Abstract
Objective
Prospectively investigate whether weight gain or weight loss increases risk for onset of binge eating and purging in adolescent women.
Method
Diagnostic interviews and direct measures of body mass were completed by 496 adolescent women annually for eight years.
Results
Substantial weight gain or weight loss during the study produced a 7-fold increase in risk for future onset of threshold or subthreshold bulimia nervosa (BN) relative to weight-stable participants, though the low incidence rate limited statistical power. Those who showed onset of threshold/subthreshold BN experienced greater increases in weight in the two years before onset of their eating disorder relative to healthy comparison participants.
Discussion
This is the first prospective study to demonstrate that weight gain and weight loss may both increase risk for future onset of bulimic pathology. Results suggest that young women who have difficulty limiting their dietary intake are at increased risk for BN, an eating disorder characterized by loss of control over eating.
Little is known about the relation between relative body weight and the development of BN. Russell1, in his classic paper that first formally identified this syndrome, noted that BN was “an ominous variant of anorexia” and that these patients, though typically presenting with “unremarkable” body weights, weighed significantly more prior to the development of their eating disorder. Others2-4 have presented evidence that individuals with BN are significantly weight-suppressed, that is, their current weight tends to be substantially below their retrospectively reported highest weight ever relative to controls. Because individuals with BN are typically in the normal weight range, this implies that many patients with BN were somewhat overweight at their previous highest weight. Similarly, Fairburn et al.5 and Yates6 report that individuals with BN retrospectively reported a higher premorbid body mass than healthy controls and that there was a greater history of overweight in first-degree relatives of BN individuals.
On the other hand, the normal weight status of most individuals with BN also conceals a weight history that involved weighing significantly less than they currently weigh. Garner and Fairburn3 reviewed evidence suggesting that most individuals with BN lose a great deal of weight in the process of developing their disorder, with roughly a third reaching body weights characteristic of anorexia nervosa before gaining weight and transitioning to BN.7 Further, case study and non-experimental research suggests that significant weight loss precedes – and presumably spurs the development of – binge eating.8-10 In sum, many individuals with BN report a history of both higher and lower body weights relative to their weights when they come to the attention of researchers or clinicians. However, little is known about whether weight gain or weight loss increases risk for the development of BN.
A fundamental weakness in existing studies of the possible role of weight history in BN is that the relevant data are collected retrospectively. Such retrospective reports are often inaccurate because of natural forgetting that happens over long periods of time, because memories are influenced by current mood state and subsequent events, people tend to distort memories in a more socially desirable direction, people may reinvent the past to suit their current needs and circumstances, and people tend to remember events as happening earlier than they actually did.(e.g., 11,12) It would be preferable to examine the relation of weight change to the development of bulimic pathology prospectively by (a) examining bulimic symptom frequency before and after individuals have gained or lost a significant amount of weight and (b) by identifying individuals who develop bulimic pathology and determining whether weight loss or weight gain preceded the emergence of this pathology relative to a similar group that did not develop bulimic pathology. No prospective study has tested whether significant weight loss or weight gain increases risk for future development of bulimic pathology or tested whether those who develop bulimic pathology experience accelerated weight loss or weight gain prior to the development of this pathology. Because self-reported weight and height, whether collected contemporaneously or retrospectively, have questionable accuracy, (c.f., 13) it would be preferable to rely on directly measured height and weight.
This study prospectively examined the relation between weight change and onset of threshold or subthreshold BN in two different ways. The first aim was to identify participants in this data set who experienced a substantial weight loss or weight gain during the 8 years and determine if either weight change pattern was associated with increased risk for onset of threshold/subthreshold BN in the year during or soon after the weight change occurred relative to a control group who was weight stable across the 8 years. The second aim was to test whether individuals who showed onset of threshold/subthreshold BN evidence a history of significant weight loss or weight gain previous to onset of the eating disorder relative to those who remained free of this eating disorder.
Methods
Participants and Procedures
Participants were 496 adolescent girls from public and private middle schools in a large US city. These data were collected yearly for 8 years as part of a larger study.14 Adolescents ranged from 12 to 15 years of age (M = 13) and were in 7th or 8th grade at baseline (year 1). Pubertal development was not assessed in detail, but some individuals (n = 127) had not experienced menarche by the time of the baseline assessment. The sample included 2% Asian/Pacific Islanders, 7% African Americans, 68% Caucasians, 18% Hispanics, 1% Native Americans, and 4% who specified other/mixed racial heritage, which was representative of the schools from which we sampled. Average parental education, a proxy for socioeconomic status, was 29% high school graduate or less, 23% some college, 33% college graduate, and 15% graduate degree, which was representative of the city from which we sampled.
