Abstract
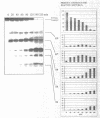
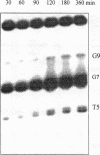
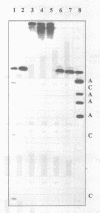
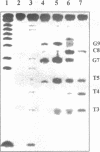
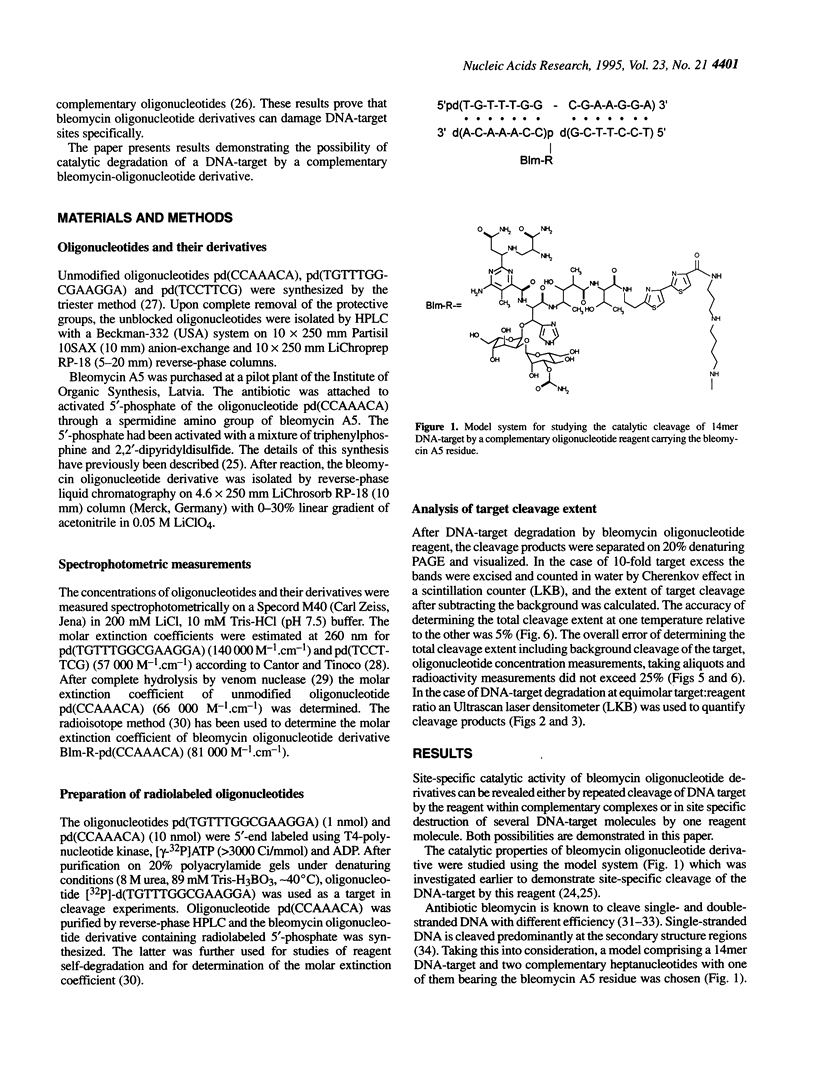
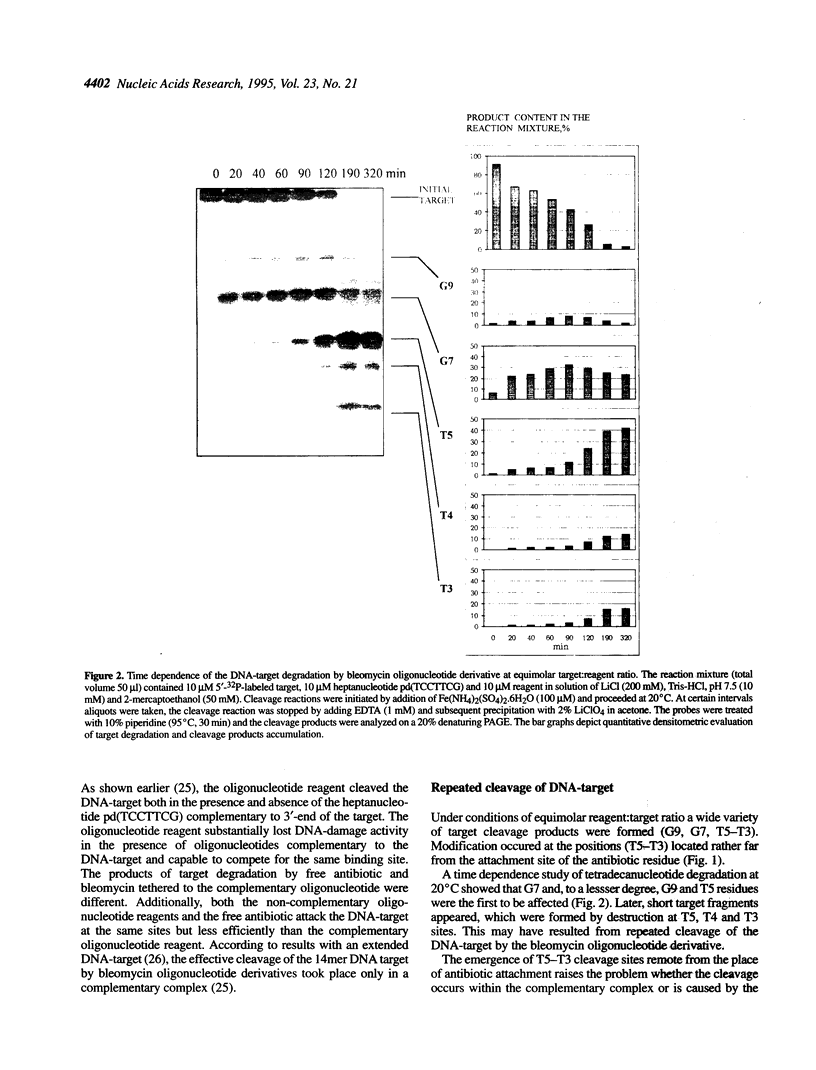
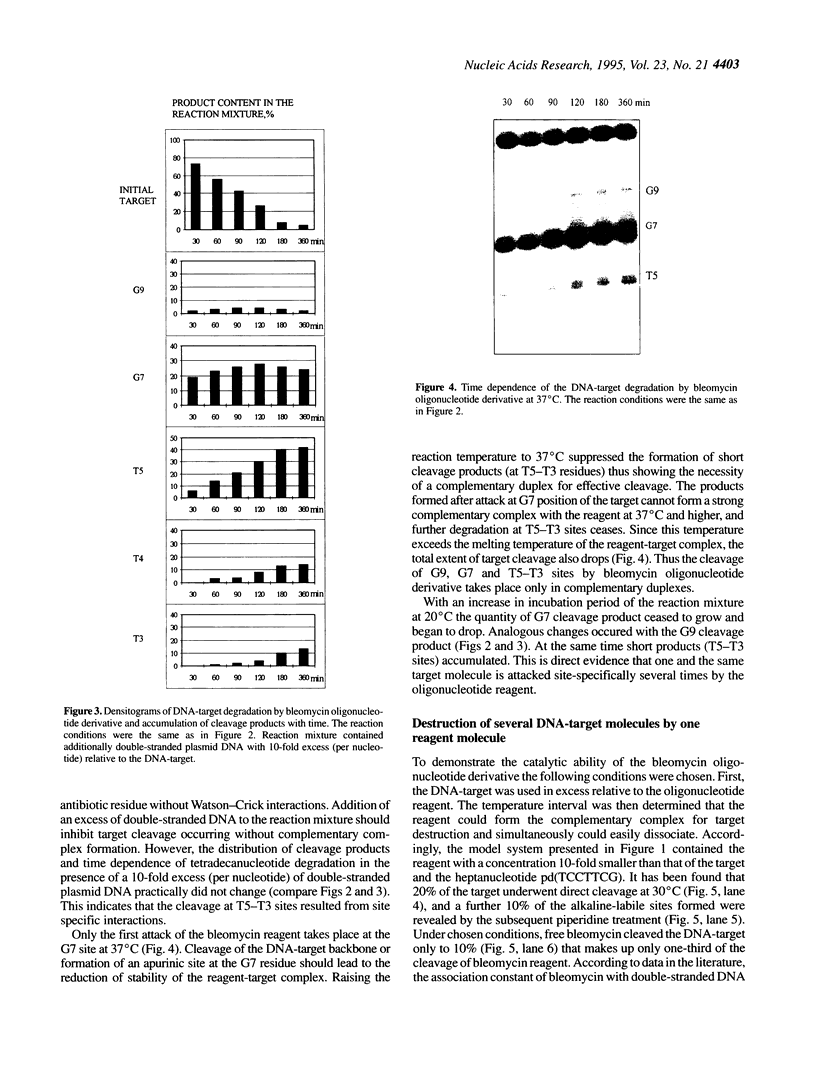
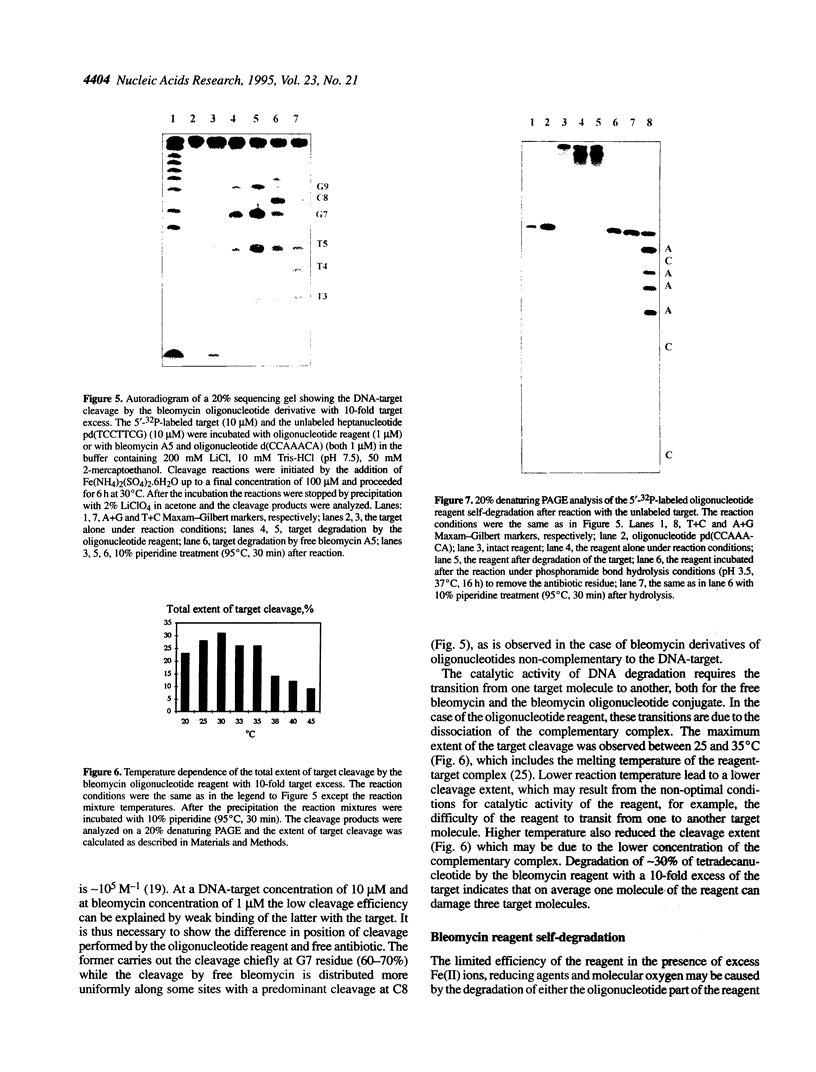
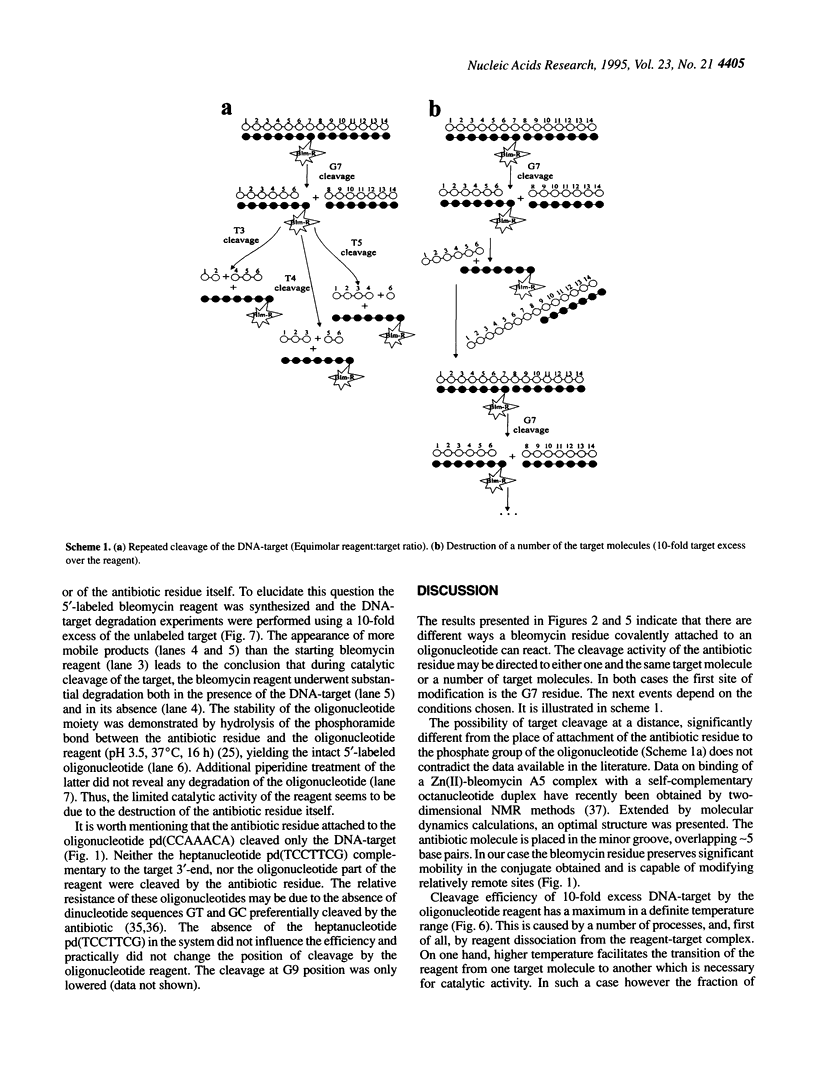
Oligonucleotide reagents have been created which are capable of catalytic site-specific cleavage of DNA-targets. The oligonucleotide reagent Blm-R-pd(CCAAACA) bearing the bleomycin A5 (Blm-RH) residue was used to degrade the DNA-target pd(TGTTTGGCGAAGGA). It has been shown that at equimolar reagent: target concentration the bleomycin oligonucleotide derivative can repeatedly cleave the target at G9, G7, T5, T4 and T3 in site-specific manner. This paper demonstrates that with a 10-fold excess of the DNA-target relative to the reagent 30% degradation of the target was observed primarily at a single position G7. The paper also shows that one reagent molecule containing bleomycin A5 residue was capable to degrade three molecules of the DNA-target. The catalytic activity of Blm-R-pd(CCAAACA) was the highest in the temperature range close to the melting temperature of the reagent-target complex, that is under conditions where the oligonucleotide reagent can form a complementary complex and easily dissociate to interact with the next molecule of the target. The number of target molecules degraded by the bleomycin reagent is limited by the degradation of the antibiotic residue itself.