Abstract
Bone marrow mesenchymal stem cells (MSCs) are a valuable cell source for tissue engineering and regenerative medicine. Transforming growth factor β (TGF-β) can promote MSC differentiation into either smooth muscle cells (SMCs) or chondrogenic cells. Here we showed that matrix stiffness modulated these differential effects. MSCs on soft substrates had less spreading, fewer stress fibers and lower proliferation rate than MSCs on stiff substrates. MSCs on stiff substrates had higher expression of SMC markers α-actin and calponin-1; in contrast, MSCs on soft substrates had a higher expression of chondrogenic marker collagen-II and adipogenic marker lipoprotein lipase (LPL). TGF-β increased SMC marker expression on stiff substrates. However, TGF-β increased chondrogenic marker and suppressed adipogenic marker on the soft substrates, while adipogenic medium and soft substrates induced adipogenic differentiation effectively. Rho GTPase was involved in the expression of all aforementioned lineage markers, but did not account for the differential effects of matrix stiffness. In addition, soft substrates did not significantly affect Rho activity, but inhibited Rho-induced stress fiber formation and α-actin assembly. Further analysis showed that MSCs on soft matrices had weaker cell adhesion, and that the suppression of cell adhesion strength mimicked the effects of soft substrates on the lineage marker expression. These results provide insights of how matrix stiffness differentially regulates stem cell differentiation, and have significant implications for the design of biomaterials with appropriate mechanical property for tissue regeneration.
Keywords: Extracellular matrix, cell adhesion, mesenchymal stem cells, smooth muscle cell, chondrocyte, matrix rigidity
INTRODUCTION
Bone marrow mesenchymal stem cells (MSCs) are a valuable cell source for tissue engineering and regenerative medicine applications. MSCs are expandable and can differentiate into a variety of cell types, including smooth muscle cells (SMCs), chondrocytes, osteoblasts and adipocytes. The interactions of MSCs with the extracellular matrix (ECM) play an important role in MSC differentiation and function. ECM can be designed to guide MSC differentiation, deliver MSCs for therapies and construct tissues using MSCs. In addition to ECM cues, MSC differentiation is regulated by growth factors. It is noted that transforming growth factor β (TGF-β) promotes MSC specification into a smooth muscle (SM) lineage on stiff substrates [1, 2]. In contrast, TGF-β induces chondrogenic differentiation of MSCs in hydrogels [3]. This phenomenon suggests that the ECM could play an important role in the divergence of cell fate. We postulated that ECM stiffness modulated MSC differentiation into SMC and chondrogenic lineages.
Cells are not only sensitive to dynamic mechanical loading in ECM [4-7], but are also sensitive to the mechanical properties of the ECM such as the stiffness [8-11]. The range of ECM stiffness in the body is enormous, from soft, pliable brain tissue with an elastic modulus of tenths of a kilopascal (kPa) to hard, calcified bone with a modulus of hundreds of megapascals (MPa). With many orders of magnitude separating the softest and stiffest tissue in the body, these tissues contain cells that are tuned to the specific mechanical environments in which they reside. Engler et al. have demonstrated that ECM guides MSC differentiation into osteoblastic, skeletal muscular and neural lineages in a manner dependent on ECM stiffness [11]. However, whether and how ECM stiffness regulates MSC differentiation into other cell types such as SMCs and chondrogenic cells are not clear. In this study we investigated how ECM stiffness modulates MSC differentiation into SMC and chondrogenic lineages in response to TGF-β, and explored the underlying mechanisms.
MATERIALS AND METHODS
The commercial sources of reagents are listed in Supplementary Table I.