The study was described as an investigation of adolescent mental and physical health. An active parental consent procedure was used to recruit participants, wherein an informed consent letter describing the study was sent to parents of eligible girls (a second mailing was sent to non-responders). This resulted in an average participation rate of 56%, which was similar to rates in other school-recruited samples that used active consent procedures and structured interviews (e.g., 61%).15 Participants completed a structured interview assessing eating disorder symptoms, depression,16 and had their weight (via a SECA scale, model 770) and height measured by female assessors at baseline (year 1) and at seven annual follow-ups (year 2 to year 8). Female assessors with at least a bachelor's degree in psychology conducted interviews. They attended 24 hours of training, wherein they received instruction in structured interview skills, reviewed diagnostic criteria for relevant DSM-IV disorders,17 observed simulated interviews, and role-played interviews. Assessors had to demonstrate an inter-rater agreement (kappa [k] > .80) with supervisors using tape-recorded interviews before collecting data. A randomly selected subset of interviews (5%) was recorded annually to ensure that assessors continued to show acceptable inter-rater agreement (k > .80) with experts. Assessments took place at schools during or immediately after school hours or at participants' houses. Participants received a gift certificate or cash payment for completing each assessment.
In the first set of analyses, we identified adolescents who experienced either a 10% weight gain or weight loss over a 1-year period (based on age-adjusted normative weight charts showing an average increase in BMI of 2% per year) during the first 5 years of the study. Regardless of when this weight change occurred, this year was considered an index year for either weight gain or weight loss. A third group of weight stable adolescents, who never experienced a weight change greater than 5% year-to-year across the 8 years, were identified and referred to as weight stable participants. These participants' scores on a measure of bulimic symptoms were then compared at the end of the year when the weight change occurred and for the two subsequent yearly assessments. This allowed us to determine if a large increase or decrease in body mass increased the risk of subsequently developing threshold/subthreshold BN relative to a weight stable control group. In the second set of analyses, individuals who showed onset of threshold or subthreshold BN after year 2 were identified. Then the weight change trajectory experienced by this group during the two annual visits preceding the onset of bulimic pathology was calculated. These weight changes were compared to those of all other participants who never showed onset of bulimic pathology. In this way we were able to determine if the bulimic pathology group experienced a different two-year weight trajectory prior to their identification than did the non-disordered control group.
Measures
Eating pathology
The Eating Disorder Diagnostic Interview,18 a semi-structured interview that was adapted from the Eating Disorder Examination,19 assessed DSM-IV criteria for anorexia nervosa (AN), BN, and binge eating disorder (BED) at each of the 8 annual assessments. These data also allowed us to determine whether participants met criteria for threshold or subthreshold BN at each assessment point. In accordance with previous studies,18,20,21 participants who endorsed symptoms from each domain for BN, but who endorsed a subthreshold level on at least one symptom were given subthreshold diagnoses. For a threshold BN diagnosis we required participants to report at least 24 uncontrollable binge eating episodes and 24 compensatory behavior episodes (self-induced vomiting, laxatives or diuretics, fasting, and excessive exercise intended to compensate for overeating) over a 3-month period, and to report that weight and shape was definitely one of the main aspects of self-evaluation. For a subthreshold BN diagnosis we required participants to report at least 6 uncontrollable binge eating episodes and 6 compensatory behavior episodes over a 3-month period, and to report that weight and shape was definitely an aspect of self-evaluation. Excessive exercise was defined as at least 1 hour of vigorous exercise or 2 hours of moderate exercise that was engaged in specifically to compensate for a binge eating episode. Fasting was defined as abstaining from caloric intake (meals or snacks) for approximately 24 hours or more for the purpose of weight control (e.g., only eating dinner for two consecutive days).17
To assess the test-retest reliability for eating disorder diagnoses for this adapted interview, a randomly selected subset of 137 participants who were interviewed by the assessors for this study and another ongoing study18 were re-interviewed by the same assessor within a 1-week period, resulting in high test-retest reliability for threshold and subthreshold diagnoses of AN, BN, or BED (κ = .96). To assess the inter-rater agreement for these threshold and subthreshold eating disorder diagnoses, a randomly selected subset of 149 participants who were interviewed by the assessors for this study and the other ongoing study were re-interviewed by a second blinded assessor, resulting in high inter-rater agreement (κ = .86). The EDDI has also been shown to be sensitive to detecting intervention effects and has shown predictive validity for future onset of depression in past studies.16,18,22
Body mass
The body mass index (BMI= kg/m2) was used to reflect relative weight.23 Height was measured to the nearest millimeter using portable stadiometers. Weight was assessed to the nearest 0.1 kg using digital scales with the girls wearing light indoor clothing without shoes or coats. Two measures of height and weight were obtained and averaged. The BMI shows convergent validity (r = .80 – .90) with direct measures of body fat such as dual energy x-ray absorptiometry.23
Results
Classification of Participants
The number of participants providing data at each assessment is reported in Table 1. An attempt was made to assess each participant yearly, even if she missed a previous assessment. Participants missing data at any assessment point did not differ significantly from the remaining participants on demographic factors or any of the study variables, suggesting that attrition should not introduce bias.