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor C. R., Tinoco I., Jr Absorption and optical rotatory dispersion of seven trinucleoside diphosphates. J Mol Biol. 1965 Aug;13(1):65–77. doi: 10.1016/s0022-2836(65)80080-8. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper: sequence-specific targeting. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7147–7151. doi: 10.1073/pnas.83.19.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova E. I., Fedorova O. S., Knorre D. G. Kinetic study of the addressed modification by hemin derivatives of oligonucleotides. Biochimie. 1993;75(1-2):5–11. doi: 10.1016/0300-9084(93)90018-n. [DOI] [PubMed] [Google Scholar]
- Frolova E. I., Ivanova E. M., Zarytova V. F., Abramova T. V., Vlassov V. V. Porphyrin-linked oligonucleotides. Synthesis and sequence-specific modification of ssDNA. FEBS Lett. 1990 Aug 20;269(1):101–104. doi: 10.1016/0014-5793(90)81129-c. [DOI] [PubMed] [Google Scholar]
- Gajewski E., Aruoma O. I., Dizdaroglu M., Halliwell B. Bleomycin-dependent damage to the bases in DNA is a minor side reaction. Biochemistry. 1991 Mar 5;30(9):2444–2448. doi: 10.1021/bi00223a021. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kross J., Henner W. D., Hecht S. M., Haseltine W. A. Specificity of deoxyribonucleic acid cleavage by bleomycin, phleomycin, and tallysomycin. Biochemistry. 