Cell Culture and Characterization
Human MSCs were maintained and characterized for surface markers and differentiation potential as described previously [1]. Briefly, MSCs were acquired from Lonza Corp. and cultured in MSC growth medium (MSCGM, Cambrex) for expansion. After cell expansion, MSCs were subjected to flow cytometry analysis of surface marker expression (positive for CD105, CD166, CD29, and CD44 and negative for CD34, CD14, and CD45) to confirm the undifferentiated state of MSCs. The osteogenic and chondrogenic differentiation of MSCs was tested by using the protocol from Lonza Corp. The adipogenic differentiation of MSCs was induced by in Minimum Essential Medium-α (αMEM) containing 10% fetal bovine serum (FBS), insulin (10 μg/ml), dexamethasone (1 mM) and isobutylxanthine (0.5 mM). SMC lineage was induced by TGF-β1 (10 ng/ml). The differentiation of MSCs into Schwann cells was induced in N2 medium supplemented with CNTF (10 ng/ml), bFGF (10 ng/ml), 1 mM dibutyryl-cAMP and neuregulin (20 ng/ml).
For all experiments, MSCs (passage 8-11) with 70-80% confluency were used. Unless specified, experiments were performed in DMEM supplemented with 1% FBS (low FBS) and 1% penicillin/streptomycin.
Fabrication of Substrates with Different Stiffness
The first method involved the control of collagen-I gel thickness. Collagen-I gel (1 mg/ml) was prepared by neutralizing collagen-I solution with NaOH as described previously [12]. The soft substrate had collagen-I gel with about 500 μm thickness. For stiff substrates, collagen-I gel solution (before polymerization) was used to rinse the culture dishes, and excess solution was removed, resulting in a thin layer (microns) coating of polymerized collagen.
The second method was to make polyacrylamide (PA) gels with different amount of crosslinker as described previously [9]. Briefly, glass slides were treated with 3-aminopropyltrimethoxysilane and 0.5% glutaraldehyde. PA gel solution with the desired concentration of acrylamide and bis-acrylamide was allowed to polymerize to form 200 μm thick gel on the slides. Sulfo-SANPAH was used to link collagen-I (10 μg/cm2) to the PA gel surface. Stiff substrates (glass slides) were coated with the same density of collagen-I. All substrates were sterilized by UV. To determine the elastic modulus of PA gels, a Comparator (B.C. Ames Company, Waltham, MA) was used. The Comparator measured the displacement of a gel in response to a known force.
Fluorescent Staining, Quantitative Polymerase Chain Reaction (qPCR), Immunoblotting and Cell Proliferation Analysis
Fluorescence staining, microscopy, quantitative polymerase chain reaction (qPCR) and immunoblotting analysis were performed as described previously [1, 2, 4, 7]. The primer sequences for the genes of interest are listed in Supplemental Table 2. The level of gene expression was normalized with the amount of 18S ribosomal RNA in the same sample. Rho activity was measured by using a Rho affinity precipitation assay kit. Cell proliferation rate was quantified by measuring BrdU incorporation using a flow cytometer [7].
Transfection and Generation of MSC lines
The DNA plasmids encoding green fluorescence protein (GFP) or GFP-RhoA(v14) [13] were transfected into MSCs using the Lipofectamine™2000 reagent [1]. The transfected cells were selected by using G418 (500 μg/ml).
Measurement and Manipulation of Cell Adhesion Strength
The adhesion strength of cells was measured by using laminar flow chambers [14] with the application of a shear stress at 80 dyn/cm2 for 30 minutes. The percentage of cells remaining on the substrate was quantified by counting cells in multiple areas (in phase contrast images) before and after the flow experiments.
To decrease cell adhesion strength on stiff substrates coated with collagen-I, cells were pretreated with an integrin β1 antibody (5 μg/ml) (mAb13) or mouse IgG (as control) in suspension for 30 minutes, and seeded for 6 hours in the presence of the respective antibody. Cells were either fixed for immunostaining of actin and vinculin or lysed for qPCR analysis.
Statistical analysis
Statistical analysis was performed as described previously [2, 7]. Student's unpaired t-test was used to compare two groups. Analysis of variance (ANOVA) and Holm's t-test were used to compare more than two groups. A log-transformed one-sample t-test was used to analyze normalized ratios. Data from all experiments is presented as mean±standard deviation.
RESULTS
Matrix Stiffness and Cytoskeleton Organization
Expanded MSCs were characterized by the expression of surface markers and the capability of differentiation into osteogenic, chondrogenic and Schwann cells (Fig. S1).