Table 1. Group Membership and Attrition.
| Study Year | Assessed | Not Assessed | Weight Losers Identified | Weight Gainers Identified | BN Spectrum Identified |
|---|---|---|---|---|---|
| 1 | 496 (100%) | 0 (0%) | |||
| 2 | 482 (97.2%) | 13 (2.6%) | 13 (2.7%) | 30 (6.2%) | |
| 3 | 477 (96.2%) | 18 (3.6%) | 16 (3.4%) | 23 (4.8%) | 0 (0%) |
| 4 | 486 (98.0%) | 9 (1.8%) | 19 (3.9%) | 10 (2.1%) | 5 (1.0%) |
| 5 | 480 (96.8%) | 15 (3.0%) | 14 (2.9%) | 9 (1.9%) | 5 (1.0%) |
| 6 | 431 (86.9%) | 34 (6.9%) | 14 (3.3%) | 14 (3.3%) | 7 (1.6%) |
| 7 | 452 (91.1%) | 43 (8.7%) | 4 (0.9%) | ||
| 8 | 451 (90.9%) | 44 (8.9%) |
Note. Weight Losers = participants with a 10% age-adjusted BMI decrease; Weight Gainers = participants with a 10% age-adjusted BMI increase, BN Spectrum = participants who met criteria for clinical or subclinical Bulimia Nervosa.
As the goal of the first analysis was to investigate risk for onset of threshold/subthreshold BN during or after a considerable weight gain or loss, participants were identified who experienced a 10% age-adjusted weight increase or decrease during any of the first five one-year intervals of the study. The number of participants who showed a 10% change in age adjusted BMI at each of these intervals is reported in Table 1. Because an average of only 17 participants per year showed a 10% age-adjusted decrease, and an average of 18 participants showed a 10% age-adjusted increase, in BMI scores over each of the one-year intervals, we pooled participants who showed a 10% age-adjusted weight change across any of the five intervals (year 1 to year 2, year 2 to year 3, year 3 to year 4, year 4 to year 5, or year 5 to year 6) to maximize statistical power. In total, there were 84 adolescent girls who evidenced a 10% age-adjusted reduction in BMI over any of the five one-year detection intervals in this study, though only 76 of these individuals provided interview data at one and two years following their weight loss. Similarly, there were 92 adolescent girls who evidenced a 10% age-adjusted weight increase in BMI over any of the five one-year detection intervals, but only 86 of them had two years of follow-up data. For the pooled data, the two assessments that encompass the 10% age-adjusted BMI change are referred to as T1 and T2. The two annual assessments that follow the BMI change are referred to as T3 and T4. A similar procedure has been used in previous research.24
A third group of “weight stable” participants was created, which consisted of individuals who experienced no more than a 5% age-adjusted change in BMI across any of the seven one-year intervals of the study. Eighty-five participants were identified as “weight stable” across the 8-year study. This number is likely an underestimate of the true number of weight stable individuals, as only participants who had complete data from all eight yearly assessments were considered eligible for the weight stable group. This was done to avoid including participants who may have experienced a 10% weight change during an interval during which they were not assessed. As the participants in the weight loss and weight gain groups could have been identified at any of the first five one-year intervals of the study, the detection interval for the weight stable participants was randomly assigned from one of the first five years of the study in order to maintain equivalence among detection intervals for the three groups. Analyses confirmed that the weight stable group did not differ significantly from the weight gain and weight loss groups on demographic factors.
