Abstract
Aims
To investigate the association of CYP2A6 genetic polymorphisms with smoking-related phenotypes in Chinese smokers.
Design
Case-only genetic association study.
Setting
Southern China
Participants
A total of 1,328 Han Chinese smokers who participated in a community-based chronic disease screening project in Guangzhou and Zhuhai from 2006 to 2007.
Measurements
All participants were answered a structured questionnaire about socio-demographic status and smoking behaviors and informative alleles for the cytochrome P450 2A6 (CYP2A6) gene (CYP2A6 *4, *5, *7, *9 and *10) were genotyped.
Findings
The frequencies of CYP2A6 *4, *5, *7, *9 and *10 alleles were 8.5%, 1.2%, 6.3%, 13.5% and 2.4%, which corresponded to 48.9%, 15.4%, 24.2% and 11.5% of participants being classified as normal, intermediate, slow and poor metabolizers, respectively. Multivariate analyses demonstrated that compared with normal metabolizers, poor metabolizers reported smoking fewer cigarettes per day (adjusted OR = 0.49; 95% CI: 0.32–0.76), started smoking regularly later in life (adjusted OR = 1.55; 95% CI: 1.06–2.26) and, amongst former smokers, reported smoking for a shorter duration prior to quitting (adjusted OR = 0.33; 95% CI: 0.12–0.94). However, poor metabolizers were less likely to quit smoking and remain abstinent than normal metabolizers (OR = 0.54; 95% CI: 0.34–0.86).
Conclusions
Reduced metabolism function of CYP2A6 in smokers appears to be associated with fewer cigarettes smoked, later initiation of smoking regularly, shorter smoking duration and lower likelihood of smoking cessation.
Keywords: CYP2A6, genetic polymorphisms, smoking behavior, Chinese smokers
INTRODUCTION
China is the largest tobacco producer and consumer in the world with more than 300 million Chinese smokers consuming roughly one-third of the world’s cigarettes (1–3). It is estimated that one million smokers die from tobacco-related diseases each year in China and this number is expected to rise to 2.2 million death by 2020 (4, 5). Approximately 66% of Chinese males and 4% of Chinese females are current smokers (4); fewer than 30% of smokers in China report that they intend to quit smoking and less than 10% report quitting successfully and remaining abstinent (6). Therefore, there is an urgent need to advance our understanding of risk factors in smoking and cessation, to complement current population-based tobacco control efforts in China.
Nicotine dependence is influenced by multiple environmental and genetic factors (7–11). Approximately 80% of inhaled nicotine is metabolically inactivated to cotinine in the liver by the cytochrome 2A6 (CYP2A6) enzyme (12). Variations in the rate of metabolic inactivation of nicotine, as mediated by CYP2A6, are associated with alterations in nicotine’s bioavailability, which in turn can alter smoking behaviors (13, 14).
Twin and family studies indicate that approximately 80% of the variation in the amount smoked is heritable and variation in some genes has been associated with heaviness of smoking (7–11). Variation in the CYP2A6 gene (chromosome 19q13.2) moderate CYP2A6 enzymatic activity (15, 16). More than 30 CYP2A6 alleles have been reported (http://www.imm.ki.se/CYPalleles/cyp2a6.htm). Some alleles are associated with absent (e.g., *4) (17, 18), reduced (e.g., *9) (19), normal (e.g., *8) (20), or increased (e.g., *1B) (21) CYP2A6 activity with markedly different frequencies among ethnic groups (22–31).
Previous studies have reported that reduced activity CYP2A6 genotypes were associated with altered age of onset (22, 32, 33), shorter duration of smoking (22, 34, 35), fewer cigarettes per day (22–25, 34, 36, 37), fewer pack-years (22, 24, 35), and likelihood of quitting smoking (35). However two early studies, that used older assays and evaluated fewer alleles, found no association with cigarettes per day (38, 39). Among smokers of European-ancestry two clinical trials (40–44) and two case-control studies (33, 45) have shown that reduced activity CYP2A6 genotypes were associated with higher abstinence rates or history of smoking cessation, respectively. However, in the one published report of CYP2A6 genotype and smoking cessation in Asians, Minematsu and colleagues reported a lower likelihood of smoking cessation in smokers with reduced activity CYP2A6 genotypes (35). Thus the influence of CYP2A6 genotype on smoking behaviours is not completely clear, particularly among less well-studied populations such as the Chinese.