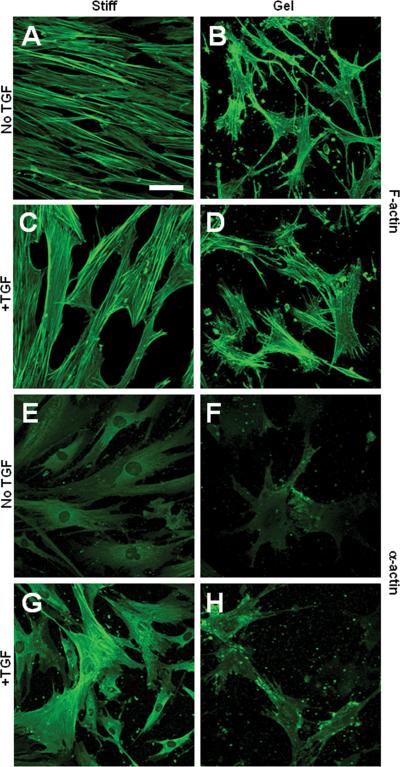
To minimize the compounding effects of soluble factors, the studies of substrate stiffness and MSC differentiation were performed in low-serum (1%) medium unless otherwise specified. We first determined the effects of substrate stiffness on MSCs by using collagen gels. MSCs on stiff substrates exhibited extensive stress fibers (Fig. 1A), while cells on collagen gels had less spreading, fewer stress fibers but more filopodia (Fig. 1B). TGF-β induced thicker stress fibers in MSCs on stiff substrates (Fig. 1C) but not on collagen gels (Fig. 1D).
Figure 1.
Effects of collagen gel and TGF-β on actin cytoskeleton in MSCs. MSCs were grown on collagen-coated culture dishes (stiff substrates) or collagen gel for 1 day in the absence or presence of TGF-β. The cells were stained for F-actin by using phalloidin (A-D) or stained for SM α-actin (E-H). Scale bar=50 μm.
MSCs had a low level of SM α-actin expression on stiff substrates (Fig. 1E) and the expression of α-actin was lower in MSCs on collagen gels (Fig. 1F). In either case, α-actin was not assembled into stress fibers, implying that α-actin was not a functional part of the cytoskeleton. TGF-β treatment resulted in an increase of α-actin expression and the assembly of α-actin into stress fibers in MSCs on a stiff substrate (Fig. 1G), suggesting TGF-β induced a contractile phenotype in MSCs. In contrast, TGF-β failed to induce the contractile phenotype in MSCs on collagen gels (Fig. 1H).
Effect of Matrix Stiffness and TGF-β on Lineage Specifications
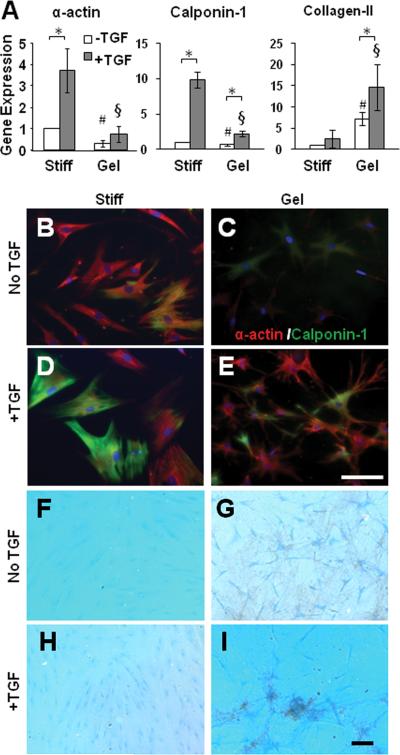
We used qPCR to determine the gene expression of differentiation markers. Consistent with immunostaining results, MSCs grown on collagen gels had lower expression levels of SMC markers α-actin and calponin-1 than MSCs grown on a stiff substrate (Fig. 2A). TGF-β significantly induced the expression of α-actin and calponin-1 in MSCs on stiff substrates but not on collagen gels. In contrast, collagen-II, a chondrogenic marker, increased dramatically in MSCs grown on collagen gels compared to MSCs grown on stiff substrates. TGF-β further increased collagen-II expression on collagen gels but not on stiff substrates. These striking differential effects suggest that substrate stiffness plays an important role in the divergence of lineage specification into SMC and chondrogenic lineages.
