Abstract
Obesity and type 2 diabetes (T2D) are two prevalent chronic diseases that have become a major public health concern in industrialized countries. T2D is characterized by hyperglycemia and islet beta cell dysfunction. Glucagon-like peptide 1 (GLP-1) promotes β cell proliferation and neogenesis and has a potent insulinotropic effect. Leptin receptor deficient male rats are obese and diabetic and provide a model of T2D. We hypothesized that their treatment by sustained expression of GLP-1 using encapsulated cells may prevent or delay diabetes onset. Vascular smooth muscle cells (VSMC) retrovirally transduced to secrete GLP-1 were seeded into TheraCyteTM encapsulation devices, implanted subcutaneously and rats monitored for diabetes. Rats that received cell implants showed mean plasma GLP-1 level of 119.3±10.2 pM that was significantly elevated over control values of 32.4±2.9 pM (P<0.001). GLP-1 treated rats had mean insulin levels of 45.9±2.3 ng/ml that were significantly increased over control levels of 7.3±1.5 ng/ml (P<0.001). In rats treated before diabetes onset elevations in blood glucose were delayed and rats treated after onset became normoglycemic and showed improved glucose tolerance tests. Untreated diabetic rats possess abnormal islet structures characterized by enlarged islets with β-cell infiltration and multifocal vacuolization. GLP-1 treatment induced normalization of islet structures including a mantle of β-cells and increased islet mass. These data suggest encapsulated transduced cells may offer a potential long term treatment of patients.
Keywords: Diabetes, bioisolator, GLP-1, encapsulated cells, β cells
Introduction
Obesity and type 2 diabetes (T2D) are two prevalent chronic diseases that have become a major public health concern in industrialized countries affecting at least 16 million people in the United States alone (1). T2D is characterized by hyperglycemia, islet beta cell dysfunction, insulin resistance, hyperglucagonemia, and increased hepatic glucose production. By the time T2D patients present with hyperglycemia, both insulin resistance and β-cell dysfunction are usually present (2). At the present time there are no singly effective treatments for T2D. Tight blood glucose control is required to delay or prevent the onset of late complications that are debilitating and associated with five-fold overmortality (3). Diabetes mellitus increases the risk for hypertension and associated cardiovascular diseases that include coronary, cerebrovascular, renal and peripheral vascular disease (4).
Glucagon-like peptide-1 (GLP-1) is produced by intestinal enteroendocrine L cells, is secreted in response to food ingestion and is a regulator of insulin secretion (5). GLP-1 is produced by posttranslational processing of proglucagon in intestinal L cells and secreted in two biologically active forms, GLP-1 (7-36) amide and GLP-1 (7-37). GLP-1 has pleiotropic biological and clinical effects that have importance for T2D patients. These include stimulation of glucose-dependent insulin secretion, insulin biosynthesis, β cell proliferation and neogenesis and inhibition of β cell apoptosis (5). In both humans and rodents infusion of GLP-1 has been shown to normalize blood glucose and to reduce post-prandial blood glucose excursions (5). However, because of the extremely short circulating half life, continuous 24 hour per day infusion was required. GLP-1 delivery to diabetic mouse, rat and non-human primate models has been shown to have short term beneficial effects (5).
The congenic DR.lepr-/lepr- rat is a model of obesity-associated gender-dependent diabetes (6). The onset of diabetes is rapid in the males while only the females are diabetes resistant. The animals progress from normoglycemia to hyperglycemia within a week and histological analyses of DR.lepr-/lepr- rat pancreas indicates the presence of enlarged islets with β-cell infiltration and multifocal vacuolization compared with DR.+/+ rats (6). We hypothesized that treatment of these rats by sustained GLP-1 expression may increase islet mass and delay diabetes onset or reverse hyperglycemia. We chose to investigate vascular smooth muscle cells as vehicles for GLP-1 delivery because we have shown this cell type will allow sustained expression of retrovirally transduced genes in rodents, dogs and primates (7-11). The encapsulation of GLP-1 secreting cells in an immunoprotective device circumvents the side-effects of immunosuppressants.
