Abstract
Background
It is generally assumed that T cells do not produce active TGF-β since active TGF-β as measured in supernatants by ELISA without acidification is usually not detectable. However, it is possible that active TGF-β from T cells may take a special form which is not detectable by ELISA.
Methodology/Principal Findings
We constructed a TGF-β bioassay which can detect both soluble and membrane-bound forms of TGF-β from T cells. For this bioassay, 293T cells were transduced with (caga)12 Smad binding element-luciferase along with CD32 (Fc receptor) and CD86. The resulting cells act as artificial antigen presenting cells in the presence of anti-CD3 and produce luciferase in response to biologically active TGF-β. We co-cultured pre-activated murine CD4+CD25− T cells or CD4+CD25+ T cells with the 293T-caga-Luc-CD32-CD86 reporter cells in the presence of anti-CD3 and IL-2. CD4+CD25− T cells induced higher luciferase in the reporter cells than CD4+CD25+ T cells. This T cell-produced TGF-β is in a soluble form since T cell culture supernatants contained the TGF-β activity. The TGF-β activity was neutralized with an anti-mouse LAP mAb or an anti-latent TGF-β/pro-TGF-β mAb, but not with anti-active TGF-β Abs. An anti-mouse LAP mAb removed virtually all acid activatable latent TGF-β from the T cell culture supernatant, but not the ability to induce TGF-β signaling in the reporter cells. The induction of TGF-β signaling by T cell culture supernatants was cell type-specific.
Conclusions/Significance
A newly developed 293T-caga-Luc-CD32-CD86 reporter cell bioassay demonstrated that murine CD4 T cells produce an unconventional form of TGF-β which can induce TGF-β signaling. This new form of TGF-β contains LAP as a component. Our finding of a new form of T cell-produced TGF-β and the newly developed TGF-β bioassay system will provide a new avenue to investigate T cell function of the immune system.
Introduction
TGF-β is an immunoregulatory cytokine that controls immune responses by multiple mechanisms [1]. TGF-β-deficient mice manifest an autoimmune syndrome and do not survive longer than 3–4 wks after birth [2], [3]. Moreover, it has been shown that TGF-β initiates Th17 differentiation in combination with IL-6 or IL-21 [4], [5], [6], [7], [8]. Although IL-17 is a dominant factor in the induction of autoimmune diseases such as experimental autoimmune encephalomyelitis [9] and collagen-induced arthritis [10], IL-17 production is not seen in TGF-β1 −/− mice [5]. Although many cell types produce TGF-β, T cell-produced TGF-β is plays an important role in the control of autoimmune responses and Th17 differentiation. Thus, T cell-specific TGF-β conditional knockout mice develop fatal autoimmune disease even though they survive longer than TGF-β−/− mice [11], and Th17 differentiation is hampered in these mice [11], indicating that TGF-β produced by T cells themselves is required for Th17 differentiation.
TGF-β is produced as a pro-form (pro-TGF-β), and is intracellularly processed by furin proprotein convertase into latent TGF-β. Latent TGF-β is a non-covalently associated complex consisting of latency-associated peptide (LAP) which is the N-terminal portion of pro-TGF-β, and mature TGF-β which is made of the C-terminal of pro-TGF-β. Latent TGF-β cannot bind TGF-β receptors and thus further activation processes are required for biological activity [12]. It is unknown how T cell-produced TGF-β is activated.
Murine T cell culture supernatants usually do not contain active TGF-β when measured by ELISA without acidification. Thus, it is generally believed that T cells do not produce active TGF-β.
Nakamura et al. [13] first reported that murine CD4+CD25+ regulatory T cells (Tregs) expressed surface LAP and/or TGF-β (LAP/TGF-β), and they proposed that the membrane-bound TGF-β mediated suppressive activity of Tregs. We also confirmed that Foxp3+ Tregs express surface LAP/TGF-β by using our newly developed anti-mouse LAP/TGF-β mAbs [14]. Human FOXP3+ Tregs have also been shown to express surface LAP [15], [16], [17]. It is possible that surface LAP/TGF-β on T cells can trigger TGF-β signaling in target cells by a cell-cell contact manner, give that active TGF-β is usually not detectable from T cell culture supernatants by ELISA. Alternatively, active TGF-β may be a rapidly-consumed, short-lived cytokine in T cell culture. Although there is no experimental evidence thus far, it is also possible that T cells produce biologically active TGF-β in a form that is not detectable by ELISA.
Given these possibilities, we developed a bioassay system which detects TGF-β activity, rather than the specific molecular form (the 25 kDa free TGF-β dimer) that an ELISA detects. This new bioassay consists of reporter cells that have direct contact with T cells and which can sense both short-lived and membrane-bound forms of TGF-β. 293T cells were transduced with a TGF-β reporter vector which has repeated the CAGA Smad binding elements in the promoter followed by luciferase, and with CD32 (Fc receptor) and CD86.
By using the newly developed 293T-caga-Luc-CD32-CD86 reporter cells, we found that pre-activated murine CD4 T cells induced high luciferase signals in the reporter cells. Although Foxp3+ T cells expressed surface LAP/TGF-β [14], pre-activated CD4+CD25− T cells induced much higher TGF-β signal than pre-activated CD4+CD25+ T cells. The T cell-produced TGF-β was a soluble form since T cell culture supernatants contained TGF-β activity. The T cell-produced TGF-β is not the canonical 25 kDa mature TGF-β since a TGF-β ELISA did not detect the 25 kDa mature TGF-β form in the same T cell culture supernatants. Surprisingly, the TGF-β activity in T cell culture supernatants was neutralized with an anti-LAP mAb and with an anti-pro-TGF-β/latent TGF-β mAb, but not with anti-mature TGF-β Abs. TGF-β activity remained in culture supernatants even after the culture supernatant was treated with immobilized anti-LAP mAb by which latent TGF-β detected by TGF-β ELISA after acidification was virtually all depleted. Thus, T cell-produced TGF-β takes a unique molecular form which contains LAP as a component, and from which 25 kDa mature TGF-β is not released even after acidification. T cell-produced TGF-β initiated TGF-β signaling not in all cell types, suggesting that cell type-specific factors are required to sense the T cell-produced TGF-β.
