Abstract
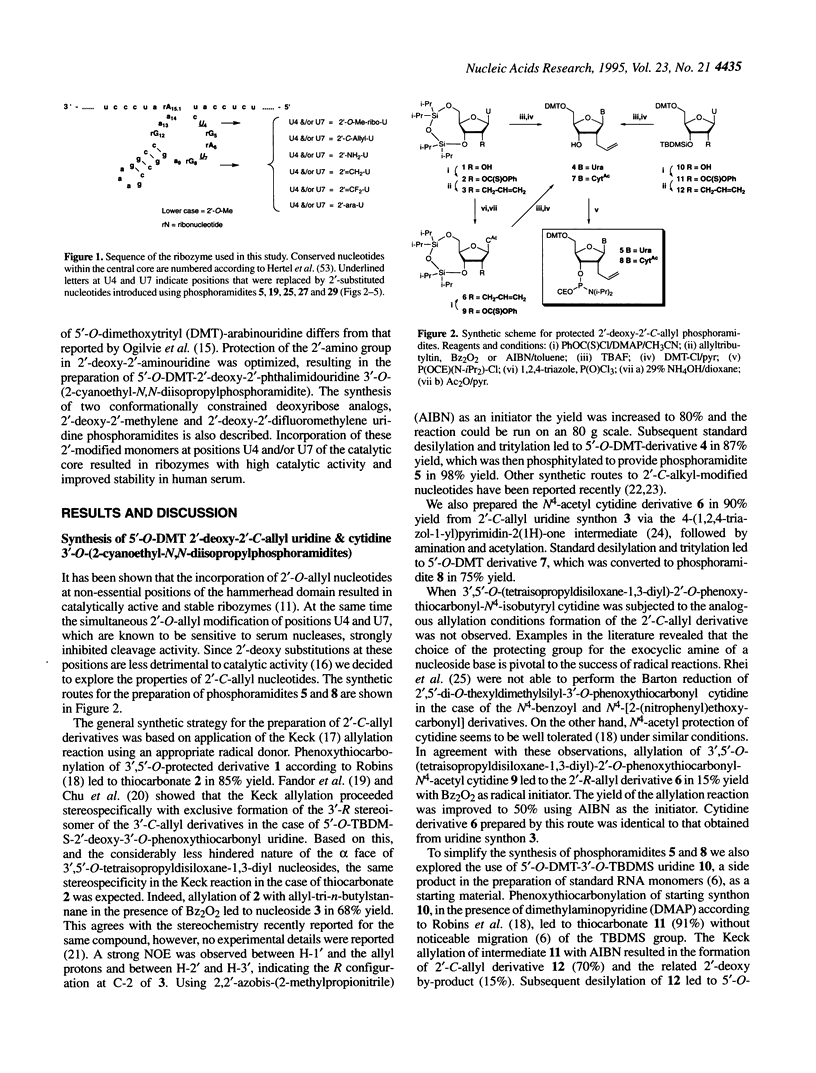
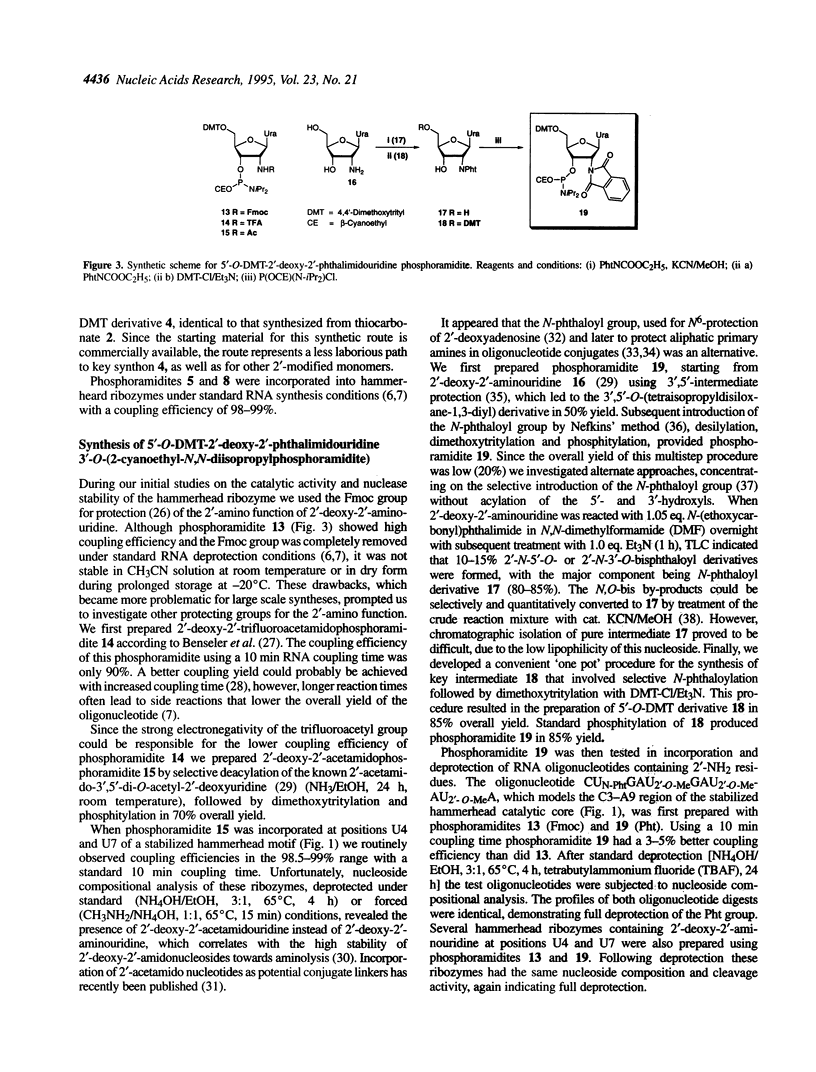
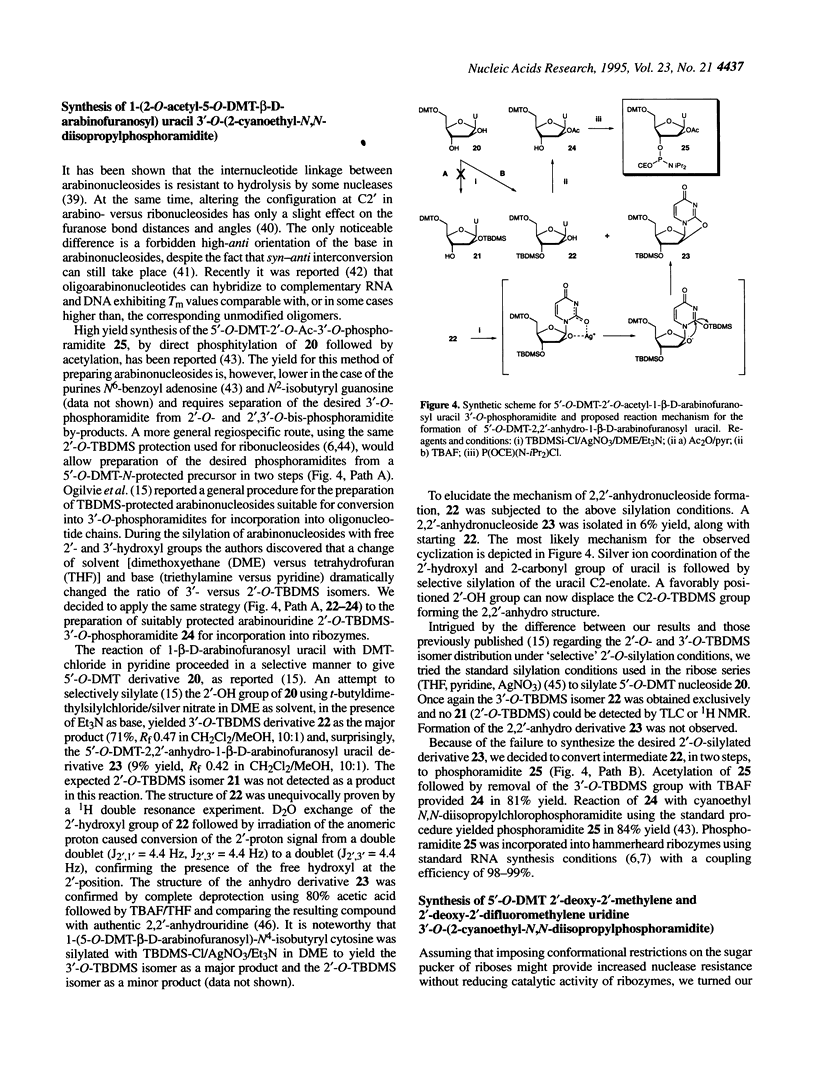
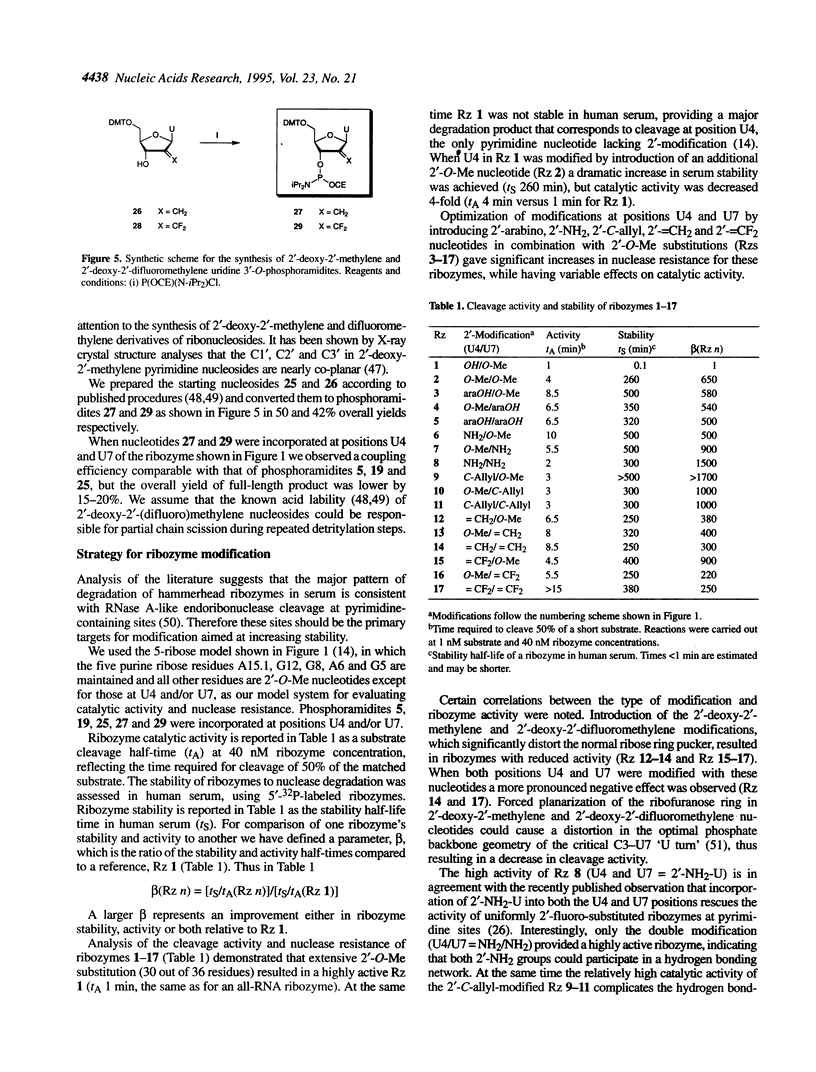
Several 2'-modified ribonucleoside phosphoramidites have been prepared for structure-activity studies of the hammerhead ribozyme. The aim of these studies was to design and synthesize catalytically active and nuclease-resistant ribozymes. Synthetic schemes for stereoselective synthesis of the R isomer of 2'-deoxy-2'-C-allyl uridine and cytidine phosphoramidites, based on the Keck allylation procedure, were developed. Protection of the 2'-amino group in 2'-deoxy-2'-aminouridine was optimized and a method for the convenient preparation of 5'-O-dimethoxytrityl-2'-deoxy-2'-phthalimidouridine 3'-O-(2-cyanoethyl-N,N-diisopropylphosphoramidite) was developed. During the attempted preparation of the 2'-O-t-butyldimethylsilyl-3'-O-phosphoramidite of arabinouridine a reversed regioselectivity in the silylation reaction, compared with the published procedure, was observed, as well as the unexpected formation of the 2,2'-anhydronucleoside. A possible mechanism for this cyclization is proposed. The synthesis of 2'-deoxy-2'-methylene and 2'-deoxy-2'-difluoromethylene uridine phosphoramidites is described. Based on a '5-ribose' model for essential 2'-hydroxyls