Materials and methods
Transduction of rat vascular smooth muscle cells (VSMC)
To obtain GLP-1 expression we constructed an expression plasmid designated LhIL-GLP-1-SN and generated amphotropic pLhIL-GLP-1-SN retrovirus using PA317 packaging cells (12). We transduced, selected and cloned Wistar rat VSMC and a clone giving hormone expression of 72.8pM GLP-1 per 107 cells per 24 hrs. and was used in all implantation experiments (12).
Subcutaneous implantation of bioisolator devices into leptin receptor deficient rats
Leptin receptor deficient rats were bred at the University of Washington (6, 13). 40μl (4.5cm × 1cm × 2mm) TheraCyte Bioisolator devices obtained from TheraCyteTM Inc. Irvine, CA were loaded with 107 transduced VSMC and implanted subcutaneously in anesthetized DR.lepr-/lepr- rats (11, 12). Control rats received mock surgery consisting of anesthesia, subcutaneous incision, pocket dissection and wound closure without implantation of a bioisolator device. All animal procedures were performed under approved protocols and in accordance with ethical guidelines by the Institutional Animal Care and Use Committee (IACUC) at the University of Washington.
Diagnosis of diabetes, glucose and weight monitoring
Starting at 35 days of age, all rats were monitored daily by weight and starting at 37 days of age daily blood glucose was monitored using a blood glucose meter (Ascencia Contour, Bayer, Leverkusen, Germany). Diabetes was diagnosed when blood glucose levels exceeded 200mg/dL on two consecutive days. DR.+/+ normal control rats, untreated DR.lepr-/lepr- rats and GLP-1 treated DR.lepr-/lepr- rats were monitored and did not receive exogenous insulin.
Insulin and GLP-1 assays
For insulin assays 0.2 ml of blood was collected into EDTA containing tubes. Plasma was separated and stored at −20°C. For GLP-1 levels, 0.2 ml of blood was collected into EDTA containing tubes with the addition of 5μl of DPP-4 inhibitor (Linco Research Inc., Missouri) and plasma was separated and stored at −20°C. ELISA was used to measure insulin (Crystal Chem Inc, Illinois) and GLP-1 (Linco Research Inc, Missouri) levels.
Pancreas histology
Pancreatic tissue was subjected to staining using insulin and glucagon specific antibodies for the identification of islet β and α cells respectively (12). Pancreata were fixed in 10% paraformaldehyde/PBS and embedded in paraffin and sections were stained with hematoxylin and eosin and immunostained using guinea pig anti-insulin (Dako), or a murine monoclonal anti rat glucagon antibody (Sigma), followed by biotinylated secondary antibody, ABC-Elite (Vector Laboratories, Burlingame CA) and 3,3′-diaminobenzidine.
Intraperitoneal glucose tolerance test (IPGTT)
Untreated DR.lepr-/lepr- rats, GLP-1 treated DR.lepr-/lepr- rats and DR+/+ wild type rats were fasted for 6-8 h and injected IP with a 2.8M glucose solution to receive 2.5g glucose/kg. Blood glucose levels were determined at base-line (fasting) and at 30, 60 and 120 min by tail-vein puncture and Accu-check glucose meter.