Results
Generation of a TGF-β reporter cell line
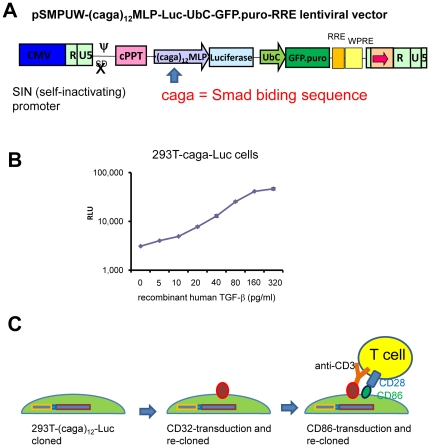
It has been reported that CAGA is a Smad binding element and a promoter assay vector containing tandem repeats of CAGA in the minimum promoter region ((caga)12-MLP-Luc) is a sensitive and specific TGF-β reporter vector [18], [19]. We inserted the (caga)12-MLP-Luc segment into a promoterless lentiviral vector (pSMPUW) to construct a lentivirus based universal TGF-β reporter vector (pSMPUW-(caga)12-MLP-Luc-UbC-EGFP.puro-RRE) (Figure 1A).
Figure 1. Generation of a TGF-β reporter cell line.
(A) Schematic diagram of pSMPUW-(caga)12-Luc lentivirus based TGF-β reporter vector. (B) Representative luciferase response of 293T-caga cells to recombinant TGF-β. 293T cells were transduced with the pSMPUW-(caga)12-Luc vector, and cultured in the presence of recombinant human TGF-β for 16 hrs. Luciferase activity was measured from the cell lysates. Mean ± S.D. from duplicates are shown. (C) Schematic diagram of generation of 293T-caga-Luc-CD32-CD86 cells and how the cells function as artificial antigen presenting cells.
Human embryonic kidney 293T cells were then transduced with the TGF-β reporter vector (293T-caga-Luc cells). The response curve of 293T-caga-Luc cells to recombinant TGF-β was able to detect as little as 2 pg/ml recombinant human TGF-β (Figure 1B). To make the 293T-caga-Luc cells function as artificial antigen presenting cells, the 293T-caga-Luc cells were transduced with mouse CD32 (Fc receptor) and mouse CD86 retroviral vectors (Figure 1C). Serpinb9 (granzyme B inhibitor) and Serpinb9b (granzyme M inhibitor) were also retrovirally expressed. These granzyme inhibitor genes suppressed CD4 T cell-mediated killing of CD32- and CD86-transduced mink lung epithelial cells (MLEC) (data not shown), and it is expected that the granzyme inhibitor genes protect 293T cells from CD4 T cell-mediated killing, too, although 293T cells are relatively resistant to CD4 cell-mediated killing without these gene transductions. The transduced 293T cells were cloned at each step and high responding or high expressing clones were selected. The resulting TGF-β reporter cell line is termed as 293T-caga-Luc-CD32-CD86.
Detection of TGF-β activity from CD4 T cells in coculture
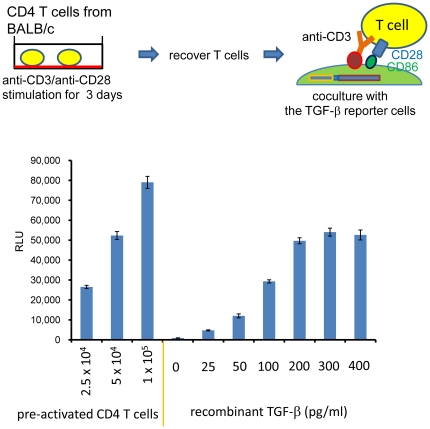
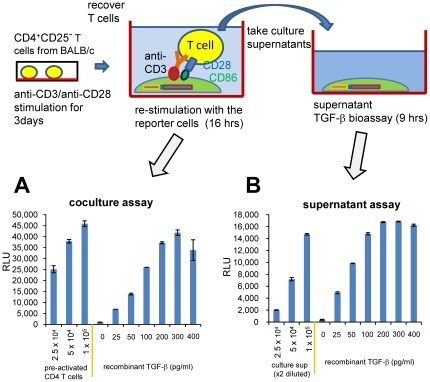
We tested whether murine CD4 T cells have TGF-β activity by using the 293T-caga-Luc-CD32-CD86 TGF-β reporter cells. For these experiments we co-cultured T cells with 293T-caga-Luc-CD32-CD86 TGF-β reporter cells in presence of anti-CD3 antibody. In preliminary experiments, we found that freshly prepared CD4 T cells barely induced luciferase during 24 hr co-culture. Then, we tested pre-activated CD4 T cells. Thus, mouse CD4 T cells were stimulated with plate-bound anti-CD3/CD28 for two days, rested for one day and then co-cultured with 293T-cgga-Luc-CD32-CD86 TGF-β reporter cells with anti-CD3 for 16 hrs (Figure 2A). As shown Figure 2B, CD4 T cells induced high luciferase activity in a cell number dependent manner. Interestingly, although the response to recombinant TGF-β became saturated above 200 pg/ml, CD4 T cells often induced higher luciferase activity than the saturation point. It should be noted that the re-stimulation at this time point minimally induced activation-induced cell death, while re-stimulation at later time points induced notable cell death under microscopic observation. Higher luciferase signal was observed at later time points when anti-FasL mAb was added to block the activation-induced cell death (Figure S1). Thus, it is not likely that the TGF-β activity is generated from dying T cells.
Figure 2. TGF-β bioassay in co-culture.
Mouse CD4 T cells were stimulated with plate-bound anti-CD3/CD28 for 2 days, and rested for 1 day. The pre-activated CD4 T cells were recovered, and the indicated numbers of T cells were added to 293T-caga-Luc-CD32-CD86 cells with 0.5 µg/ml anti-CD3 antibody. Recombinant human TGF-β was also added as a standard. After 16 hr of culture, the reporter cells were lysed and the luciferase activity was measured. Error bars represent mean ± S.D. of duplicates.