Change in BMI by Group
Weight loss group
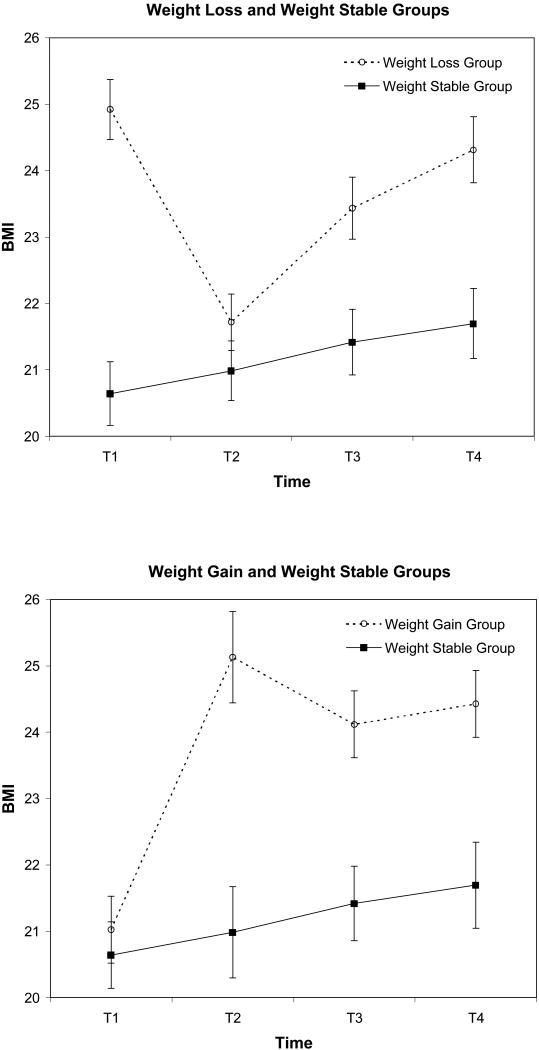
The overall trend for the weight loss group compared to the weight stable group is depicted in the top panel of Figure 1. As expected given the strategy used to create the comparison groups, the weight loss group showed significantly greater decreases in BMI over the one-year detection interval relative to the weight stable group, as indicated by a significant Time by Group interaction in a repeated measures analysis of variance model (F(1, 159) = 330.00, p < .001, ηp2 = 0.68). Post-hoc within subjects t-tests revealed a significant decrease in BMI in the weight loss group (M = -3.20, SD = 1.64; t (75) = 17.00, p < .001, ηp2 = 0.79), and a comparatively small, but statistically significant BMI increase in the weight stable group (M = 0.34, SD = 0.70; t (84) = 4.54, p < .001, ηp2 = 0.20). (A change of one BMI unit equals approximately 4-9 lbs, depending on height). At the initial assessment of the detection interval, the BMI of the weight loss group (M = 24.92, SD = 4.50) was significantly higher than the BMI of the weight stable group (M = 20.64, SD = 3.88; t (159) = 6.48, p < .001, ηp2 = 0.21). However, at the assessment following the weight change, there was no significant difference in BMI between the weight loss group (M = 21.72, SD = 3.72) and the weight stable group (M = 20.99, SD = 4.09; t (159) = 1.19, p = .238, ηp2 = 0.01). Over the two, one-year intervals following the weight change, the weight loss group showed significantly greater increases in BMI (M = 2.60, SD = 2.99) than were shown in the weight stable group (M = 0.71, SD = 1.08), as indicated by a significant Time by Group interaction in a repeated measures analysis of variance model (F(2, 318) = 14.75, p < .001, ηp2 = 0.09). Post-hoc t-tests indicated no difference between the two groups on BMI at the assessment directly following the weight change (as described above). However, the weight loss group exhibited a significantly higher BMI than the weight stable group at both yearly assessments following the BMI decrease (p < .005 for both comparisons). In sum, before experiencing weight loss, the weight loss group was significantly heavier than the weight stable group. After losing weight, the BMI of the weight loss group was equivalent to the BMI of the weight stable group. However, members of the weight loss group tended to regain the majority of the weight that was lost over the following two years.
Figure 1.
BMI trajectory of the weight loss group (participants with a 10% age-adjusted BMI decrease) and the weight gain group (participants with a 10% age-adjusted BMI increase) compared to the weight stable group (no year-to-year weight change greater than 5% across the 8 year study). Participants in the two weight change groups were pooled by fixing the year of weight change at T1 to T2. Points represent mean BMI; vertical lines depict standard errors of the means.
Weight gain group
The overall trend for the weight gain group compared to the weight stable group is depicted in the bottom panel of Figure 1. Again, as expected given the strategy used to create the comparison groups, the weight gain group showed significantly greater increases in BMI over the one-year reference period than were shown in the weight stable group, as indicated by a significant Time by Group interaction in a repeated measures analysis of variance model (F(1, 169) = 41.25, p < .001, ηp2 =0.20). A post-hoc within subjects t-test revealed a significant increase in BMI in the weight gain group (M = 4.11, SD = 5.36; t (85) = 7.11, p < .001, ηp2 = .37). As noted, there was a small but statistically significant BMI increase in the weight stable group over the same period. At the assessment preceding the weight change interval, the BMI of the weight gain group (M = 21.03, SD = 5.28) was not significantly different than the BMI of the weight stable group (M = 20.64, SD = 3.88; t (169) = 0.54, p = 0.591, ηp2 = 0.002). However, at the assessment following the weight change, there was a significant difference between the BMI of the weight gain group (M = 25.13, SD = 8.00) and the weight stable group (M = 20.99, SD = 4.09; t (169) = 4.26, p < .001, ηp2 = 0.10). Over the two one-year intervals following the weight change, there was a significant difference in the trajectory of weight change between the two groups, as evidenced by a significant Time by Group interaction in a repeated measures analysis of variance model (F(2, 338) = 4.15, p = .017, ηp2 = 0.02). A Post-hoc t-test showed a small, but statistically significant, increase in BMI in the weight stable group in the two years following the detection interval (M = 0.71, SD = 1.08; t(84) = 6.06, p < .001, ηp2 = 0.26). The weight gain group showed a non-significant weight decrease over the same period (M = 0.70, SD = 6.12; t(85) = 1.07, p = .195, ηp2 = 0.02). The weight gain group exhibited a significantly higher BMI than the weight stable group at both yearly assessments following the BMI increase (p < .005 for both comparisons). In sum, before experiencing weight gain, the BMI of the weight gain group is equivalent to the BMI of the weight stable group. After gaining weight, the BMI of the weight gain group is significantly greater than that of the weight stable group, and tends to remain at that high level over the following two years.