Statistical analysis
Data were expressed as means± SEM, and evaluated by Student’s t test. Significance was determined as P <0.01
Results
Delivery of encapsulated VSMC expressing GLP-1 to DR.lepr-/lepr- rats before diabetes onset
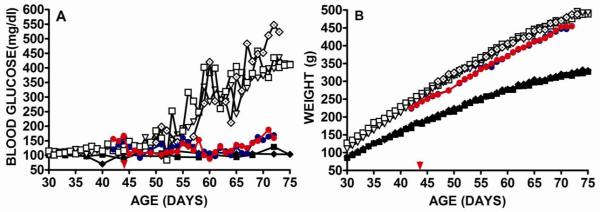
Two DR.lepr-/lepr- rats received implants containing 107 transduced cells at 44 days of age before diabetes onset and were serially monitored for weight and blood glucose (Fig. 1). Untreated DR.lepr-/lepr- control rats showed elevated blood glucose starting at 55 days of age and became hyperglycemic by 59 days of age (Fig. 1A). Blood glucose levels of DR.lepr-/lepr- rats before onset were 114.6±1.7 mg/dl (n=2) and were significantly elevated post onset at 278.6±14.1 mg/dl (n=2) P<0.001. An untreated DR.lepr-/lepr- rat that was a littermate of the two treated rats showed elevated blood glucose levels at 52 days of age (Fig. 1A, open black diamond). In contrast, GLP-1 treated rats showed blood glucose levels of 123.1±3.0 mg/dl (n=2) that were elevated over preonset levels of 114.6±1.7 mg/dl (p=0.04) that was maintained for 73 days of age, beyond the time of diabetes onset of untreated control rats (Fig. 1A). Although the blood glucose levels of GLP-1 treated DR.lepr-/lepr- rats were above normal they were controlled and were not considered hyperglycemic. By 30 days of age untreated DR.lepr-/lepr- rats showed weight gain above DR.+/+ control rats that increased through the study period up to 75 days of age (Fig. 1B). The weight gain of a DR.lepr-/lepr- rat that was a littermate control of the treated rats and were housed together to receive the same access to food, showed the same weight increases (Fig. 1B open black diamond). The pre-onset delivery of GLP-1 caused significantly less weight gain in comparison to untreated DR.lepr-/lepr- rats (Fig. 1B). Over the period of 48 to 72 days of age, the mean weight of GLP-1 treated rats was 376.8±7.9 g (n=2) and untreated 403.4±6.5 g (n=3), p=0.005. This is probably a result of a satiety signal mediated by GLP-1 expression. In contrast, over the period of 48 to 72 days of age, normal untreated DR.+/+ rats showed weights of 274.8±32.6 g that was statistically significantly less than untreated or GLP-1 treated DR.lepr-/lepr- rats (P<0.001) (Fig. 1B). In our statistical analysis we did not include weight and blood glucose values for the 3 days immediately following implant surgery because of the effects of surgery and to allow the encapsulated cells to stabilize within the device. These data suggest the use of encapsulated cells to deliver GLP-1 before the onset of diabetes will delay, or even prevent the onset of diabetes and reduce weight gain.
Fig 1. Male DR.lepr-/lepr- rats receiving encapsulated VSMC secreting GLP-1 before onset of diabetes.
Two GLP-1 treated DR.lepr-/lepr- rats (red and blue closed circles) and three untreated DR.lepr-/lepr- rats (open black symbols) were monitored for blood glucose (A) and weight (B). One untreated DR.lepr-/lepr- control rat (open black diamond) was a littermate of the two treated rats. Implant surgery was at 44 days of age before diabetes onset (red arrow). Untreated DR.+/+ control rats were monitored for blood glucose (A) and weight (B) black closed symbols.
Delivery of encapsulated VSMC expressing GLP-1 to DR.lepr-/lepr- rats after diabetes onset
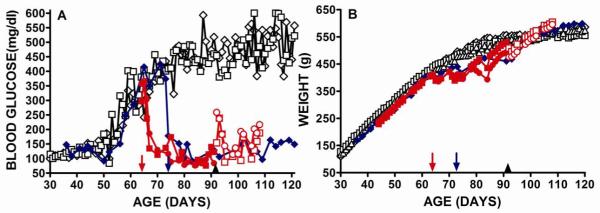
Single Theracyte devices seeded with 107 transduced cells were implanted subcutaneously into three ad libitum fed male DR.lepr-/lepr- rats after diabetes onset defined as blood glucose levels exceeding 200mg/dL on two consecutive days (Fig. 2). Two rats received encapsulation devices at 64 days of age (red symbols) and one at 73 days of age (blue symbol). As noted above, untreated male DR.lepr-/lepr- rats show elevations of blood glucose at about 50 days of age and by 65 days of age were hyperglycemic (Fig. 2A). In GLP-1 treated rats the blood glucose levels decreased after surgery and became in the normal range three days after cell implantation, probably reflecting the time for vascularization of the devices to give cell-mediated GLP-1 delivery. Reduced blood glucose was observed for up to 121 days (Fig. 2A). The mean blood glucose levels of GLP-1 treated rats were 115.2±4.3 mg/dl (n=3) and these were not significantly different from the blood glucose levels of DR.lepr-/lepr- rats before diabetes onset of 114.6±1.7 mg/dl (n=2) (p=0.92). To investigate the duration of the effect of GLP-1 expression on blood glucose we removed the encapsulation devices from two treated rats 27 days after they were implanted (Fig. 2A red symbols). Although blood glucose levels became elevated to a mean of 160.7±46.1 mg/dl (n=2) (Fig. 2A open red symbols) they did not return to the hyperglycemic levels of >350 mg/dl recorded before surgery (Fig. 2A). Hypoglycemia was not detected in any of the treated rats and this was anticipated from the known glucose regulated action of GLP-1 (5, 14, 15).