TGF-β activity from Tregs
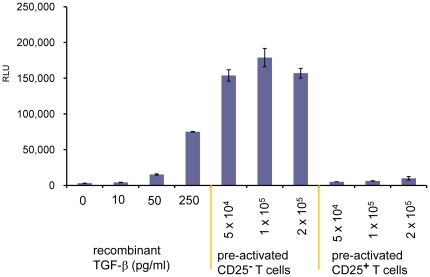
We previously reported that activated Fox3+ CD4 T cells express surface LAP/TGF-β using our newly developed anti-mouse LAP/TGF-β mAbs [14]. We tested whether these surface LAP/TGF-β-expressing Foxp3+ CD4 T cells were CD4 T cells with TGF-β activity. CD4+CD25+ (>90% Foxp3+) cells and CD4+CD25− (>98% Foxp3−) cells were isolated and stimulated with plate-bound anti-CD3/CD28 in the presence of IL-2 for two days and rested for one day in presence of IL-2. They were then co-cultured with 293T-caga-Luc-CD32-CD86 TGF-β reporter cells in presence of anti-CD3 and IL-2 for 16 hrs. The T cells have tight cell to cell contact with the reporter cells both by the CD32-anchored anti-CD3 Ab and by CD86; thus the reporter cells should be able to sense membrane-bound TGF-β activity if present. As shown in Figure 3, CD4+CD25+ T cells had less TGF-β activity in the co-culture bioassay than CD4+CD25− T cells. These results demonstrate that non-Tregs are the main CD4 T cells with TGF-β activity by the reporter assay.
Figure 3. TGF-β bioassay of CD4+CD25+ Tregs.
Mouse CD4+CD25+ T cells and CD4+CD25− T cells were stimulated with plate-bound anti-CD3/CD28 for 2 days, and rested for 1 day in presence of 100 U/ml IL-2. These pre-activated CD4 T cells were recovered, and the indicated numbers of T cells were added to 293T-caga-Luc-CD32-CD86 cells with 0.5 µg/ml anti-CD3 antibody and 100 U/ml IL-2 for 16 hrs after which luciferase activity was measured. Error bars represent mean ± S.D. of duplicates.
No requirement for direct contact with T cells for induction of TGF-β signaling
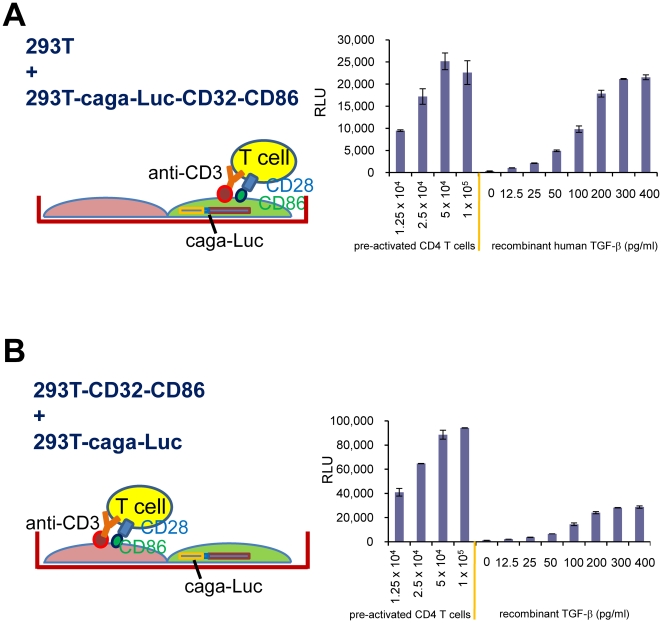
Although we initially anticipated that membrane-bound TGF-β on Foxp3+ CD4 T cells would trigger TGF-β signaling in the reporter cells, as described above, this was not the case. To further investigate the role of membrane-bound TGF-β, we asked whether direct contact between T cells and the reporter cells was required to initiate TGF-β signaling. In order to address this question, 293T-caga-Luc cells that did not have surface CD32 or CD86 were mixed with CD32- and CD86-trandsduced 293T cells that did not have the (caga)12-Luc reporter (Figure 4B). If T-reporter contact was required to present T cell-produced TGF-β to TGF-β receptors on the reporter cells, there would be diminished induction of luciferase in this condition. We found, however, that pre-activated CD4 T cells stimulated with 293T-CD32-CD86 cells in presence of anti-CD3 induced TGF-β signaling in 293T-caga-Luc cells as well as or better than the condition in which 293T-caga-Luc-CD32-CD86 cells were used as reporter cells (Figure 4A). This result indicates that the T cell-produced TGF-β that results in TGF-β activity as measured in our reported system is soluble and can diffuse to adjacent cells.
Figure 4. Investigation of requirement for direct contact between T cells and TGF-β reporter cells.
(A) 293T-caga-Luc-CD32-CD86 cells were mixed with an equal number of un-manipulated 293T cells. Under this condition, a single 293T-caga-Luc-CD32-CD86 cell stimulates CD4 T cells and responds to TGF-β activity produced by the same T cells. (B) 293T-caga cells that did not have CD32 or CD86 were mixed with 293T-CD32-CD86 cells lacking the (caga)12-Luc reporter. Under this condition the 293T-caga reporter cells have minimal contact with CD4 T cells.
TGF-β activity in T cell culture supernatants
After demonstrating that T cell-produced TGF-β is in a soluble form, we next asked whether this form accumulates in culture supernatants. To address this, pre-activated CD4+CD25− T cells (anti-CD3/28 for 3 days) were re-stimulated with the reporter cells plus anti-CD3 for 16 hrs as the co-culture assay (Figure 5A), and the culture supernatants were also taken at the end of the assay. The culture supernatants were added to fresh 293T-caga-Luc-CD32-CD86 TGF-β reporter cells (without live T cells), and the reporter cells were cultured for 9 hrs (Figure 5B). We found that the T cell culture supernatant from the 1×105 cells/well induced luciferase activity equivalent to approximately 200 pg/ml of recombinant human TGF-β. Thus, TGF-β activity accumulates in T cell culture supernatants. We also measured the same culture supernatant by TGF-β ELISA without acidification which detects the 25 kDa free TGF-β dimer and found that the amount of active TGF-β as measured by the TGF-β ELISA was 10 pg/ml. “Total” TGF-β as measured by ELISA after acidification was found to be 486 pg/ml (Table 1). These results indicate that the T cell-produced TGF-β activity takes an unconventional form which is different from the 25 kDa free TGF-β dimer and is not detectable by ELISA.