In the present study, we studied five CYP2A6 alleles (CYP2A6*4, *5, *7, *9 and *10 alleles) known to reduce the rate of nicotine metabolism and that are prevalent in Chinese (20, 22, 30, 46, 47). Our aim was to explore associations between variation in the CYP2A6 gene and age of onset of smoking regularly, smoking duration, cigarette consumption and smoking cessation in Han Chinese in southern China (1, 3, 48). We hypothesized that reduced activity CYP2A6 genotypes would be associated with later onset of smoking regularly, fewer cigarettes per day, a shorter smoking duration and increased likelihood of smoking cessation.
METHODS
Design
The present study was a case-only genetic association study of smokers in Han Chinese of southern China.
Participants
A total of 7,293 local residents aged 20 years or over participated in a community-based chronic disease-screening project conducted in Guangzhou and Zhuhai, China from July 2006 to June 2007. They were randomly selected by a stratified multistage sampling method described in our previous publication (49) resulting in N = 1,328 Han Chinese smokers being selected for genotyping [n = 970 (73%) current smokers; n = 358 (27%) former smokers]. The Ethics Committees of Sun Yat-sen University approved the study and written informed consent was obtained from all participants.
Data collection
Data collection methods incorporated face-to-face interviewing conducted by trained medical students or clinical doctors using a structured questionnaire for acquiring socio-demographic characteristics (e.g. age, gender, income, education level, marital status, occupation) and smoking behaviors. The survey was completed, and blood samples obtained, at local health care centers.
Measurement and definition of smoking behaviors
A ‘current regular smoker’ was defined by having smoked greater than 100 cigarettes in ones lifetime and having smoked at least one cigarette daily at the time of the interview. A ‘former smoker’ was someone who had smoked greater than 100 cigarettes in their lifetime but reported that they had quit smoking (50). ‘Daily cigarette consumption’ was the average number of cigarettes per day reported by current regular smokers. ‘Age of starting smoking regularly’ referred to the age when an individual started smoking at least one cigarette per day and was analyzed in current and former smokers. ‘Smoking cessation’ was defined by participant responses of having quit smoking and having reported being abstinent at the time of the interview (former smokers). ‘Duration of smoking’ was calculated by subtracting the age of onset of daily smoking from the age of smoking cessation among former smokers only.
CYP2A6 genotyping
Blood was collected and transported on ice to a −80° freezer within three hours of collection. Genomic DNA was extracted using the Takara kit according to manufacturer’s instructions [Blood Genome DNA Extraction Kit, TaKaRa Biotechnology (Dalian) CO., Ltd. China]. The genotyping of CYP2A6 *4, *9, *7 and *10 alleles was conducted using methods previously described (20, 22, 40) with the modification of the first and second amplification primer steps for *7 and *10, respectively (51, 52).
A two-step allele-specific PCR assay was developed to genotype for CYP2A6*5. The 3,128 bp PCR product from the first step of genotyping *7 and *10 was used as a template in the second step PCR for the *5 allele. The 25 μl PCR mixture consisted of 1 μl the first PCR product, 80 nM each primer (26, 52), 0.2 mM dNTPs, 2.5 μl 10×buffer [containing Tris-Hcl (PH 8.3) 100 mM, KCl 500 mM and MgCl2 1.5 mM] and 0.5 U of Taq DNA polymerase. The reaction conditions were as the follows: an initial denaturation at 94° for 1 minutes, followed by 30 cycles of denaturation at 94° for 30 seconds, annealing at 57° for 30 seconds, and extension at 72° for 2 minutes, before a final extension at 72° for 10 minutes. The product of the second step PCR was 1,215 bp. Taq polymerase was obtained from TaKaRa [TaKaRa Biotechnology (Dalian) CO., Ltd. China]. Primers were synthesized by Sangon (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. China).