Fig 2. Male DR.lepr-/lepr- rats receiving encapsulated VSMC secreting GLP-1 after onset of diabetes.
Treated (closed colored symbols) and untreated (black open symbols) rats were monitored for blood glucose (A) and weight (B). After diabetes onset two rats received implants at 64 days of age (red symbols, red arrow) and one at 73 days of age (blue symbol, blue arrow). Theracyte implants were removed from two rats at 91 days of age (black arrow head) and subsequent blood glucose and weights are shown as open colored symbols.
The treated rats showed an initial weight loss that we believe resulted from the appetite suppressant properties of GLP-1 (Fig. 2B). The two rats that received encapsulated cells on 64 days of age had a mean weight of 427.5±5.5 g (n=2) over the 67-87 days of age following implantation and this was significantly reduced from the mean weight of 495.2±3.5 g ( n=3) P<0.0001 of untreated DR.lepr-/lepr- rats over the same time period (Fig. 2B). The DR.lepr-/lepr- rat that received encapsulated cells at 73 days of age lost weight and over the period of 77-97 days of age had a mean weight of 460.5±4.0 g that was significantly less than the mean weight of 527.7±2.2 g (n=3) recorded for untreated DR.lepr-/lepr- control rats over the same age period (P<0.001). After this period of weight loss the weights of the GLP-1 treated DR.lepr-/lepr- rats approached and then exceeded that of untreated DR.lepr-/lepr- animals and this may be due to increased well being and return of appetite that resulted from their blood glucose levels being in the normal range.
Plasma GLP-1 and insulin levels
Plasma GLP-1 levels in untreated DR.lepr-/lepr- rats ranged from 25.5 to 39.5 pM with a mean of 32.4±2.9 pM (n=4) and after implant surgery GLP-1 levels were significantly elevated and ranged from 94.0 pM to 144.5 pM with mean of 119.3±10.2 pM (n=5) (P<0.001). The levels of GLP-1 before surgery were within the normal range for rats (16). At sacrifice, 17 days after device removal from a GLP-1 treated DR.lepr-/lepr- rat (Fig. 1) plasma GLP-1 level was 34.5 pM, similar to GLP-1 levels before treatment. These data show significantly elevated GLP-1 levels in plasma of rats receiving encapsulated cells (P<0.001) and also a return to baseline level after device removal. Normal rat insulin levels were 1-5 ng/ml and untreated male DR.lepr-/lepr- rats had insulin levels between 2.9 and 12.1 ng/ml with a mean of 7.3±1.5 ng/ml (n=5) as we have previously reported (6). GLP-1 treated DR.lepr-/lepr- rats had insulin levels ranging from 38.6 to 51.4 ng/ml with a mean of 45.9±2.3 ng/ml (n=6) that were significantly increased over untreated DR.lepr-/lepr- rats (P<0.001). Insulin levels of two GLP-1 treated rats 17 days after implant removal remained elevated with a mean of 38.3 ng/ml.
