptionally induced by acid pH and other stress stimuli. J Bacteriol. 1992 Feb;174(4):1189–1196. doi: 10.1128/jb.174.4.1189-1196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naczynski Z. M., Mueller C., Kropinski A. M. Cloning the gene for the heat shock response positive regulator (sigma 32 homolog) from Pseudomonas aeruginosa. Can J Microbiol. 1995 Jan;41(1):75–87. doi: 10.1139/m95-010. [DOI] [PubMed] [Google Scholar]
- Nagai H., Yuzawa H., Kanemori M., Yura T. A distinct segment of the sigma 32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10280–10284. doi: 10.1073/pnas.91.22.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Yuzawa H., Yura T. Interplay of two cis-acting mRNA regions in translational control of sigma 32 synthesis during the heat shock response of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F., Bahl H. Cloning, sequencing, and molecular analysis of the groESL operon of Clostridium acetobutylicum. J Bacteriol. 1992 May;174(10):3282–3289. doi: 10.1128/jb.174.10.3282-3289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G., Ron E. Z. Heat shock transcription of the groESL operon of Agrobacterium tumefaciens may involve a hairpin-loop structure. J Bacteriol. 1993 May;175(10):3083–3088. doi: 10.1128/jb.175.10.3083-3088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengart M. L., Fatscher H. P., Fuchs E. The initiation of translation in E. coli: apparent base pairing between the 16srRNA and downstream sequences of the mRNA. Nucleic Acids Res. 1990 Apr 11;18(7):1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature. 1987 Sep 24;329(6137):348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzstein M., Völker U., Dedio J., Löbau S., Zuber U., Schiesswohl M., Herget C., Hecker M., Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992 May;174(10):3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Imai M., Yura T. The use of operon fusions in studies of the heat-shock response: effects of altered sigma 32 on heat-shock promoter function in Escherichia coli. Mol Gen Genet. 1987 Apr;207(1):24–28. doi: 10.1007/BF00331486. [DOI] [PubMed] [Google Scholar]
- Yura T., Nagai H., Mori H. Regulation of the heat-shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]
- Yura T., Tobe T., Ito K., Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzawa H., Nagai H., Mori H., Yura T. Heat induction of sigma 32 synthesis mediated by mRNA secondary structure: a primary step of the heat shock response in Escherichia coli. Nucleic Acids Res. 1993 Nov 25;21(23):5449–5455. doi: 10.1093/nar/21.23.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. N., Kusukawa N., Erickson J. W., Gross C. A., Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor sigma 32. J Bacteriol. 1988 Aug;170(8):3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber U., Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994 Mar;176(5):1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]