1982 Aug 31;21(18):4310–4318. doi: 10.1021/bi00261a021. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Helene C., Chassignol M., Thuong N. T. Targeted cleavage of polynucleotides by complementary oligonucleotides covalently linked to iron-porphyrins. Biochemistry. 1986 Nov 4;25(22):6736–6739. doi: 10.1021/bi00370a002. [DOI] [PubMed] [Google Scholar]
- Lin S. B., Blake K. R., Miller P. S., Ts'o P. O. Use of EDTA derivatization to characterize interactions between oligodeoxyribonucleoside methylphosphonates and nucleic acids. Biochemistry. 1989 Feb 7;28(3):1054–1061. doi: 10.1021/bi00429a020. [DOI] [PubMed] [Google Scholar]
- Lokhov S. G., Podyminogin M. A., Sergeev D. S., Silnikov V. N., Kutyavin I. V., Shishkin G. V., Zarytova V. P. Synthesis and high stability of complementary complexes of N-(2-hydroxyethyl)phenazinium derivatives of oligonucleotides. Bioconjug Chem. 1992 Sep-Oct;3(5):414–419. doi: 10.1021/bc00017a010. [DOI] [PubMed] [Google Scholar]
- Murugesan N., Ehrenfeld G. M., Hecht S. M. Oxygen transfer from bleomycin-metal complexes. J Biol Chem. 1982 Aug 10;257(15):8600–8603. [PubMed] [Google Scholar]
- Nakamura M., Peisach J. Self-inactivation of Fe(II)-bleomycin. J Antibiot (Tokyo) 1988 May;41(5):638–647. doi: 10.7164/antibiotics.41.638. [DOI] [PubMed] [Google Scholar]
- Petering D. H., Byrnes R. W., Antholine W. E. The role of redox-active metals in the mechanism of action of bleomycin. Chem Biol Interact. 1990;73(2-3):133–182. doi: 10.1016/0009-2797(90)90001-4. [DOI] [PubMed] [Google Scholar]