Intraperitoneal glucose tolerance tests
As a measure of the effect of GLP-1 treatment on glucose metabolism we performed glucose tolerance tests on DR.+/+ normal control rats, GLP-1 treated and untreated DR.lepr-/lepr- rats (Fig. 3). Rats were fasted for 8 hours before intraperitoneal glucose administration. Wild type DR.+/+ control rats showed increased blood glucose levels at 30 mins after glucose administration that returned to baseline by one hour (Fig. 3). untreated DR.lepr-/lepr- rats showed elevated blood glucose levels of 350-450 mg/dl that became elevated to >650 mg/dl after glucose administration and then gradually became reduced to an average of 550 mg/dl by 120 mins (Fig. 3). In contrast, GLP-1 treated DR.lepr-/lepr- rats showed blood glucose levels in the normal range after 8 hours fast that were increased to about 400 mg/dl levels at 30 mins after glucose administration and then reduced to 250 mg/dl at 60 mins and after 2 hours were at 200 mg/dl (Fig. 3). These data showed GLP-1 treated rats were able to metabolize the administered glucose at rates that, although not normal, were greatly improved over untreated DR.lepr-/lepr- rats.
Fig 3. IPGTT of DR.+/+ wild Type, GLP-1 Treated and Untreated DR.lepr-/lepr- rats.
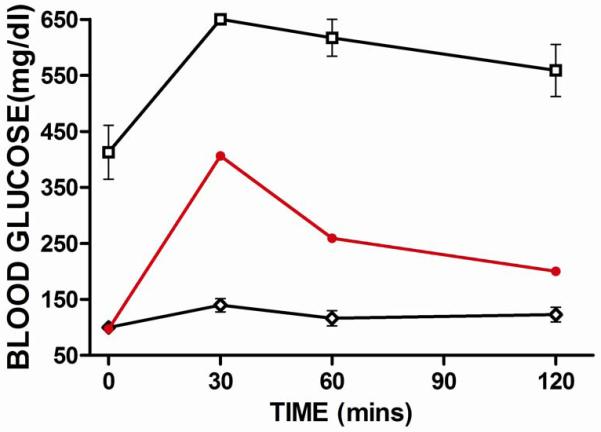
Fasted rats received glucose (2.5g/kg) IP and blood glucose was monitored for 2 hours. Untreated DR.lepr-/lepr- rats (n=3, open square); GLP-1 treated DR.lepr-/lepr- rats (n=2, closed circle); DR wild type rats (n=4, open diamond). Mean and standard error are shown.
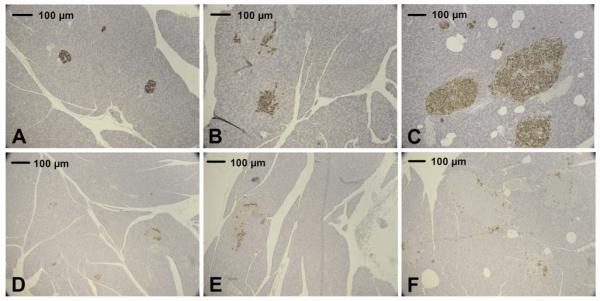
Insulin and glucagon staining of pancreatic tissue
Pancreatic sections from DR.+/+ control, untreated and GLP-1 treated DR.lepr-/lepr- rats were stained for insulin and glucagon (Fig. 4). The pancreas shown was from a GLP-1 treated rat 17 days after removal of implant (Fig. 1). The untreated DR.+/+ control rat showed insulin staining β-cells (Fig. 4A) and, as is typical of rodents, glucagon staining β cells around the periphery (Fig. 4D). However, in the untreated DR.lepr-/lepr- rats insulin staining islets were enlarged (Fig 4B) and this has been previously reported for untreated DR.lepr-/lepr- rats (6). Pancreatic sections from untreated DR.lepr-/lepr- rats stained for glucagon showed α-cells infiltrating throughout the islet α-cells (Fig. 4E). Most notably, the DR.lepr-/lepr- rats treated with GLP-1 showed islets that were without multifocal vacuolization with normal morphology showing β-cells located on the islet periphery and were enlarged (Fig. 4C). Pancreatic tissue from GLP-1 treated DR.lepr-/lepr- rats showed glucagon positive α cells around the periphery of islets (Fig. 4F) and this is typical of wild type rat islets (Fig. 4D) and different from untreated DR.lepr-/lepr- rats (Fig. 4F). Normalized pancreatic islet morphology was observed in rats sacrificed with implant and those sacrificed 17 days after implant removal and this is probably related to the long-lasting effects of GLP-1 on β cell proliferation and neogenesis (17).
Fig 4. Pancreas tissue from wild type DR.+/+, untreated and treated DR.lepr-/lepr- rats.