The CYP2A6*9 allele was considered a “decrease of function” allele (D) and *4, *5, *7 and *10 were considered ‘loss of function’ (L) alleles (14, 17, 18, 20, 26, 47, 53–58) resulting in the following genotype grouping based on these predicted pharmacokinetic impacts. (1) ‘Normal metabolizers’ were defined as having neither a D nor an L allele (i.e., *1/*1); (2) ‘Intermediate metabolizers’ had only one D allele (e.g., *1/*9) which was associated with approximately 75% of the activity of normal metabolizers); (3) ‘Slow metabolizers’ had either one L allele or two D alleles (e.g. *1/*4 or *9/*9) which was associated with 50% of the activity of normal metabolizers; and (4) ‘Poor metabolizers’ had either one L and one D allele or two L alleles (e.g. *9/*4, or *4/*4) which was associated with less than 25% of the activity of normal metabolizers (16, 22).
Statistical analysis
Means and standard deviations (SD) were used to describe continuous variables, while percentages were used to describe categorical variables. Binary logistic regression analyses were conducted to assess the associations between CYP2A6 genotype and smoking phenotypes (age of starting to smoke regularly, duration of smoking, daily cigarette consumption and smoking cessation); in these analyses, each phenotype was classified into binary variables as dependent variables according to their median (0 ≤ median, 1 > median) and for smoking cessation, (0 = current regular smokers, 1 = former smokers). The means of smoking behavior phenotypes were compared among the four CYP2A6 genotype groups by Kruskal-wallis test, followed by Least-Significance-Difference test for multiple comparisons. Confounding was defined as a change in the odds ratio (OR) of more than 10%. Only variables meeting the criteria for confounding were included in a final model [age (categorical), nationality, occupation, education level, and family income] and the only covariate meeting this criterion for confounding was age. Thus, age was adjusted for in all logistic regression models.
The frequencies of CYP2A6 genotypes for each allele were each consistent with the Hardy-Weinberg distribution. All p values were two-sided, and statistical significance was p = 0.05. We used SPSS 13.0 software package (SPSS, Inc., Chicago, IL USA) for all analyses and calculation.
RESULTS
Socio-demographic characteristics
The mean age of participants was 54.6 (SD = 11.9) years, ranging from 20 to 85. The majority of smokers (92.4%) were males; other demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics in smokers from southern China (N = 1,328)
| Demographic characteristics | N | % |
|---|---|---|
| Age (years) | ||
| 20–29 | 38 | 2.9 |
| 30–39 | 120 | 9.0 |
| 40–49 | 247 | 18.6 |
| 50–59 | 434 | 32.7 |
| 60–69 | 357 | 26.9 |
| 70–85 | 132 | 9.9 |
| Gender | ||
| Male | 1,227 | 92.4 |
| Female | 101 | 7.6 |
| Occupation | ||
| Person in charge | 156 | 11.8 |
| Technician | 183 | 13.8 |
| Service personnel | 167 | 12.6 |
| Operator | 306 | 23.0 |
| Retired personnel | 368 | 27.7 |
| Jobless | 148 | 11.1 |
| Education | ||
| Illiteracy or elementary school | 278 | 20.9 |
| Junior middle school | 446 | 33.6 |
| Senior middle school or vocational secondary school | 418 | 31.5 |
| College or above | 186 | 14.0 |
| Marital status | ||
| Single | 51 | 3.8 |
| Married | 1,224 | 92.2 |
| Divorce or widowed | 53 | 4.0 |
| Family monthly total income (yuan) | ||
| <1,000 | 172 | 13.0 |
| 1,000~ | 452 | 34.0 |
| 3,000~ | 363 | 27.3 |
| 5,000~ | 212 | 16.0 |
| Don’t know/refuse to answer | 129 | 9.7 |
| CYP2A6 allele | ||
| *4 allele | 227 | 8.5 |
| *5 allele | 33 | 1.2 |
| *7 allele | 168 | 6.3 |
| *9 allele | 358 | 13.5 |
| *10 allele | 64 | 2.4 |
Frequency CYP2A6 genotypes and alleles
The frequency of the CYP2A6*4 allele was 8.5%, the *5 allele was 1.2%, the *7 allele was 6.3%, the *9 allele was 13.5% and the *10 allele was 2.4%. The most common genotypes were CYP2A6*1/*1 (48.9%), *1/*9 (15.4%), and *1/*4 (10.6%). Allele and genotype-predicted metabolic group frequencies are presented in Table 2. Based on the predicted pharmacokinetic impact of the variant alleles, 48.9% of the subjects were regarded as normal metabolizers, 15.4% as intermediate metabolizers, 24.2% as slow metabolizers, and 11.5% as poor metabolizers.