- Povirk L. F. Catalytic release of deoxyribonucleic acid bases by oxidation and reduction of an iron.bleomycin complex. Biochemistry. 1979 Sep 4;18(18):3989–3995. doi: 10.1021/bi00585a023. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Stein R. W., Peisach J., Horwitz S. B. Properties and products of the degradation of DNA by bleomycin and iron(II). Biochemistry. 1978 Jul 11;17(14):2746–2754. doi: 10.1021/bi00607a008. [DOI] [PubMed] [Google Scholar]
- Sergeev D. S., Zarytova V. F., Mamaev S. V., Godovikova T. S., Vlassov V. V. Sequence-specific cleavage of single-stranded DNA by oligonucleotides conjugated to bleomycin. Antisense Res Dev. 1992 Fall;2(3):235–241. doi: 10.1089/ard.1992.2.235. [DOI] [PubMed] [Google Scholar]
- Sergeyev D. S., Godovikova T. S., Zarytova V. F. Direct cleavage of a DNA fragment by a bleomycin-oligonucleotide derivative. FEBS Lett. 1991 Mar 25;280(2):271–273. doi: 10.1016/0014-5793(91)80309-q. [DOI] [PubMed] [Google Scholar]
- Shabarova Z. A., Dolinnaya N. G., Drutsa V. L., Melnikova N. P., Purmal A. A. DNA-like duplexes with repetitions. III. Efficient template-guided chemical polymerization of d(TGGCCAAGCTp). Nucleic Acids Res. 1981 Nov 11;9(21):5747–5761. doi: 10.1093/nar/9.21.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steighner R. J., Povirk L. F. Bleomycin-induced DNA lesions at mutational hot spots: implications for the mechanism of double-strand cleavage. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8350–8354. doi: 10.1073/pnas.87.21.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nagai K., Yamaki H., Tanaka N., Umezawa H. On the mechanism of action of bleomycin: scission of DNA strands in vitro and in vivo. J Antibiot (Tokyo) 1969 Sep;22(9):446–448. doi: 10.7164/antibiotics.22.446. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita T., Muraoka Y., Nakatani T., Fujii A., Iitaka Y., Umezawa H. Chemistry of bleomycin. XXI. Metal-complex of bleomycin and its implication for the mechanism of bleomycin action. J Antibiot (Tokyo) 1978 Oct;31(10):1073–1077. doi: 10.7164/antibiotics.31.1073. [DOI] [PubMed] [Google Scholar]
- Ueda K., Kobayashi S., Sakai H., Komano T. Cleavage of stem-and-loop structure DNA by bleomycin. Reaction on the bacteriophage G4 origin of complementary strand synthesis. J Biol Chem. 1985 May 10;260(9):5804–5807. [PubMed] [Google Scholar]
- Van Atta R. B., Bernadou J., Meunier B., Hecht S. M. On the chemical nature of DNA and RNA modification by a hemin model system. Biochemistry. 1990 May 22;29(20):4783–4789. doi: 10.1021/bi00472a006. [DOI] [PubMed] [Google Scholar]
- Zarytova V. F., Sergeyev D. S., Godovikova T. S. Synthesis of bleomycin A5 oligonucleotide derivatives and site-specific cleavage of the DNA target. Bioconjug Chem. 1993 May-Jun;4(3):189–193. doi: 10.1021/bc00021a001. [DOI] [PubMed] [Google Scholar]