Untreated control wild type (A, D); untreated DR.lepr-/lepr- rat (B, E); GLP-1 treated DR.lepr-/lepr- rat (C, F). Pancreas sections were stained for insulin (A, B, C) or glucagon (D, E, F). Pancreas from treated DR.lepr-/lepr- rats was obtained 17 days after implant removal.
Discussion
DR.lepr-/lepr- rats exposed to sustained GLP-1 before the onset of diabetes did not become hyperglycemic for up to 73 days of age whereas untreated rats showed elevations of blood glucose that by 60 days of age had increased to levels >400 mg/dl. DR.lepr-/lepr- rats treated before diabetes onset showed reduced weight gain as has been observed in GLP-1 treated ZDF rats (16, 18) and is ascribed to the appetite suppressant properties of GLP-1 (5). Although treated rats were obese the subcutaneous localization of Theracytes did not prevent vascularization and GLP-1 secretion. Our data show that constitutive expression of GLP-1 will delay the onset of diabetes in the DR.lepr-/lepr- rat and is also associated with a statistically significant reduced weight gain. Blood glucose levels of DR.lepr-/lepr- rats treated after diabetes onset decreased immediately after surgery and became in the normal range three days after cell implantation, probably reflecting the time for vascularization of the devices to give cell-mediated GLP-1 delivery and this has been reported for implants of transduced cells expressing erythropoietin (11). In treated diabetic rats blood glucose levels were in a normal range without hypoglycemia, probably because GLP-1 induced increase in islet insulin secretion is inactive during normoglycemia (5). This property of GLP-1 suggests that glucose regulated GLP-1 expression may not be necessary and introduces a safety factor in constitutive, unregulated GLP-1 secretion by cell therapy. However, GLP-1 has effects on tissues other than pancreas, such as brain, heart and stomach and their exposure to long term expression of GLP-1 may be detrimental (5).
Normal rat insulin levels were 1-5 ng/ml and untreated male DR.lepr-/lepr- rats had mean insulin levels of 7.3±1.5 ng/ml as we have previously reported (6). GLP-1 treated DR.lepr-/lepr- rats had mean insulin levels of 45.9±2.3 ng/ml that probably resulted from their increased islet mass that resulted in more normal glucose tolerance tests. Pancreatic tissue from untreated DR.lepr-/lepr- rats showed disorganization of islets with dispersed glucagon staining α-cells, as has been reported for other rat models of T2D (16, 19). The α-cell infiltration has been ascribed to persistent hyperglycemia (16, 19). However, after GLP-1 treatment pancreas tissue from GLP-1 DR.lepr-/lepr- rats showed enlarged islet mass with normal morphology and normal peripheral distribution of glucagon secreting α cells (20).
In summary, the subcutaneous implantation of transduced allogeneic cells in Theracyte™ encapsulation devices is a safe and simple procedure, provided sustained delivery of GLP-1 and did not require immunosupression. In treated diabetic and obese rats the normalization of islet structure mediated by GLP-1 led to normoglycemia, improved glucose tolerance and weight control.