Figure 2.
Effects of collagen gel and TGF-β on gene expression and MSC differentiation. (A) MSCs were cultured on collagen-coated stiff substrates or collagen gel for 2 days in the absence or presence of TGF-β. Cells were lysed for qPCR analysis. * indicates significant difference (P<0.05). # indicates significant difference from the gene expression in MSCs on stiff substrate without TGF-β (P<0.05). § indicates significant difference from the gene expression in MSCs on stiff substrate with TGF-β (P<0.05). (B-E) Differentiation of MSCs into SMC lineage. MSCs were cultured on collagen-coated stiff substrates or collagen gel for 2 weeks in the absence or presence of TGF-β. Cells were double-stained for SM α-actin (red) and calponin-1 (green). (F-I) Under the same culture condition as in B-E, GAGs were stained by Alcian Blue. Scale Bars are 100 μm.
Long-term (2 weeks) culture of MSCs on stiff substrates or collagen gels showed that MSCs (~15%) on stiff substrates, but not on collagen gels, developed stress fibers containing both α-actin and calponin-1 (Fig. 2B-C). TGF-β increased the number of cells (~77%) with α-actin/calponin-1 containing stress fibers on stiff substrates but did not induce a contractile phenotype on collagen gels (Fig. 2D-E).
In contrast, MSCs on collagen gels, but not on stiff substrates, exhibited higher level of GAG staining, suggesting chondrogenic differentiation (Fig. 2F-G). The supplement of TGF-β further enhanced this difference (Fig. 2H-I).
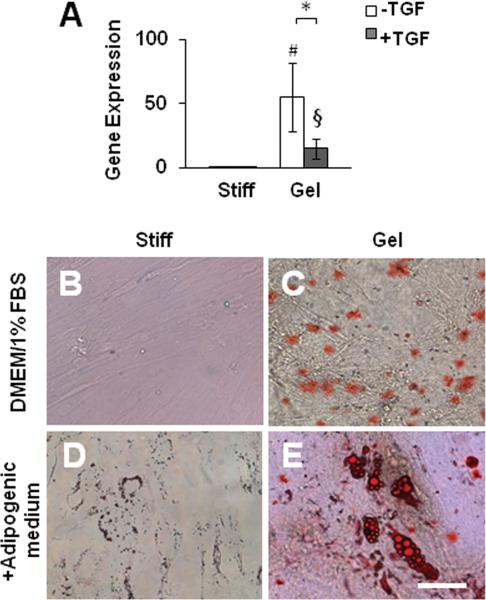
Of note, collagen gels also drastically induced the expression of lipoprotein lipase (LPL), an adipogenic linage marker (Fig. 3A), suggesting that the effect of a soft substrate was not specific and lineage specification might require other microenvironmental factors. Indeed, TGF-β suppressed LPL expression on collagen gels (Fig. 3A). Long-term culture showed that soft substrate induced immature lipid droplets (Fig. 3B-C). With the addition of adipogenic supplements, adipogenic differentiation was detected on collagen gels but not on stiff substrates (Fig. 3D-E).
Figure 3.
Effect of collagen gel on MSC differentiation into adipogenic lineage. (A) MSCs were cultured on collagen-coated stiff substrates or collagen gel for 2 days in the absence or presence of TGF-β. Cells were lysed for qPCR analysis as described in Figure 2A. (B-E) Adipogenic differentiation of MSCs. MSCs were cultured on collagen-coated stiff substrates or collagen gel for 2 weeks in the absence or presence of adipogenic medium. Lipids were stained by Oil Red. Scale Bar=50 μm.