Onset of Threshold/subthreshold BN by Group
We next tested whether the two weight-change groups showed differential onset of threshold/subthreshold BN relative to the weight stable group. As in the case of the BMI change analyses, the detection interval and the two following years were examined separately. First, the three groups were probed for participants who developed threshold or subthreshold BN at any time during the study. In the weight loss group, eight such participants were identified (10.5% of weight losers); one was threshold, seven were subthreshold (one progressed to threshold at a later assessment). Two of these cases first met criteria before the detection interval (the average time of BN onset was 1.5 years before the 10% weight loss), and six first met criteria following the detection interval (the average delay in BN onset was 3 years after the 10% weight loss). A further eight participants meeting criteria for onset of threshold/subthreshold BN were identified in the weight gain group (9.3% of weight gainers); all were subthreshold (two progressed to threshold at a later assessment). One of these cases first met criteria three years before the detection interval, two cases were identified during the detection interval, and five cases were identified following the detection interval (the average delay in BN onset for these five cases was 4.6 years after the 10% weight gain). One case of subthreshold BN was identified in the weight stable group (1.2% of weight stables). The relation with the detection interval is not reported for this case, as the detection interval was randomly assigned for members of the weight stable group.
Fisher's exact test was used to determine whether an association exists between weight change group and frequency of diagnosis with threshold or subthreshold BN. Cases from the two weight change groups were included in these analyses only if they were first diagnosed during or after the 10% age-adjusted weight change. Cases in which the disorder was diagnosed prior to the weight change were eliminated from these analyses. Threshold or subthreshold BN was identified in 8.1% of the weight loss group, compared to 1.2% of the weight stable group (Odds Ratio = 7.41, p = 0.39, Fisher's exact test). Likewise, the disorder was identified in 8.2% of the weight gain group, compared to 1.2% of the weight stable group (Odds Ratio = 7.54, p = 0.32, Fisher's exact test).
Weight Change Preceding Onset of BN
Twenty-seven participants were identified who first showed onset of threshold/subthreshold BN during years three through seven of the study (i.e., year 3 through year 7). Of those, 21 had weight information available for two years preceding, and one year following, the onset of bulimic pathology (see Table 1 for the number of participants detected in years 3 through 7). All 21 cases were subthreshold (four progressed to threshold at a later assessment). Participants who met criteria for bulimic pathology during years 1, 2, and 8 would have had insufficient data for the following analyses, and were excluded. As in the previous analyses, all participants who were diagnosed with bulimic pathology during years 3 to 7 were pooled to increase statistical power. The rates of other threshold and subthreshold eating disorders in this sample have been reported elsewhere.25
A non-BN group was created that included all of the participants with adequate weight data that did not show onset of threshold/subthreshold BN (n = 420) at any time during the study. For the bulimic pathology group, participants had to show onset at during years 3 through 7. Therefore, the detection year for the participants in the non-BN group was randomly assigned from years 3 through 7.
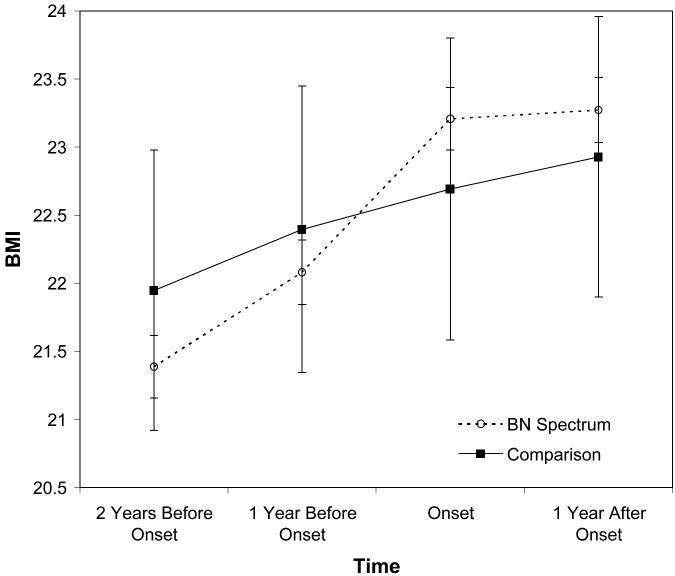
The overall trend for the bulimic pathology group compared to the non-BN group is depicted in Figure 2. The former showed significantly greater increases in BMI over the two years preceding the onset of bulimic pathology relative to the comparison group, as indicated by a significant Time by Group interaction in a repeated measures analysis of variance model (F(2, 878) = 3.72, p = .008, ηp2 = 0.01). There was no difference in weight trajectory for the two groups in the year following onset of bulimic pathology (F(1, 439) = 0.16, p = .686, ηp2 < .01). The BMI of the bulimic pathology group was not significantly different from the comparison group at any of the yearly assessments (p > .55 for all comparisons). Thus, in the two years before experiencing the onset of bulimic pathology BMI increased at a greater rate than that of the non-eating disordered comparison group. After the onset of bulimic symptoms, the BMI of both the bulimic pathology and comparison group increased at an equivalent rate.