in the hammerhead ribozyme these 2'-modified monomers were incorporated at positions U4 and/or U7 of the catalytic core. A number of these ribozymes had almost wild-type catalytic activity and improved stability in human serum, compared with an all-RNA molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beigelman L., McSwiggen J. A., Draper K. G., Gonzalez C., Jensen K., Karpeisky A. M., Modak A. S., Matulic-Adamic J., DiRenzo A. B., Haeberli P. Chemical modification of hammerhead ribozymes. Catalytic activity and nuclease resistance. J Biol Chem. 1995 Oct 27;270(43):25702–25708. doi: 10.1074/jbc.270.43.25702. [DOI] [PubMed] [Google Scholar]
- FOX J. J., WEMPEN I. NUCLEOSIDES. XXVI. A FACILE SYNTHESIS OF 2,2' -ANHYDRO-ARABINO PYRIMIDINE NUCLEOSIDES. Tetrahedron Lett. 1965 Mar;11:643–646. doi: 10.1016/s0040-4039(00)90011-x. [DOI] [PubMed] [Google Scholar]
- Fu D. J., Rajur S. B., McLaughlin L. W. Activity of the hammerhead ribozyme upon inversion of the stereocenters for the guanosine 2'-hydroxyls. Biochemistry. 1994 Nov 22;33(46):13903–13909. doi: 10.1021/bi00250a045. [DOI] [PubMed] [Google Scholar]
- Gibson K. J., Benkovic S. J. Synthesis and application of derivatizable oligonucleotides. Nucleic Acids Res. 1987 Aug 25;15(16):6455–6467. doi: 10.1093/nar/15.16.