Figure 5. TGF-β bioassay from T cell culture supernatants.
(A) Pre-activated CD4+CD25− T cells were re-stimulated with 293T-caga-Luc-CD32-CD86 cells in the presence of anti-CD3 as a co-culture assay. (B) Supernatants were taken from the co-culture assay, added to new wells of 293T-caga-Luc-CD32-CD86 reporter cells and luciferase activity was measured. Error bars represent mean ± S.D. of duplicates.
Table 1. TGF-β in a T cell culture supernatant was measured by the 293T-caga-CD32-CD86-caga bioassay (TGF-β activity), by ELISA without acidification (the 25 kDa free TGF-β dimer), and by ELISA after acidification ( “total TGF-β”).
| TGF-β in culture supernatant | ||
| 293T-(caga)12-Luc | ELISA | ELISA |
| bioassay | without acidification | after acidification |
| (activity) | (25 kDa free dimer) | (“total”) |
| ∼200 pg/ml | 10 pg/ml | 486 pg/ml |
T cell-produced TGF-β activity induces Smad2 phosphorylation in 293T-caga-Luc-CD32-CD86 cells
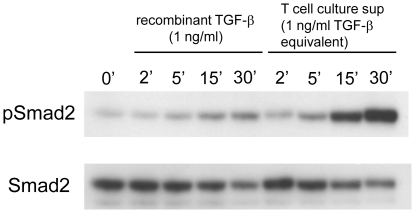
To confirm that the luciferase activity was a consequence of TGF-β signaling, we determined Smad2 phosphorylation in 293T-caga-Luc-CD32-CD86 cells after exposure to T cell culture supernatants. T cell culture supernatants from anti-CD3/CD28-stimulated cultures of pre-activated CD4+CD25− T cells contained higher TGF-β activity (2–3 ng/ml recombinant TGF-β equivalent) than cultures re-stimulated with 293T-caga-Luc-CD32-CD86 reporter cells/anti-CD3 (data not shown). Thus, we used culture supernatants from plate-bound anti-CD3/CD28 re-stimulated CD4+CD25− T cells for further studies. 1 ng/ml recombinant human TGF-β or a diluted T cell culture supernatant adjusted to the equivalent TGF-β activity determined by the 293T-caga-Luc-CD32-CD86 TGF-β bioassay was added to 293T-caga-Luc-CD32-CD86 cells for the indicated time, and the cell lysates were run on SDS-PAGE and blotted with anti-phospho-Smad2 antibody. Although the T cell culture supernatant was diluted to normalize the TGF-β activity, the T cell culture supernatant induced stronger Smad2 phosphorylation than recombinant TGF-β at all the time points (Figure 6). This result suggests that T cell-produced TGF-β activity is qualitatively different from the 25 kDa free TGF-β dimer.
Figure 6. Smad2 phosphorylation by T cell culture supernatants.
Recombinant TGF-β (1 ng/ml) or a T cell culture supernatant containing equivalent amount of TGF-β activity was added to 293T-caga-Luc-CD32-CD86 cells. After 2, 5, 15, and 30 min, the cells were lysed, and the lysates were run on SDS-PAGE. After transfer to a PVDF membrane, the membrane was blotted with anti-phospho-Smad2 Ab, and reblotted with anti-Smad2/3 Ab.
T cell-produced TGF-β activity is not found in the ELISA detectable “total” TGF-β fraction
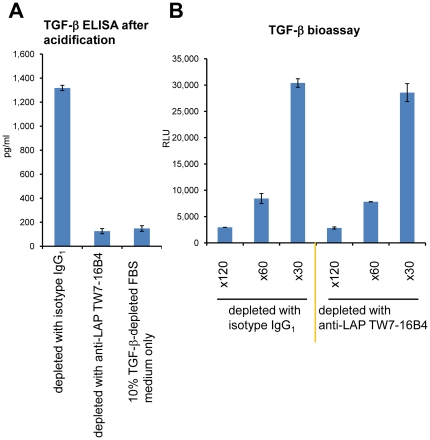
TGF-β measured by ELISA after acidification is termed “total” TGF-β. This is based on the assumption that following acidification, the 25 kDa free TGF-β dimer is released from any form of TGF-β, such as the small latent TGF-β complex and the large latent TGF-β complexes. We thus asked whether T-cell produced TGF-β activity resides in the “total” TGF-β fraction. Since FBS-derived latent TGF-β affects TGF-β ELISA after acidification, we used TGF-β-depleted FBS for the culture medium [20]. The background bovine TGF-β remaining in the 10% TGF-β-depleted FBS-supplemented medium was 148 pg/ml. We treated a T cell culture supernatant with anti-LAP mAb TW7-16B4-coupled magnetic beads (or an IgG1 isotype control mAb MOPC21) to deplete latent TGF-β in the supernatant. The amount of “total” TGF-β detected by ELISA after acidification in control MOPC21-treated T cell culture supernatant was 1,318 pg/ml, whereas the anti-LAP TW7-16B4-treated T cell culture supernatant contained 126 pg/ml “total” TGF-β (Figure 7A). Since anti-murine LAP TW7-16B4 mAb does not cross-react with bovine LAP (data not shown), we conclude that all T cell-derived “total” TGF-β was removed by the TW7-16B4-coupled magnetic bead treatment. However, TGF-β activity in the TW7-16B4-treated T cell culture supernatant was intact since the anti-LAP TW7-16B4-treated T cell culture supernatant induced an identical TGF-β response in 293T-caga-Luc-CD32-CD86 reporter cells as the control MOPC21-treated T cell culture supernatant (Figure 7B). These data indicate that T cell-produced TGF-β activity is not contained in the ELISA detectable “total” TGF-β fraction.
Figure 7. Immunologic depletion of “total” TGF-β from culture supernatants.