Figure 2.
BMI trajectory of the BN spectrum group (participants who met criteria for clinical or subclinical Bulimia Nervosa) relative to year of first diagnosis, compared to the comparison group (all other participants not meeting a diagnosis of a BN spectrum disorder during the study). Points represent mean BMI; vertical lines depict standard errors of the means.
Discussion
Research on the relation between weight change and BN has been limited by its reliance on retrospective data, which have questionable validity. This is the first study to use prospective data to evaluate if a large weight loss or weight gain increases risk for onset of threshold/subthreshold BN. Overall, there was a 7-fold increase in risk for onset of bulimic pathology for participants who had gained or lost at least 10% of their weight compared to participants who were weight stable. The low incidence of BN onset resulted in low power to detect statistically significant effects in this analysis. Nevertheless, the effect sizes are quite large.
This is also the first prospective study to suggest that weight gain typically occurs prior to BN onset. Specifically, participants who showed an onset of threshold or subthreshold BN, compared to those who never met criteria for a diagnosis, showed significantly greater increases in BMI over the two years preceding the onset of the diagnosis. In the two years following the diagnosis, the BMI change in the bulimic pathology group did not differ significantly from that of the comparison group.
These data indicate that many participants who developed bulimic pathology experienced a significant increase in weight in the two years before the onset of their BN symptoms. This finding raises that possibility that young women who have difficulty limiting their dietary intake are also at increased risk for BN, an eating disorder that is characterized by overeating and a loss of control of eating. The effect sizes (though not the statistical significance) of other analyses also indicate that for some participants, substantial weight loss is associated with concurrent or subsequent onset of BN. Young women who experience a large weight loss often have a history of being overweight, so it is possible these participants shared the same vulnerability to overeating, which was temporarily controlled, presumably by strict dieting. In fact, results indicated that in the two years prior to the 10% age-adjusted BMI change, the BMIs of the weight loss group and the weight stable group accelerated significantly faster than that of the weight gain group. There was no significant difference in the BMI trajectory of the weight loss group and the weight stable group in the two years before the 10% age-adjusted BMI change. In sum, we can conclude that the onset of BN symptoms is associated with elevated BMI and substantial weight fluctuation. It is possible that weight stability itself offers protection against the risk of BN onset, or that particular characteristics of young women who remain weight stable throughout adolescence offer some protection from risk (e.g., caloric intake is motivated by homeostatic need rather than by hedonic desire).
There are several possible interpretations of the present findings. One possibility is that marked weight loss or weight gain disconnects homeostatic feeding controls and increases risk for hedonic eating that may drive binge episodes. A second possibility is that the two weight change groups were in the process of developing different weight-related problems. Most studies that have compared the onset of dieting and binge eating in individuals with BN have found that dieting (and, presumably, weight loss) precedes the development of binge eating by 1-2 years26-29 whereas studies of obese individuals who engage in binge eating find that the onset of dieting and binge eating occur at about the same time, with dieting following binge eating about as often as it precedes it.30-32 In the current study, elevated bulimic symptoms in the group that lost weight may signal the development of threshold or subthreshold BN, whereas the elevated symptoms in the group that gained weight (and the weight gain that preceded the development of BN in those that developed it) may reflect the early phases of developing overweight and threshold or subthreshold BED. In the latter case, any compensatory behaviors this group used initially to counter the effects of binge eating would presumably fade over time. A third possibility is that all three findings from the present study reflect weight gain proneness among those susceptible to the development of a bulimic-spectrum disorder. That is, those who experienced BN symptoms after a weight loss (first graph in Fig. 1) had significantly higher BMIs than controls before losing weight, those who experienced BN symptoms after a weight gain (second graph in Fig. 1) had significantly higher BMIs than controls following their weight gain, and those selected based on the first emergence of BN symptoms (Fig. 2) experienced a significantly larger weight gain in the year prior to developing BN symptoms than the control group. From this perspective, a “primary defect” that makes certain young women susceptible to the development of BN may be their propensity toward weight gain.33 Many of those who gain weight may work assiduously to lose it; such weight loss may coincide with, but be causally unrelated to, the development of BN, or it could combine synergistically with a propensity toward weight gain to trigger the binge-purge cycle. Additional research, preferably using prospective designs, will be needed to evaluate these alternative explanations for the current findings.