Table 2.
Frequencies of CYP2A6 genotypes (above) and genotype-predicted metabolizer status (below)
| CYP2A6 genotype | N | Proportion (%) |
|---|---|---|
| *1/*1 | 649 | 48.9 |
| *1/*4 | 141 | 10.6 |
| *1/*5 | 12 | 0.9 |
| *1/*7 | 103 | 7.8 |
| *1/*9 | 205 | 15.4 |
| *1/*10 | 37 | 2.8 |
| *5/*7 | 5 | 0.4 |
| *5/*5 | 1 | 0.1 |
| *5/*9 | 7 | 0.5 |
| *5/*10 | 1 | 0.1 |
| *7/*9 | 35 | 2.6 |
| *7/*10 | 5 | 0.4 |
| *7/*7 | 9 | 0.7 |
| *9/*10 | 12 | 0.9 |
| *9/*9 | 28 | 2.1 |
| *10/*10 | 1 | 0.1 |
| *4/*5 | 5 | 0.4 |
| *4/*7 | 3 | 0.2 |
| *4/*9 | 49 | 3.7 |
| *4/*10 | 8 | 0.6 |
| *4/*4 | 11 | 0.8 |
| *1/*5 and *7/*7 | 1 | 0.1 |
| CYP2A6 genotype-predicted metabolizer status | ||
| Normal metabolizers | 649 | 48.9 |
| Intermediate metabolizers | 205 | 15.4 |
| Slow metabolizers | 321 | 24.2 |
| Poor metabolizers | 153 | 11.5 |
Smoking history
The mean age of becoming a regular smoker was 21.9 (SD = 7.7) years with a mean cigarette consumption for current smokers of 14.6 (SD = 9.0) cigarettes per day. The mean smoking duration for former smokers was 29.8 (SD = 12.8) years; former smokers were older than current smokers [59.7 (SD = 10.2) years vs. 52.7 (SD = 11.9) years; p < 0.001]. There were 928 (70.0%) individuals who reported that they had never quit smoking and 358 (26.9%) reported they quit smoking in the past and were currently not smoking (former smokers); and there were 42 (3.1%) current smokers who reported quitting in the past. The mean age of quitting smoking amongst former smokers was 49.3 (SD = 12.6) years.
Association of CYP2A6 genotypes with smoking behavior
Table 3 presents the results of logistic regression analyses of the association of CYP2A6 with smoking behaviors stratified by CYP2A6 genotype-predicted metabolic category (i.e., normal, intermediate, slow, poor metabolizer) and sex. The logistic regression analyses are also reported by genotype (e.g., *1/*4, etc.) in the Supplementary Table.
Table 3.