(A) A T cell culture supernatant from plate-bound anti-CD3/CD28 re-stimulated CD4+CD25− T cells cultured in 10% TGF-β-depleted FBS IMDM was treated with control IgG1 mAb-coated magnetic beads or anti-mouse LAP TW7-16B4 mAb-coated magnetic beads. The remaining “total” TGF-β in the culture supernatant was measured by TGF-β ELISA after acidification. Error bars represent mean ± S.D. of duplicates. (B) TGF-β activity in the same control IgG1-treated or anti-LAP TW7-16B4-treated T cell culture supernatant was measured by the 293T-caga-Luc-CD32-CD86 bioassay after ×30, ×60 and ×120 dilutions.
Neutralization of T cell-produced TGF-β activity with anti-LAP/latent TGF-β mAbs
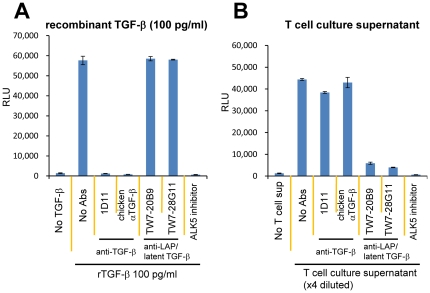
Since our results above indicate that T cell-produced TGF-β activity takes a form that is different from the 25 kDa free TGF-β dimer, we tested anti-active TGF-β Abs and anti-mouse LAP/latent TGF-β mAbs to better understand the structure of T cell-produced TGF-β. Recombinant TGF-β (100 pg/ml) and a T cell culture supernatant diluted to the equivalent activity were treated with 50 µg/ml of anti-TGF-β Abs (1D11 or chicken polyclonal anti-TGF-β), anti-mouse LAP mAb (TW7-20B9), or anti-latent TGF-β/pro-TGF-β mAb (TW7-28G11), and then tested for TGF-β activity by the 293T-caga-Luc-CD32-CD86 bioassay. As expected anti-TGF-β Abs neutralized recombinant TGF-β activity, whereas anti-LAP/anti-latent TGF-β/pro-TGF-β mAbs did not (Figure 8A). Surprisingly, anti-TGF-β Abs did not neutralize TGF-β activity in the T cell culture supernatant (Figure 8B). The ALK5 inhibitor II [14] completely blocked luciferase induction by the T cell culture supernatant (Figure 8B), confirming that the luciferase production was downstream of the TGF-β signaling. On the other hand, anti-LAP mAb TW7-20B9 and anti-latent TGF-β/pro-TGF-β TW7-28G11 inhibited TGF-β activity in the culture supernatant (Figure 8B). Thus, T-cell produced TGF-β activity as measured by the 293T-caga-Luc-CD32-CD86 bioassay takes a molecular form that contains LAP as a component. Since anti-LAP mAb TW7-20B9 does not cross-react with human LAP (Figure S2), the new form of active TGF-β is truly produced from murine CD4 T cells, but not from the human 293T reporter cells.
Figure 8. Effects of anti-LAP or anti-latent TGF-β/pro-TGF-β mAbs on TGF-β activity in T cell culture supernatants.
(A) Recombinant human TGF-β was pre-mixed with anti-active TGF-β Abs (1D11 or chicken anti-TGF-β), an anti-LAP mAb (TW7-20B9), an anti-latent TGF-β/pro-TGF-β mAb (TW7-28G11) (final 50 µg/ml), or ALK5 inhibitor II (final 1 µM), and then added to 293T-caga-Luc-CD32-CD86 reporter cells. (B) A T cell culture supernatant containing an equivalent amount of TGF-β was pre-mixed with the indicated Abs or the ALK5 inhibitor, and added to 293T-caga-Luc-CD32-CD86 cells. The luciferase activity was measured after 16 hr culture. Error bars represent mean ± S.D. of duplicates.
T cell-produced TGF-β is not latent TGF-β
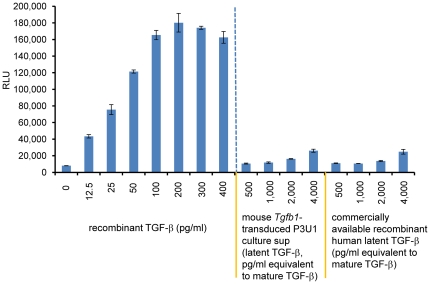
Since the T cell-produced TGF-β contains LAP, it may simply represent the latent TGF-β complex and the 293T-caga-Luc-CD32-CD86 reporter cells have a TGF-β activation machinery which can initiate TGF-β signaling in response to latent TGF-β. To exclude this possibility, we added a commercially available recombinant human latent TGF-β or a culture supernatant of mouse TGF-β-transduced P3U1 cells to the 293T-caga-Luc-CD32-CD86 reporter cells. We found that mouse TGF-β-transduced P3U1 cells produced high amounts of latent TGF-β, as judged by a TGF-β ELISA with or without acidification. However, both latent TGF-β samples barely induced TGF-β signaling in the 293T-caga-Luc-CD32-CD86 cells (Figure 9). Even at 4,000 pg/ml, recombinant human latent TGF-β and mouse latent TGF-β-containing supernatants induced only faint luciferase signals (equivalent to less than 10 pg/ml recombinant TGF-β), which is likely explained by the presence of contaminating active TGF-β which was detected by ELISA without acidification (data not shown).
Figure 9. Response of 293T-caga-Luc-CD32-CD86 cells to latent TGF-β.
Recombinant TGF-β, commercially available recombinant human latent TGF-β or a diluted culture supernatant of mouse Tgfb1-transduced P3U1 cells was added to 293T-caga-Luc-CD32-CD86 cells and the resultant luciferase activity was measured after 11 hrs. Error bars represent mean ± S.D. of duplicates.