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Heidenreich O., Benseler F., Fahrenholz A., Eckstein F. High activity and stability of hammerhead ribozymes containing 2'-modified pyrimidine nucleosides and phosphorothioates. J Biol Chem. 1994 Jan 21;269(3):2131–2138. [PubMed] [Google Scholar]
- Heidenreich O., Pieken W., Eckstein F. Chemically modified RNA: approaches and applications. FASEB J. 1993 Jan;7(1):90–96. doi: 10.1096/fasebj.7.1.7678566. [DOI] [PubMed] [Google Scholar]
- Hendrix C., Devreese B., Rozenski J., van Aerschot A., De Bruyn A., Van Beeumen J., Herdewijn P. Incorporation of 2'-amido-nucleosides in oligodeoxynucleotides and oligoribonucleotides as a model for 2'-linked conjugates. Nucleic Acids Res. 1995 Jan 11;23(1):51–57. doi: 10.1093/nar/23.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles D. L., Miles D. W., Redington P., Eyring H. A conformational basis for the selective action of ara-adenine. J Theor Biol. 1977 Aug 7;67(3):499–514. doi: 10.1016/0022-5193(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Matteucci M. D., Martin J. C. Current concepts in antisense drug design. J Med Chem. 1993 Jul 9;36(14):1923–1937. doi: 10.1021/jm00066a001. [DOI] [PubMed] [Google Scholar]