This study makes an important contribution to the literature for two reasons. First, information on weight change and onset of bulimic pathology was collected prospectively. The participants ranged in age from 12 to 15 years at year 1 and 19 to 22 years at year 8, so they were evaluated during the period of highest risk of the onset of BN.25 The sample was sufficiently large to capture the onset of disordered eating in many participants. Bulimic symptoms were assessed with a clinical interview, the EDDI, which appears to be a reliable and valid measure. Second, this is the first prospective study to show that, on average, young women gain weight prior to BN onset. This finding provides contrast to previous (primarily retrospective) research, and the typical conceptualization of the development of BN, which emphasize the importance of weight loss prior to BN onset.
Limitations of the study include a moderate participant recruitment rate and the possibility that the number of individuals who showed onset of bulimic pathology was too small to detect statistical significance in some analyses. Also, it is not possible to document that the participants who lost weight did so because they were dieting, because self-report measures of dietary restriction do not identify individuals who restrict their dietary intake.34 However, previous research has demonstrated that young women who experience significant weight loss typically do so because of dieting, not for other reasons, such as health problems.35
In sum, the present study indicates that both substantial weight loss and weight gain are likely to precede the development of threshold and sub-threshold bulimia nervosa in female adolescents. On one hand these findings indicate that large weight fluctuations are an important feature of – and a possible contributor to – bulimia nervosa. On the other hand it is quite challenging to try to understand how opposite weight change patterns could produce the same pathological outcome. Additional prospective research studies are needed to replicate these findings. Experimental research that promotes weight stability, especially weight gain prevention, in adolescent girls also would provide more information about the causal link between weight stability of BN risk.
Acknowledgments
This research was supported by two grants awarded to Eric Stice by the National Institute of Mental Health: Risk and Maintenance Factors for Bulimic Pathology (MH64560) and Etiology of Bulimic Pathology: Multimethod Investigation (MH01708).
References
- 1.Russell G. Bulimia nervosa: An ominous variant of anorexia nervosa. Psychol Med. 1979;9:429–48. doi: 10.1017/s0033291700031974. [DOI] [PubMed] [Google Scholar]
- 2.Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. J Abnorm Psychol. 2006;115:62–67. doi: 10.1037/0021-843X.115.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Garner DM, Fairburn CG. Relationship between anorexia nervosa and bulimia nervosa: Diagnostic implications. In: Garner DM, Garfinkel PE, editors. Diagnostic issues in anorexia nervosa and bulimia nervosa. New York: Brunner/Mazel; 1988. pp. 56–79. [Google Scholar]
- 4.Lowe MR, Davis W, Lucks D, Annunziato R, Butryn M. Weight suppression predicts weight gain during inpatient treatment of bulimia nervosa. Physiol Behav. 2006;87:487–92. doi: 10.1016/j.physbeh.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Fairburn CG, Welch SL, Doll HA, Davies B, O'Connor ME. Risk factors for bulimia nervosa: A community-based case-control study. Arch Gen Psychiatry. 1997;54:509–17. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- 6.Yates WR. Weight factors in normal weight bulimia nervosa: A controlled family study. Int J Eat Disord. 1992;11:227–34. [Google Scholar]
- 7.Eddy KT, Dorer DJ, Franko DL, Tahilani KK, Thompson-Brenner H, Herzog DB. Should bulimia nervosa be subtyped by history of anorexia nervosa?. A longitudinal validation. Int J Eat Disord. 2007;40:S67–71. doi: 10.1002/eat.20422. [DOI] [PubMed] [Google Scholar]
- 8.Keys A, Brozek K, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation. Minneapolis (MN): University of Minnesota Press; 1950. [Google Scholar]
- 9.Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: A study of former prisoners of war. J Abnorm Psychol. 1994;103:409–11. doi: 10.1037//0021-843x.103.2.409. [DOI] [PubMed] [Google Scholar]
- 10.Polivy J, Herman CP. Dieting and binging: A causal analysis. Am Psychol. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 11.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. J Child Psychol Psychiatry. 2004;45:260–73. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 12.Henry B, Moffitt TE, Caspi A, Langley J, Silva PA. On the “remembrance of things past:” A longitudinal evaluation of the retrospective method. Psychol Assess. 1994;6:92–101. [Google Scholar]