Cell type-specific responses to T cell-produced TGF-β
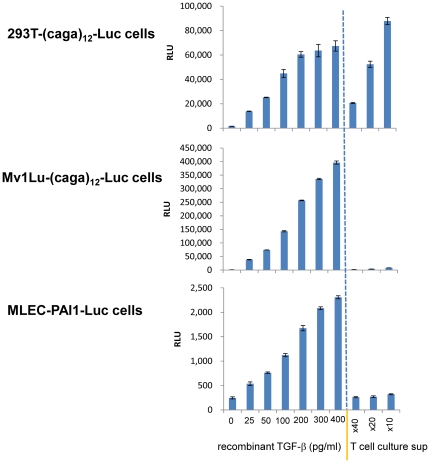
Since T cell-produced TGF-β activity takes an unconventional TGF-β form, it is possible that it requires specific sensing machineries and that only certain cell types respond to T cell-produced TGF-β. To test this, we assayed T cell culture supernatants on other TGF-β reporter cell lines. The Mv1Lu-(caga)12-Luc cell line has the same the (caga)12-Luc reporter as the 293T-caga-Luc-CD32-CD86 cell line, and responds to recombinant TGF-β in a dose-dependent manner. However, we found that Mv1Lu-(caga)12-Luc cells did not respond to T cell culture supernatants (Figure 10, middle). Another well-known TGF-β bioassay reporter line is the mink lung epithelial cell (MLEC)-PAI-1-Luc cell line which has a PAI-1 promoter-driven luciferase reporter [21]; we found that MLEC-PAI-1-Luc cells also did not respond to T cell-produced TGF-β (Figure 10, bottom). These results indicate that T cell-produced TGF-β does not initiate TGF-β signaling in all cell types, but it requires cell type-specific molecular machineries to initiate TGF-β signaling.
Figure 10. Cell type-specific responses to T cell-produced TGF-β.
293T-caga-Luc-CD32-CD86 cells (top), Mv1Lu-(caga)12-Luc cells (middle), or MLEC-PAI-1-Luc cells (bottom) were exposed to recombinant TGF-β or diluted T cell culture supernatants. The luciferase activity was measured after 11 hr culture. Error bars represent mean ± S.D. of duplicates.
Discussion
We have previously reported [22] a TGF-β bioassay for T cells using the MLEC-PAI-1-Luc cell line, in which T cells were cocultured with reporter cells and stimulated with anti-CD3/CD28-coated beads attached to the reporter cell surface. This bioassay demonstrated that CD4+CD25+ Tregs produced active TGF-β which was neutralized with anti-TGF-β mAb 1D11. In order to improve on this bioassay, we first modified the bioassay so that the reporter cells behaved as artificial antigen presenting cells by transducing MLEC-PAI-1-Luc cells with mouse CD32 (Fc receptor) for anti-CD3 capture and with mouse CD86 for costimulation. Preliminary experiments showed that these CD32/CD86-transduced MLEC-PAI-1-Luc cells were killed by murine pre-activated CD4 T cells when T cells were co-cultured with the reporter cells in the presence of anti-CD3. Thus, we chose 293T cell line as TGF-β reporter cells as this cell line is resistant to CD4-mediated killing. We made a lentivirus based TGF-β reporter vector which contains repeated CAGA Smad binding elements in the promoter region linked luciferase (pSMPUW-(caga)12-Luc). 293T cells were transduced with (caga)12-Luc, mouse CD32 and mouse CD86. Serpinb9 and Serpinb9b were also transduced as we found in preliminary experiments that these granzyme inhibitor genes acted to lessen CD4-mediated killing in MLEC-PAI-1-Luc cells.
When we used this new 293T-caga-Luc-CD32-CD86 reporter cell to assay activated T cells, we found that activated T cells produced high TGF-β activity in the 293T-caga-Luc-CD32-CD86 reporter cell assay even though we could not detect active TGF-β production by ELISA. Furthermore, we found that activated Foxp3+ CD4 T cells that express surface LAP/TGF-β [14] had lower TGF-β activity in the 293T-caga-Luc-CD32-CD86 reporter cell assay than activated CD25− T cells. This TGF-β activity was detected in T cell culture supernatants indicating that the T cell-produced TGF-β was in the soluble form and was not membrane-bound. These findings are in contrast to our previous observations using MLEC-PAI-1-Luc cells [22] that when compared to CD4+CD25− T cells, activated CD4+CD25+ T cells had higher TGF-β activity and this activity was linked to membrane bound TGF-β. We believe that this difference is because the T cell-produced TGF-β activity we observed using the 293T-caga-Luc-CD32-CD86 reporter cell assay consists of an unconventional form of TGF-β which can be measured by the 293T-caga reporter cells but not by MLEC-PAI-1-Luc cells. Thus it appears that Foxp3+ Tregs produce the canonical form of mature TGF-β which can be neutralized with anti-TGF-β mAb 1D11, whereas Foxp3− non-Tregs produce a new form of TGF-β which we have identified with our new reporter assay and which can induce TGF-β signaling in a cell type-specific manner.
Whether all activated CD4+CD25− T cells produce this newly described form of TGF-β or it is produced by a subset of CD4+CD25− cells is an important question. In addressing this question, we found that CD4 T cells that produce this new form of TGF-β reside primarily in the CD62L−CD44hi memory fraction as opposed to the CD62L+CD44lo naive fraction (Figure S3). Whether differentiated Th cell subsets such as Th1, Th2, Th17, or Tr1 cells preferentially produce this form of TGF-β is an interesting future question.
The molecular structure and/or composition of this new form of TGF-β are unknown. It contains LAP as a component since the TGF-β activity measured by the 293T-caga-Luc-CD32-CD86 reporter cell is neutralized by an anti-LAP mAb and by an anti-latent TGF-β/pro-TGF-β mAb. Since mature TGF-β is not released from the new form of TGF-β by acidification, this suggests strong binding of the LAP segment to the TGF-β segment. This may be because LAP is linked to TGF-β by covalent bonding. Alternatively, disulfide bonding inside mature TGF-β may take irregular forms that make the TGF-β segment undetectable by ELISA even if the TGF-β segment is released by acidification. Whatever the case, it should be noted that one cannot detect the new form of TGF-β by ELISA even after acidification. Thus, this new form of TGF-β does not appear to be part of the “total” TGF-β detected by ELISA after acidification.
At this time we do not know what cell properties and/or molecules are required to initiate TGF-β signaling by the new form of TGF-β we have identified. In preliminary experiments, we did not find that T cell-produced TGF-β induced Smad2 phosphorylation in CD4 T cells or in bone marrow-derived DCs. However, it is possible that sub-populations of T cells or DCs would respond T cell-produced TGF-β under special conditions.
In conclusion, our work demonstrates that murine CD4 T cells produce an unconventional form of TGF-β which has biological activity as measured by 293T-caga-Luc-CD32-CD86 reporter cells but not by other assay systems and is not produced in significant amounts by conventional Treg cells. Our finding of a new form of T cell-produced TGF-β and the newly developed TGF-β bioassay system will provide a new avenue to investigate T cell function of the immune system.