- Paolella G., Sproat B. S., Lamond A. I. Nuclease resistant ribozymes with high catalytic activity. EMBO J. 1992 May;11(5):1913–1919. doi: 10.1002/j.1460-2075.1992.tb05244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault J. P., Labuda D., Usman N., Yang J. H., Cedergren R. Relationship between 2'-hydroxyls and magnesium binding in the hammerhead RNA domain: a model for ribozyme catalysis. Biochemistry. 1991 Apr 23;30(16):4020–4025. doi: 10.1021/bi00230a029. [DOI] [PubMed] [Google Scholar]
- Pieken W. A., Olsen D. B., Benseler F., Aurup H., Eckstein F. Kinetic characterization of ribonuclease-resistant 2'-modified hammerhead ribozymes. Science. 1991 Jul 19;253(5017):314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- Pley H. W., Flaherty K. M., McKay D. B. Three-dimensional structure of a hammerhead ribozyme. Nature. 1994 Nov 3;372(6501):68–74. doi: 10.1038/372068a0. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimayama T., Nishikawa F., Nishikawa S., Taira K. Nuclease-resistant chimeric ribozymes containing deoxyribonucleotides and phosphorothioate linkages. Nucleic Acids Res. 1993 Jun 11;21(11):2605–2611. doi: 10.1093/nar/21.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaralingam M. Structure and conformation of nucleosides and nucleotides and their analogs as determined by x-ray diffraction. Ann N Y Acad Sci. 1975 Aug 8;255:3–42. doi: 10.1111/j.1749-6632.1975.tb29211.x. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Usman N., Cedergren R. Exploiting the chemical synthesis of RNA. Trends Biochem Sci. 1992 Sep;17(9):334–339. doi: 10.1016/0968-0004(92)90306-t. [DOI] [PubMed] [Google Scholar]