MSCs on Polyacrylamide (PA) Gels
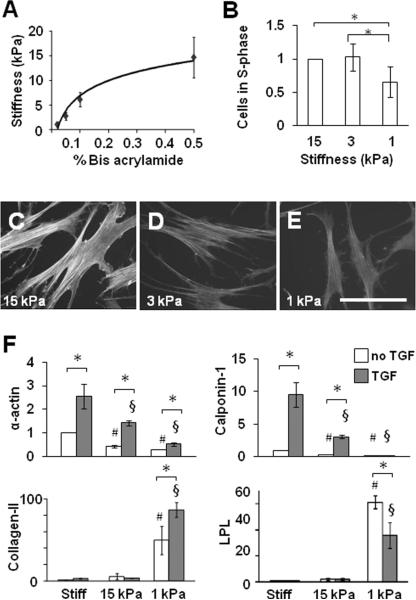
To control substrate stiffness, we made collagen-I-coated PA gels with various stiffnesses by adjusting the amount of acrylamide and bis-acrylamide cross linker, as exemplified in Fig. 4A. MSCs had a similar proliferation rate on substrates with stiffness between 3 and 15 kPa, and showed a 30% decrease in the proliferation rate on a 1-kPa substrate (Fig. 4B); these results were correlated with a decrease in ERK1/2 phosphorylation (Fig. S2A). Furthermore, MSCs had less spreading (Fig. S2B-D), fewer stress fibers (Fig. 4C-E), lower expression of α-actin and no assembly of α-actin into stress fibers (Fig. S2E-G) on soft substrates in a stiffness-dependent manner.
Figure 4.
Modulation of MSC proliferation and differentiation by matrix stiffness. (A) Stiffness of PA gels using 6% acrylamide and different bis-acrylamide concentrations. (B) MSCs were grown on PA gel for 1 day. Proliferation of MSCs on PA gels was quantified by BrdU incorporation. The number of cells in S phase for each sample was normalized to that on 15 kPa substrate to show fold-changes. * indicates significant difference (P<0.05). (C-E) Phalloidin staining of F-actin after 1-day culture. (F) MSCs were grown on collagen-coated stiff substrates or PA gels for 2 days in the absence or presence of TGF-β, and lysed for qPCR analysis. *, # and § indicate significant differences as in Figure 2A.
Stiffness-Dependent Lineage Specifications
To confirm the effects of matrix rigidity in collagen gel experiments, the gene expression of the same set of differentiation markers were examined. The expression of SMC markers α-actin and calponin-1 decreased with substrate stiffness, while chondrogenic marker collagen-II and adipogenic marker LPL showed an opposite trend and significantly increased on a soft (1 kPa) substrate (Fig. 4F). Consistent with the results on collagen gels (Fig. 2A, 3A), TGF-β induced the expression of SMC markers on stiff substrates but the magnitude of this effect was weakened on soft substrates. In contrast, on a 1-kPa substrate, TGF-β further increased collagen-II expression, but suppressed LPL expression (Fig. 4F).
Smad2/3 and Rho GTPase in Mechanotransduction
To determine whether substrate stiffness affected TGF-β signaling, we examined the translocation and phosphorylation of Smad2/3. As shown in Figure S3, matrix stiffness did not significantly affect the activation of Smad2/3 in the absence or presence of TGF-β, suggesting that matrix stiffness regulated mechanotransduction in parallel to Smad2/3 signaling.
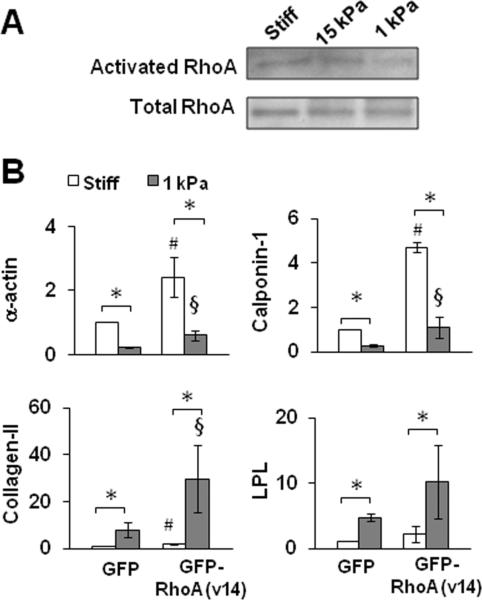
Since Rho GTPase plays an important role in stress fiber assembly and mechanotransduction, we directly examined Rho activity. However, we did not detect a significant, substrate stiffness-dependent difference of RhoA activity on stiff and soft substrates (Fig. 5A), implying that the difference in stress fibers and -actin assembly on stiff and soft substrates was not directly mediated by RhoA.