Acknowledgements
This work was supported by a University of Washington Bridge Fund (WO), Junior Faculty Award (1-05-JF-32) (D.H.M) and a Mentor Based Postdoctoral Fellowship Award (Å.L.) from the American Diabetes Association, the National Institutes of Health (AI42380), the Virus Molecular Biology and Cell Core (W.O.) of the University of Washington Diabetes and Endocrinology Research Center (DK17047) as well as by the Robert H. Williams Endowment at the University of Washington. We gratefully thank Kelly Lee Hudkins, Department of Pathology, Histology Core, University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel K, Narayan KMV. The Unite for Diabetes campaign: Overcoming constraints to find a global policy solution. Globalization and Health. 2008;4:3. doi: 10.1186/1744-8603-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 3.DCCT The Diabetes Control and Complications Trial Research Group: Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Annals of Internal Medicine. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR. Recommendations for special populations: diabetes mellitus and the metabolic syndrome. Am J Hypertens. 2003;16:41–45. doi: 10.1016/j.amjhyper.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Moralejo DH, Hansen CT, Treuting P, Hessner MJ, Fuller JM, Van Yserloo B, Jensen R, Osborne W, Kwitek AE, Lernmark A. Differential effects of leptin receptor mutation on male and female BBDR.Gimap5-/Gimap5- spontaneously diabetic rats. Physiological Genomics. 2010;41:9–20. doi: 10.1152/physiolgenomics.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geary RL, Clowes AW, Lau S, Vergel S, Dale DC, Osborne WRA. Gene transfer in baboons using prosthetic vascular grafts seeded with retrovirally-transduced smooth muscle cells: A model for local and systemic gene therapy. Hum. Gene Ther. 1994;5:1213–1218. doi: 10.1089/hum.1994.5.10-1211. [DOI] [PubMed] [Google Scholar]
- 8.Osborne WRA, Ramesh N, Lau S, Clowes MM, Dale DC, Clowes AW. Gene therapy for long-term expression of erythropoietin in rats. Proc. Natl. Acad. Sci. USA. 1995;92:8055–8058. doi: 10.1073/pnas.92.17.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lejnieks DV, Ramesh N, Lau S, Osborne WRA. Stomach implant for long term erythropoietin expression in rats. Blood. 1998;92:888–893. [PubMed] [Google Scholar]
- 10.Barry SC, Ramesh N, Lejnieks DV, Simonson WT, Kemper L, Lernmark A, Osborne WRA. Glucose-regulated insulin expression in diabetic rats. Hum. Gene Ther. 2001;12:131–139. doi: 10.1089/104303401750061195. [DOI] [PubMed] [Google Scholar]
- 11.Yanay O, Flint LY, Brzezinski M, Barry SC, Barton RW, Osborne WRA. Long term erythropoietin gene expression from transduced cells in bioisolator devices. Hum. Gene Ther. 2003;14:1587–1593. doi: 10.1089/104303403322542239. [DOI] [PubMed] [Google Scholar]
- 12.Yanay O, Moralejo M, Kernan K, Brzezinski M, Fuller JM, Barton RW, Lernmark A, Osborne WR. Prolonged survival and improved glycemia in BioBreeding diabetic rats after early sustained exposure to glucagon-like peptide 1. J Gene Med. 2010;12:538–544. doi: 10.1002/jgm.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moralejo DH, Park HA, Speros SJ, Macmurray AJ, Kwitek AE, Jacob HJ, Lander ES, Lernmark A. Genetic dissection of lymphopenia from autoimmunity by introgression of mutated Ian5 gene onto the F344 rat. J Autoimmun. 2003;21:315–324. doi: 10.1016/S0896-8411(03)00138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes, Obesity and Metabolism. 2007;9:23–31. doi: 10.1111/j.1463-1326.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Amori RE, Lau J, Pittas AG. Efficacy and Safety of Incretin Therapy in Type 2 Diabetes: Systematic Review and Meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Parsons GB, Souza DW, Wu H, Yu D, Wadsworth SG, Gregory RJ, Armentano D. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007;14:38–48. doi: 10.1038/sj.gt.3302842. [DOI] [PubMed] [Google Scholar]
- 17.Hui H, Farilla L, Merkel P, Perfetti R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur J Endocrinol. 2002;146:863–869. doi: 10.1530/eje.0.1460863. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Kwon MK, Kang ES, Park YM, Choi SH, Ahn CW, Kim KS, Park CW, Cha BS, Kim SW, Sung JK, Lee EJ, Lee HC. Adenoviral vector-mediated glucagon-like peptide 1 gene therapy improves glucose homeostasis in Zucker diabetic fatty rats. The Journal of Gene Medicine. 2008;10:260–268. doi: 10.1002/jgm.1153. [DOI] [PubMed] [Google Scholar]
- 19.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1782–1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marselli L, Thorne J, Ahn Y-B, Omer A, Sgroi DC, Libermann T, Otu HH, Sharma A, Bonner-Weir S, Weir GC. Gene Expression of Purified {beta}-Cell Tissue Obtained from Human Pancreas with Laser Capture Microdissection. J Clin Endocrinol Metab. 2008;93:1046–1053. doi: 10.1210/jc.2007-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]