Materials and Methods
Cell lines, Plasmids, and antibodies
The (caga)12-MLP-Luc vector was kindly provided from Dr. D. Vivien (the Universite' de Caen, Daix, France). Mv1Lu cells (ATCC) were stably transfected with the (caga)12-MLP-Luc plasmid (Mv1Lu-(caga)12-Luc cells). The mink lung epithelial cell line transfected with the Smad-responsive plasminogen activator inhibitor-1 promoter driving a luciferase reporter gene (MLEC-PAI-1-Luc) (originally developed by Abe et al. [21]) was obtained from Dr. L. van de Water (Massachusetts General Hospital, Boston, MA, USA). A lentivirus based TGF-β reporter vector was constructed by inserting the (caga)12-MLP-Luc segment into a promoterless lentiviral vector pSMPUW (Cell Biolabs) along with ubiquitin C promoter-driven a GFP-puro fusion gene as a selection marker (pSMPUW-(caga)12-Luc). pMCs retroviral vector was kindly provided Dr. T. Kitamura (Tokyo Univ., Tokyo, Japan) and pBMN retroviral vector was from Addgene under a MTA with Stanford University (Stanford, CA). A lentivirus supernatant and retrovirus supernatants were produced as described previously [23]. Human embryonic kidney 293T cells (Clontech) were sequentially transduced with pSMPUW-(caga)12-Luc, pBMN-mouse CD32 (without IRES), pBMN-mouse CD86 (without IRES), pMCs-Serpinb9-IRES-Thy1.1, and pMCs-Serpinb9b-IRES-Thy1.2 vectors with cloning in each step. The resultant cells were termed 293T-caga-Luc-CD32-CD86 cells. Anti-TGF-β hybridoma 1D11 was from ATCC, and chicken anti-TGF-β (AF-101-NA) was from R&D Systems. Anti-mouse LAP mAbs TW7-16B4 and TW7-20B9, and anti-latent TGF-β/pro-TGF-β mAb TW7-28G11 were described previously [14]. Anti-FasL (clone MFL3) was from BioLegend. ALK5 inhibitor II was from EMD/Calbiochem.
CD4 T cell preparation and stimulation
Mice were housed in a pathogen-free environment and the animal protocols were approved by the Committee on Animals of Harvard Medical School (Harvard Medical Area Standing Committee on Animals, Protocol No. 02683). CD4 T cells were separated from BALB/c mice (The Jackson Laboratories) using a MACS CD4 purification kit (Miltenyi Biotec). When CD4+CD25− T cells were prepared, biotinylated anti-CD25 antibody (7D4, BD Biosciences) was additionally mixed to the MCAS antibody cocktail. CD4+CD25+ T cells were prepared from the CD4 fraction by staining CD25-FITC (7D4, BD Biosciences) followed by anti-FITC MACS beads (Miltenyi Biotec), and by MS column separation. T cells were stimulated with plate-bound anti-CD3 and anti-CD28 (5 µg/ul each) for 2 days in 10% FBS-supplemented IMDM. In case of CD4+CD25+ T cell stimulation, 100 U/ml recombinant IL-2 was added both in CD4+CD25+ T cell cultures and in CD4+CD25− T cell cultures. The cells were split into non-coated wells and rested for 1 day. The cells were recovered, washed with the culture medium, counted, and used for the TGF-β bioassay, or re-stimulated with plate-bound anti-CD3/CD28 for 24 h hrs for culture supernatants. When indicated, TGF-β-depleted FBS [20] was used for the culture medium.
TGF-β ELISA
TGF-β ELISA was performed using anti-TGF-β mAb 1D11 as a coating antibody and biotinylated chicken anti-TGF-β IgY (BAF240, R&D Systems) as a detection antibody. Recombinant human TGF-β (R&D Systems) was used as a standard (0–2,000 pg/ml). Sample acidification was done by adding 1/10 volume of 1 N HCl, incubating at room temperature for 10 min, and neutralizing with 1/10 volume of 1 N NaOH/0.1 M Tris. The samples were then diluted twofold by adding 25 mM Tris buffered saline.
TGF-β bioassay
293T-caga-Luc-CD32-CD86 TGF-β reporter cells were seeded at 2×104 cells/100 µl/well on collagen-coated 96-well plates 1 day before addition of T cells. On the day of assay, 10 µl of 1 mg/ml anti-CD3 (145-2C11) (BD Biosciences) was added to each well (final 0.5 µg/ml). When CD4+CD25+ T cells were tested, IL-2 was also added to final 100 U/ml. Pre-activated CD4 T cells or recombinant human TGF-β (R&D Systems) were added at 100 µl/well (final total volume 210 µl/well) and cultured for 16 hrs. Similarly, the TGF-β bioassay from culture supernatants was conducted by adding 100 µl of diluted T cell culture supernatants to 293T-caga-Luc-CD32-CD86 cell culture wells and the reporter cells were cultured for the indicated time. When neutralizing antibodies were tested, T cell culture supernatants were diluted to be equivalent to 200 pg/ml recombinant TGF-β activity and were then premixed with the antibodies at 100 µg/ml for 30 min at room temperature. 100 µl of the mixture was added to 100 µl of 293T-caga-Luc-CD32-CD86 reporter wells. 293T-caga-Luc-CD32-CD86 cells were lysed with Glo-Lysis buffer (Promega), and luciferase activity was measured by ONE-Glo luciferease assay reagent (Promega).
Detection of Smad2 phosphorylation
293T-caga-Luc-CD32-CD86 cells were exposed to recombinant human TGF-β (1 ng/ml) or a T cell culture supernatant diluted by TGF-β activity determined by the 293T-caga-Luc-CD32-CD86 bioassay equivalent to 1 ng/ml TGF-β. After 2, 5, 15, and 30 min, the cells were lysed with 1% Triton X-100, 0.25% deoxycholate, 0.1% SDS, 1 mM NaVO4, protease inhibitor cocktail (Pierce/Thermo), 25 mM Tris buffered saline. The lysates were clarified by centrifugation at 13,000 rpm for 15 min, and run on SDS-PAGE under reducing conditions. Western blot was conducted with rabbit anti-phospho-Smad2(Ser465/467) antibody (Cell Signaling) and with rabbit anti-Smad2/3 antibody (Cell Signaling).