Figure 5.
Effects of Rho activation on the gene expression in MSCs on stiff and soft substrates. (A) MSCs were cultured for 1 day and lysed for Rho activity assay followed by immunoblotting analysis. (B) MSCs with the expression of GFP or GFP-RhoA(v14) were cultured for 2 days and lysed for qPCR analysis. * indicates significant difference (P<0.05). # indicates significant difference from GFP-MSCs on stiff substrates (P<0.05). § indicates significant difference from GFP-MSCs on soft substrates (P<0.05).
To further investigate whether Rho activation could induce stress fibers on soft substrates and thus regulate the differential expression of differentiation markers, we overexpressed the constitutively activated RhoA, RhoA(V14), in MSCs. RhoA activation significantly induced the expression of SMC markers on stiff substrates, and to a lesser extent on soft substrates (Fig. 5B). However, RhoA activation also significantly increased the expression of collagen-II and LPL on a soft substrate (Fig. 5B). These results suggested that Rho-mediated signaling positively regulated the expression of all these differentiation markers but could not account for their differential expression on stiff and soft substrates.
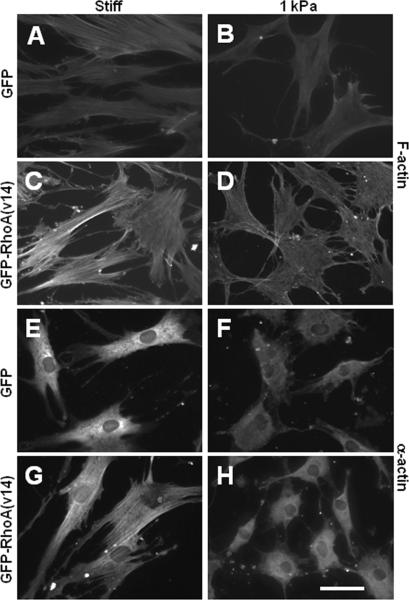
Surprisingly, actin filament immunostaining showed that RhoA activation drastically induced stress fibers and the assembly of α-actin into fibers in cells on stiff but not soft substrates (Fig. 6), suggesting that soft substrates limit the function of Rho activity.
Figure 6.
Effect of Rho activation on actin cytoskeleton and the expression and assembly of α-actin. MSCs with the expression of GFP or GFP-RhoA(v14) were cultured on collagen-coated stiff substrates or PA gels for 1 day. (A-D) MSCs were stained for actin filaments by using phalloidin. (E-H) MSCs were stained for SM α-actin. Scale bar=50 μm.
Effect of Cell Adhesion Strength on Differentiation
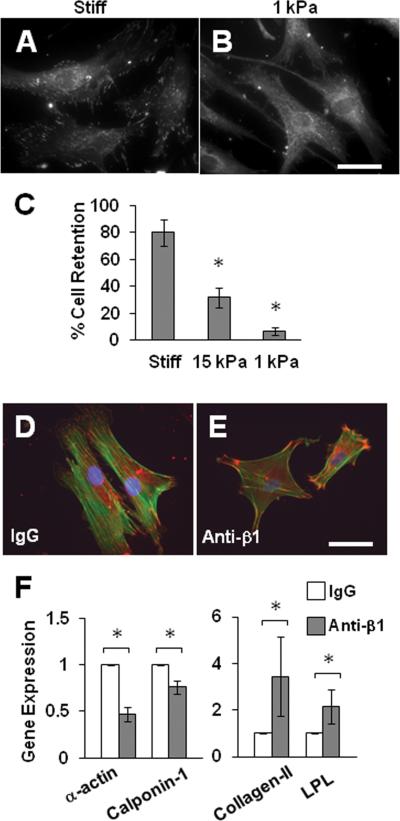
We speculated that the lack of stress fibers and differential gene expression in cells on soft substrates were due to the weaker adhesion strength, a limiting factor of soft substrates. Indeed, the staining of focal adhesions showed that focal adhesions of cells on soft substrates were fewer and smaller (Fig. 7A-B). The direct measurement of cell adhesion strength by applying fluid shear stress demonstrated a significant decrease of cell adhesion strength with substrate stiffness (Fig. 7C).