- 13.Cash TF, Grant JR, Shovlin JM, Lewis RJ. Are inaccuracies in self-reported weight motivated distortions? Percept Mot Skills. 1992;72:209–210. doi: 10.2466/pms.1992.74.1.209. [DOI] [PubMed] [Google Scholar]
- 14.Stice E, Davis K, Miller NP, Marti CN. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J Abnorm Psychol. 2008;17:941–6. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH. Natural course of adolescent major depressive disorder in a community sample: Predictors of recurrence in young adults. Am J Psychiatry. 2000;157:1584–91. doi: 10.1176/appi.ajp.157.10.1584. [DOI] [PubMed] [Google Scholar]
- 16.Stice E, Burton EM, Shaw H. Prospective relations between bulimic pathology, depression, and substance abuse: Unpacking comorbidity in adolescent girls. J Consult Clin Psychol. 2004;72:62–71. doi: 10.1037/0022-006X.72.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington (D.C.): American Psychiatric Association; 2000. [Google Scholar]
- 18.Stice E, Marti CN, Spoor S, Presnell K, Shaw H. Dissonance and healthy weight eating disorder prevention programs: Long-term effects from a randomized efficacy trial. J Consult Clin Psychol. 2008;76:329–40. doi: 10.1037/0022-006X.76.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairburn CG, Cooper ZC. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12th. New York: The Guilford Press; 1993. pp. 317–60. [Google Scholar]
- 20.Garfinkel PE, Lin E, Goering P, Spegg C. Bulimia nervosa in a Canadian community sample: Prevalence and comparison of subgroups. Am J Psychiatry. 1995;152:1052–8. doi: 10.1176/ajp.152.7.1052. [DOI] [PubMed] [Google Scholar]
- 21.le Grange D, Binford RB, Peterson CB, Crow SJ, Crosby RD, Klein MH, et al. DSM-IV threshold versus subthreshold bulimia nervosa. Int J Eat Disord. 2006;39:462–7. doi: 10.1002/eat.20304. [DOI] [PubMed] [Google Scholar]
- 22.Burton E, Stice E. Evaluation of a healthy-weight treatment program for bulimia nervosa: A preliminary randomized trial. Behav Res Ther. 2006;44:1727–38. doi: 10.1016/j.brat.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: A validation study. J Pediatr. 1998;132:204–10. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 24.Stice E, Martinez EE, Presnell K, Groesz LM. Relation of successful dietary restriction to change in bulimic symptoms: A prospective study of adolescent girls. Health Psychol. 2006;25:274–81. doi: 10.1037/0278-6133.25.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stice E, Marti CN, Shaw H, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders in a community sample of adolescents. J Abnorm Psychol. doi: 10.1037/a0016481. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewerton TD, Dansky BS, Kilpatrick DG, O'Neil PM. Which comes first in the pathologenesis of bulimia nervosa: Dieting or binging? Int J Eat Disord. 2000;28:259–64. doi: 10.1002/1098-108x(200011)28:3<259::aid-eat2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Bulik CM, Sullivan PF, Carter FA, Joyce PR. Initial manifestations of disordered eating behavior: Dieting versus binging. Int J Eat Disord. 1997;22:195–201. doi: 10.1002/(sici)1098-108x(199709)22:2<195::aid-eat12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JE, Hatsukami D, Eckert ED, Pyle RL. Characteristics of 275 patients with bulimia. Am J Psychiatry. 1985;142:482–5. doi: 10.1176/ajp.142.4.482. [DOI] [PubMed] [Google Scholar]
- 29.Mussell MP, Mitchell JE, Fenna CJ, Crosby RD, Miller JP, Hoberman HM. A comparison of onset of binge eating versus dieting in the development of bulimia nervosa. Int J Eat Disord. 1997;21:353–60. doi: 10.1002/(sici)1098-108x(1997)21:4<353::aid-eat8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Abbott DW, de Zwaan M, Mussell MP, Raymond NC, Seim HC, Crow SJ, et al. Onset of binge eating and dieting in overweight women: Implications for etiology, associated features and treatment. J Psychosom Res. 1998;44:367–74. doi: 10.1016/s0022-3999(97)00261-4. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, et al. Binge eating disorder: Its further validation in a multisite study. Int J Eat Disord. 1993;13:137–53. [PubMed] [Google Scholar]
- 32.Spurrell EB, Wilfley DE, Tanofsky MB, Brownell KD. Age of onset for binge eating: Are there different pathways to binge eating? Int J Eat Disord. 1997;21:55–65. doi: 10.1002/(sici)1098-108x(199701)21:1<55::aid-eat7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Chernyak Y, Lowe MR. Motivations for dieting: Drive for thinness is different from drive for objective thinness. J Abnorm Psychol. doi: 10.1037/a0018398. in press. [DOI] [PubMed] [Google Scholar]
- 34.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychol Assess. 2004;16:51–59. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Stice E, Cooper J, Schoeller D, Tappe K, Lowe M. Are dietary restraint scales valid measures of moderate- to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychol Assess. 2007;19:449–58. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]