Depletion of “total” TGF-β from culture supernatants with an anti-LAP Ab
400 µl of 1 mg/ml of anti-mouse IgG magnetic beads (BioMag Plus, Polysciences) was placed in a 1.5 ml microcentrifuge tube and the beads were washed with PBS three times. 400 µl PBS containing 20 µg of anti-mouse LAP mAb TW7-16B4 or isotype control MOPC21 was added to the beads and incubated with rotation for 4 hrs. After washing with PBS three times, 0.6 ml of the T cell culture supernatant from plate-bound anti-CD3/CD28 re-stimulated CD4+CD25− T cells in 10% TGF-β-depleted FBS IMDM was added to the bead pellets, and incubated at 4°C for overnight. The unbound supernatant was recovered by magnetic separation. The amount of TGF-β before and after separation was measured by TGF-β ELISA after acidification.
Latent TGF-β
Recombinant human latent TGF-β was purchased from R&D Systems. Recombinant mouse latent TGF-β was made as a culture supernatant of mouse Tgfb1-transduced P3U1 cells. The 1× culture supernatant contained 84 ng/ml total TGF-β (mostly latent TGF-β) and 142 pg/ml active TGF-β determined by TGF-β ELISA with, and without acidification, respectively.
Supporting Information
Effect of blocking anti-Fas Ligand antibody to T cell-produced TGF- β. Pre-activated CD4 T cells were harvested on day 4, which is one day delayed compared with the regular stimulation (day 3 recovery), and the CD4 T cells were co-cultured with 293T-caga-Luc-CD32-CD86 reporter cells in presence of blocking anti-FasL mAb.
(TIF)
Species specificity of TW7 antibodies. human TGFB1-transduced P3U1 cells (clone 32, without IRES-GFP) (GFP− population) and mouse Tgfb1-transduced P3U1 cells (clone 11, containing IRES-GFP) (GFP+ population) were mixed and surface stained with TW7-16B4, TW7-20B9, or TW7-28G11 mAb. TW7-16B4 and TW7-20B9 stained only mouse Tgfb1-transduced cells while TW7-28G11 stained both human TGFB1-transduced cells and mouse Tgfb1-transduced cells.
(TIF)
Production of TGF-β activity from naïve CD4 T cells and memory CD4 T cells. CD62L+CD44lo naïve CD4 T cells or CD62LloCD44hi memory CD4 T cells were stimulated with plate-bound anti-CD3/CD28 for 2 days, and rested for 1 day. The pre-activated CD4 T cells were recovered, and the indicated numbers of T cells were added to 293T-caga-Luc-CD32-CD86 cells with 0.5 µg/ml of anti-CD3 antibody. Recombinant human TGF-β was also added as a standard. After 16 hr culture, the reporter cells were lysed and the luciferase activity was measured. Error bars represent mean ± S.D. of duplicates.
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Institutes of Health Grants AI435801 and NS38037. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rubtsov YP, Rudensky AY. TGFβ signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, et al. Immune dysregulation in TGF-β1-deficient mice. J Immunol. 1994;153:1936–1946. [PubMed] [Google Scholar]
- 4.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 10.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;17:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 11.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Miyazono K, Ichijo H, Heldin CH. Transforming growth factor-β: latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oida T, Weiner HL. TGF-β induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS ONE. 2010;5:e15523. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, et al. GARP (LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-β on the surface of activated human Treg. Eur J Immunol. 2009;39:3315–3322. doi: 10.1002/eji.200939684. [DOI] [PubMed] [Google Scholar]
- 18.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, et al. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Docagne F, Colloc'h N, Bougueret V, Page M, Paput J, et al. A soluble transforming growth factor-β (TGF-β) type I receptor mimics TGF-β responses. J Biol Chem. 2001;276:46243–46250. doi: 10.1074/jbc.M010915200. [DOI] [PubMed] [Google Scholar]
- 20.Oida T, Weiner HL. Depletion of TGF-β from fetal bovine serum. J Immunol Methods. 2010;362:195–198. doi: 10.1016/j.jim.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, et al. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 22.Oida T, Xu L, Weiner HL, Kitani A, Strober W. TGF-β-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J Immunol. 2006;177:2331–2339. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- 23.Oida T, Weiner HL. Overexpression of TGF-β1 gene induces cell surface localized Glucose-Regulated Protein 78-associated Latency-associated peptide/TGF-β. J Immunol. 2010;185:3529–3535. doi: 10.4049/jimmunol.0904121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of blocking anti-Fas Ligand antibody to T cell-produced TGF- β. Pre-activated CD4 T cells were harvested on day 4, which is one day delayed compared with the regular stimulation (day 3 recovery), and the CD4 T cells were co-cultured with 293T-caga-Luc-CD32-CD86 reporter cells in presence of blocking anti-FasL mAb.
(TIF)
Species specificity of TW7 antibodies. human TGFB1-transduced P3U1 cells (clone 32, without IRES-GFP) (GFP− population) and mouse Tgfb1-transduced P3U1 cells (clone 11, containing IRES-GFP) (GFP+ population) were mixed and surface stained with TW7-16B4, TW7-20B9, or TW7-28G11 mAb. TW7-16B4 and TW7-20B9 stained only mouse Tgfb1-transduced cells while TW7-28G11 stained both human TGFB1-transduced cells and mouse Tgfb1-transduced cells.
(TIF)
Production of TGF-β activity from naïve CD4 T cells and memory CD4 T cells. CD62L+CD44lo naïve CD4 T cells or CD62LloCD44hi memory CD4 T cells were stimulated with plate-bound anti-CD3/CD28 for 2 days, and rested for 1 day. The pre-activated CD4 T cells were recovered, and the indicated numbers of T cells were added to 293T-caga-Luc-CD32-CD86 cells with 0.5 µg/ml of anti-CD3 antibody. Recombinant human TGF-β was also added as a standard. After 16 hr culture, the reporter cells were lysed and the luciferase activity was measured. Error bars represent mean ± S.D. of duplicates.
(TIF)