Figure 7.
Cell adhesion strength mediated the effects of matrix stiffness. (A-B) MSCs were cultured collagen-coated stiff substrates or PA gels for 1 day and stained for vinculin. Scale bar=50 μm. (C) MSCs were subjected to shear stress and the percentage of cells remaining on the substrates was quantified. * indicates significant difference from all other samples. (D-F) MSCs were treated with a blocking antibody against integrin β1 (anti-β1) or a control IgG, seeded on collagen-coated culture dishes, and either stained for actin filaments (green) and vinculin (red) (D-E) or lysed for qPCR analysis (F). Scale bar=50 μm. * indicates significant difference (P<0.05).
To determine whether weaker adhesion strength could result in the differential gene expression of SMC and chondrogenic genes, we used integrin antibodies to partially block MSC adhesion, which decreased cell spreading (Fig. S4), focal adhesions and stress fibers (Fig. 7D-E). As expected, the decrease in cell adhesion suppressed the expression of SMC markers and increased the expression of collagen-II and LPL (Fig. 7F), mimicking the effects of a soft substrate.
DISCUSSION
Our study has shown that MSC differentiation is responsive to two important stimuli in the cellular microenvironment: matrix stiffness and growth factors. Matrix stiffness alone can promote a certain lineage over another, i.e. SMC on stiff substrates, and cartilage and fat on soft substrates. These results are largely correlated with the mechanical properties of the native tissue in which these cells reside. For example, native arteries have MPa stiffness, while fat stiffness is at the level of kPa. Although mature cartilage may not be as soft as 1 kPa, this stiffness could be the reminiscent of the stiffness during cartilage development, thereby signaling development toward a cartilage phenotype by matrix synthesis.
It has been shown that matrix stiffness regulates MSC differentiation toward bone, muscle or neuronal lineages when grown on hard, medium or soft substrates that are akin to the native stiffness of their respective tissues [11]. Here we report the effects of matrix stiffness on smooth muscle, chondrogenic and adipogenic lineages. The difference of these observations could be attributed to the difference in culture medium, e.g., the amount of FBS and other biochemical supplements. In most of our experiments, low FBS was used to avoid the compounding effects of soluble factors in the culture medium. Nevertheless, the fact that the same matrix stiffness modulates the differentiation of cells into multiple lineages suggests that, at least in some cases (e.g., chondrogenic vs. adipogenic), matrix stiffness alone is not sufficient to determine the cell fate. The definitive commitment and terminal differentiation of the cells into a specific lineage may require the cooperation of other differentiation factors. Indeed, the effects of matrix stiffness on the expression of SMC, chondrogenic and adipogenic makers are further defined by TGF-β and adipogenic medium. TGF-β has previously been used to induce stem cell differentiation into either SMC lineage [2] or chondrogenic lineage [3, 15]. Our results indicate that this phenomenon can be explained by the growth factor effects working in concert with the physical cue of matrix stiffness. Although matrix stiffness alone is not most effective to drive a specific and terminal differentiation, it is an important determinant and co-factor for cell differentiation induced by soluble biochemical factors. Stem cells need to integrate microenvironmental cues from both soluble biochemical factors and ECM to regulate differentiation.
The differential effects of matrix stiffness on the expression of SMC and chondrogenic markers have significant implications in tissue engineering and regenerative medicine applications. For example, to construct vascular grafts, scaffolds with MPa stiffness are preferred over hydrogels that are not appropriate for SMC differentiation and are not strong enough for a functional vascular graft. On the other hand, when MSCs are engineered for cartilage repair, they are usually grown in hydrogels or as cell pellets [3, 15], of which the stiffness is low; under these conditions, three-dimensional culture microenvironment and cell-cell interactions could also play a role. The results from this study provide a rational basis for the design and selection of appropriate scaffolds.