- Verheyden J. P., Wagner D., Moffatt J. G. Synthesis of some pyrimidine 2'-amino-2'-deoxynucleosides. J Org Chem. 1971 Jan 29;36(2):250–254. doi: 10.1021/jo00801a002. [DOI] [PubMed] [Google Scholar]
- Wechter W. J. Nucleic acids. I. The synthesis of nucleotides and dinucleoside phosphates containing ara-cytidine. J Med Chem. 1967 Sep;10(5):762–773. doi: 10.1021/jm00317a003. [DOI] [PubMed] [Google Scholar]
- Wincott F., DiRenzo A., Shaffer C., Grimm S., Tracz D., Workman C., Sweedler D., Gonzalez C., Scaringe S., Usman N. Synthesis, deprotection, analysis and purification of RNA and ribozymes. Nucleic Acids Res. 1995 Jul 25;23(14):2677–2684. doi: 10.1093/nar/23.14.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrom M. L., Bhat H. B. Trichloroacetyl and trifluoroacetyl as N-blocking groups in nucleoside synthesis with 2-amino sugars. J Org Chem. 1967 Jun;32(6):1821–1823. doi: 10.1021/jo01281a025. [DOI] [PubMed] [Google Scholar]
- Yang J. H., Usman N., Chartrand P., Cedergren R. Minimum ribonucleotide requirement for catalysis by the RNA hammerhead domain. Biochemistry. 1992 Jun 2;31(21):5005–5009. doi: 10.1021/bi00136a013. [DOI] [PubMed] [Google Scholar]