Binary logistic regression analyses of smoking phenotypes stratified by CYP2A6 genotype-predicted metabolizer status and sex
| Males (N = 1,227) | ||||
|---|---|---|---|---|
| Characteristics related with smoking behavior# | N (%) | Crude OR (95% CI) | Adjusted OR† (95% CI) | Trend test† |
| Age of starting smoking regularly | ||||
| Normal metabolizer | 594 (48.4) | 1.00 (reference) | 1.00 (reference) | p = 0.015 |
| Intermediate metabolizer | 190 (15.5) | 1.08 (0.77–1.51) | 1.07 (0.76–1.51) | |
| Slow metabolizer | 299 (24.4) | 1.19 (0.89–1.58) | 1.26 (0.94–1.69) | |
| Poor metabolizer | 144 (11.7) | 1.40 (0.97–2.02) | 1.55 (1.06–2.26)* | |
| Smoking duration (years) § | ||||
| Normal metabolizer | 180 (55.7) | 1.00 (reference) | 1.00 (reference) | p = 0.002 |
| Intermediate metabolizer | 57 (17.6) | 0.81 (0.45–1.48) | 0.75 (0.38–1.48) | |
| Slow metabolizer | 59 (18.3) | 0.35 (0.18–0.68)* | 0.36 (0.17–0.75)* | |
| Poor metabolizer | 27 (8.4) | 0.32 (0.12–0.83)* | 0.33 (0.12–0.94)* | |
| Daily cigarette consumption §§ | ||||
| Normal metabolizer | 414 (45.8) | 1.00 (reference) | 1.00 (reference) | p = 0.002 |
| Intermediate metabolizer | 133 (14.7) | 0.99 (0.67–1.47) | 0.99 (0.67–1.47) | |
| Slow metabolizer | 240 (26.5) | 0.77 (0.56–1.07) | 0.77 (0.56–1.07) | |
| Poor metabolizer | 117 (12.9) | 0.49 (0.32–0.76)* | 0.49 (0.32–0.76)* | |
| Smoking cessation | ||||
| Normal metabolizer | 573 (48.1) | 1.00 (reference) | 1.00 (reference) | p < 0.001 |
| Intermediate metabolizer | 186 (15.6) | 0.97(0.67–1.38) | 0.95 (0.65–1.38) | |
| Slow metabolizer | 289 (24.2) | 0.56(0.40–0.78)* | 0.59 (0.41–0.83)* | |
| Poor metabolizer | 144 (12.1) | 0.50(0.32–0.79)* | 0.54 (0.34–0.86)* | |
|
Females (N = 101) | ||||
| Characteristics related with smoking behavior# | N (%) | Crude OR (95% CI) | Adjusted OR† (95% CI) | Trend test † |
| Age of starting smoking regularly | ||||
| Normal metabolizer | 55 (54.5) | 1.00 (reference) | 1.00 (reference) | p = 0.851 |
| Intermediate metabolizer | 15 (14.9) | 0.69 (0.22–2.21) | 0.67 (0.21–2.16) | |
| Slow metabolizer | 22 (21.8) | 1.04 (0.39–2.79) | 1.02 (0.38–2.76) | |
| Poor metabolizer | 9 (8.9) | 0.83 (0.20–3.42) | 0.82 (0.20–3.40) | |
| Smoking duration (years) § | ||||
| Normal metabolizer | 14 (40.0) | 1.00 (reference) | 1.00 (reference) | p = 0.866 |
| Intermediate metabolizer | 7 (20.0) | 1.33 (0.22–8.29) | 2.99 (0.34–26.37) | |
| Slow metabolizer | 10 (28.6) | 0.67 (0.13–3.45) | 0.87 (0.15–5.04) | |
| Poor metabolizer | 4 (11.4) | 1.00 (0.11–9.23) | 1.92 (0.18–20.93) | |
| Daily cigarette consumption §§ | ||||
| Normal metabolizer | 41 (62.1) | 1.00 (reference) | 1.00 (reference) | p = 0.164 |
| Intermediate metabolizer | 8 (12.1) | 0.31 (0.03–2.78) | 0.30 (0.03–2.72) | |
| Slow metabolizer | 12 (18.2) | 0.72 (0.17–3.10) | 0.71 (0.16–3.07) | |
| Poor metabolizer | 5 (7.6) | - | - | |
| Smoking cessation | ||||
| Normal metabolizer | 50 (53.2) | 1.00 (reference) | 1.00 (reference) | p = 0.468 |
| Intermediate metabolizer | 15 (16.0) | 0.88(0.28–2.78) | 0.91 (0.28–2.96) | |
| Slow metabolizer | 21 (22.3) | 0.75(0.27–2.09) | 0.77 (0.27–2.15) | |
| Poor metabolizer | 8 (8.5) | 0.60 (0.13–2.79) | 0.61 (0.13–2.86) | |
Note. The smoking cessation was defined by 0 = no, 1 = yes, and the other dependent variables were defined by 0 = less than or equal to the median, 1 = larger than the median;
Binary logistic regression adjusted for age; -: Insufficient small sample size for LR to generate ORs.