Our results also provide important insights into the underlying mechanisms of mechanotransduction. Matrix stiffness does not affect the Smad2/3 signaling pathway, suggesting that it may regulate mechanotransduction in parallel to TGF-β signaling. Rho GTPase plays an important role in actin assembly and focal adhesion formation. Although Rho has been suggested as a mechanotransducer of matrix stiffness, the difference in Rho activity on stiff and soft substrates is not significant under our experimental conditions with low serum. In addition, Rho activation induced SMC markers on a stiff substrate and increased chondrogenic and adipogenic markers on a soft substrate, suggesting that Rho-mediated signaling is important for the expression of all these linage markers but cannot explain the differential expression of these markers regulated by matrix stiffness. As Rho regulates multiple signaling pathways, the basal level of Rho activity may be essential for maintaining the expression of many genes.
If Rho cannot account for the differential effects of matrix stiffness, what causes the decrease of stress fibers on soft substrate and the differential gene expression in MSCs? Our data indicate that the weak cell adhesion on soft substrates is a limiting factor. Soft substrate can only withstand low levels of force exerted by cells. When cells generate traction forces higher than what the substrate can withstand, the matrix may yield and cells could adapt by decreasing the traction force, integrin binding and their own stiffness [16, 17], which modulate cell spreading and thus differentiation [18, 19]. The limit of cell adhesion strength on a soft substrate can provide feedback to cells to decrease stress fiber formation, which also explains why Rho activation fails to induce stress fibers on soft substrates. Indeed, cell adhesion is much weaker on soft substrates, and the decrease of cell adhesion strength by blocking integrin binding mimics the effect of soft substrates on the differential expression of SMC, chondrogenic and adipogenic markers in MSCs. In summary, our results support the following hypothesis: a soft matrix only allows weak cell adhesion, which decreases the formation of stress fibers and differentially regulates the expression of SMC, chondrogenic and adipogenic markers. These experiments not only underscore the importance of mechanical cues in collaborating with chemical cues to determine cell fate, but also provide a mechanistic explanation for how mechanical cues can regulate the lineage specification of MSCs and other type of stem cells.
Conclusions
In conclusion, stiff matrix promotes MSC differentiation into SMC lineage, while soft matrix (~1 kPa) promotes MSC differentiation into chondrogenic and adipogenic lineage. Although matrix stiffness is an important determinant of stem cell differentiation, its effect may not be specific for only one lineage, and biochemical factors such as TGF-β are required, together with matrix stiffness, to define a unique differentiation pathway. Furthermore, the weak cell adhesion strength on soft matrix may account for the inhibition of Rho-induced stress fibers and α-actin assembly and the differential effects of matrix stiffness on MSC differentiation.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported in part by grants (HL078534 and HL083900 to S.L.) and Predoctoral Fellowships (to A.D.T. and 1F31HL087728 to R. D.) from National Institute of Health. A.D.T. and R.D. are Siebel Scholars. A.W. is a postdoctoral CIRM Scholar of California Institute for Regenerative Medicine training grant TG2-01164.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wang D, Park JS, Chu JS, Krakowski A, Luo K, Chen DJ, et al. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor β1 stimulation. J Biol Chem. 2004;279(42):43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- 2.Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, et al. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28(4):734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 3.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9(4):679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 4.Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88(3):359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 5.Kurpinski K, Park J, Thakar RG, Li S. Regulation of Vascular Smooth Muscle Cells and Mesenchymal Stem Cells by Mechanical Strain. Mol Cell Biomech. 2006;3(1):21–34. [PubMed] [Google Scholar]
- 6.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A. 2005;102(44):15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2006;103(44):16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 9.Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204(1):198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Lao J, Chen BP, Li YS, Zhao Y, Chu J, et al. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. Faseb J. 2003;17(1):97–99. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 13.del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sánchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. European Journal of Immunology. 1999;29(11):3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203(1):166–176. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 16.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9(1):82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.