Smoking duration results for former smokers only;
Smoking quantity results for current smokers only; Results for others measures included current regular smokers and former smokers; p-value for statistical trend of age-adjusted analyses;
p<0.05.
Crude and age-adjusted ORs are presented for each phenotype of interest. CYP2A6 genotype groups were not associated with any phenotype examined in females and logistic regression analyses combining both sexes, and of males alone, did not demonstrate any appreciable differences in observed associations. Results are presented stratified by sex. However, it should be noted that the number of female smokers was quite low (n = 101) rendering analytic results relatively less reliable. The following, statistically significant, results represent age-adjusted analyses of males only: Compared with normal metabolizers, poor metabolizers reported smoking fewer cigarettes per day (current smokers) and initiating smoking regularly at an older age (current and former smokers). Slow and poor metabolizers smoked for a shorter duration (former smokers) compared to normal metabolizers. Both slow and poor metabolizers were less likely to have reported quitting smoking than normal metabolizers (former smokers).
Figure 1 illustrates differences in the mean number of cigarettes per day, age of starting smoking regularly, and smoking duration between CYP2A6 genotype-predicted metabolic groups.
Figure 1.
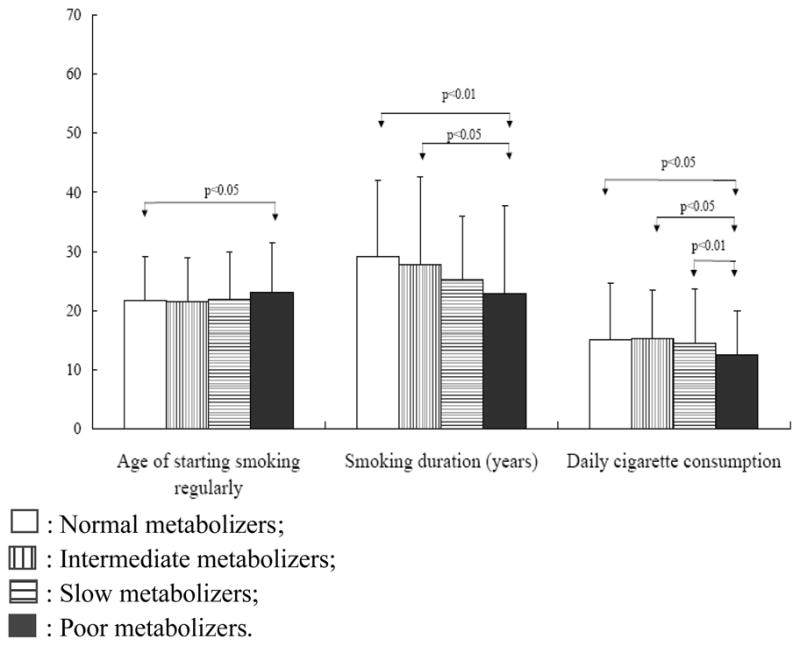
Comparison of smoking behaviors in smokers grouped according to CYP2A6 genotype-predicted metabolic status.
□: Normal metabolizers;
▥: Intermediate metabolizers;
▤: Slow metabolizers;
■: Poor metabolizers.
Note. Y scale was the mean + standard deviation of age for the age of starting smoking regularly (all smokers), years for smoking duration (former smokers) & daily cigarette consumption (current smokers).
DISCUSSION
To our knowledge, this study represents the first report of CYP2A6 genotypes and their association with smoking behaviors in southern China. CYP2A6 genotypes indicative of reduced metabolic function were associated with later smoking initiation, fewer cigarettes smoked per day, shorter smoking duration and reduced likelihood of smoking cessation.
Smoking Initiation
Gu and colleagues observed that smokers possessing a CYP2A6*2 allele (a loss of activity allele) started smoking regularly three years later than smokers not possessing a CYP2A6*2 allele (33). Moreover, in a prospective adolescent cohort study by Audrain-McGovern and colleagues (23), the reduced activity metabolizer genotype group was associated with a slower rate of escalation in nicotine dependence. In contrast to the findings here, Schoedel and colleagues reported that slow metabolizers had an earlier age of first smoking (22) and O’Loughlin and colleagues who studied early adolescents (age 13) found that poor metabolizers, once smoking, converted to nicotine dependence at a faster rate, but smoked fewer cigarettes once dependent (32). Therefore, while none of these studies assessed initiation prospectively, it would appear that those with reduced activity converted to dependence more rapidly, but escalated in level of dependence more slowly, consistent with their lower levels of smoking consumption. Differences in definitions of ‘age of starting smoking’ contribute to the apparent differences seen in the impact of CYP2A6 on this variable (59) and should be examined in the prospective adolescent cohorts.
Daily cigarette consumption
CYP2A6 poor metabolizers reported lower daily cigarette consumption than CYP2A6 normal metabolizers, which is consistent with several previous studies in subjects of a variety of ancestral backgrounds (22–25, 32, 34, 36). Cigarette consumption is the most robust phenotype associated with CYP2A6 genotype; recently it was also detected in a genome-wide meta-analysis. Thorgeirsson and colleagues examined more than 31,000 European-ancestry smokers and reported a highly significant association between a single nucleotide polymorphism (rs4105144), which is in linkage disequilibrium with CYP2A6 *2 (rs180127212), and lower smoking quantity (37).
Smoking duration and smoking cessation
Amongst former smokers, CYP2A6 poor and slow metabolizer genotypes were both associated with reduced smoking duration compared to normal metabolizer genotypes. Slow metabolizer CYP2A6 genotypes have also been reported to be associated with shorter smoking duration in European-ancestry studies (22, 33). However, the only other CYP2A6 genetic association study of Asians reporting smoking duration found no association (24).
In contrast to results from European-ancestry smokers in clinical trials (40–44) and case-control studies (33, 45), the present study demonstrated that CYP2A6 slow and poor metabolizers were less likely to report having successfully quit smoking than CYP2A6 normal metabolizers. Minematsu and colleagues found that individuals possessing a null or *2 allele were less likely to have successfully quit smoking even though, as we found, these reduced activity genotypes were lighter smokers (fewer cigarettes per day) (35).
Given the limitations of the data, we are unable to confirm why there appears to be a discrepancy in associations between CYP2A6 genotype and smoking cessation in Asian and European-ancestry smokers, but there are several potential explanations for this observation. One potential explanation is that in China and Japan, for example, smoking cessation is rarely attempted until one is confronted with a smoking-related health complication (60–63), perhaps because environmental factors including negative societal norms towards smoking (20, 22) and clinical treatment of nicotine dependence may be less prevalent (64–66). Lighter smokers would be expected to experience major morbidity prompting a smoking cessation attempt less often than heavier smokers and CYP2A6 slow and poor metabolizers tend to be lighter smokers and less prone to develop smoking-related illness than CYP2A6 normal metabolizers (67). Alternatively Minematsu and colleagues suggested that in Japanese smokers slower nicotine metabolism resulted in maintenance of reinforcing levels of nicotine for a longer period of time, thereby making it more difficult to extinguish smoking-related behavior (35). Another possible explanation is that slow metabolizers who continue to smoke may have a higher proportion of other variables associated with reduced smoking cessation such as lower education and/or may possess additional genetic variants that increase risk of nicotine dependence and may therefore be more resistant to quitting smoking (25). However, even though the present study observed that slow and poor metabolizers were less likely to report quitting smoking, our study did not examine smokers enrolled in a smoking cessation clinical trial with biochemical verification of abstinence and/or response to nicotine replacement or other pharmacotherapies. Clinical trials conducted by our group in North America have demonstrated that European-ancestry smokers with reduced activity CYP2A6 genotypes, and/or those with slower CYP2A6 activity, quit well on nicotine patch and with behavioral counseling treatment for smoking cessation (40, 42, 43), which cannot be explained entirely by higher nicotine plasma levels. Thus, the role of CYP2A6 and smoking cessation, and the variety of ways this can be evaluated, needs further evaluation among different ethnic groups.
Frequencies of CYP2A6 genotypes
The frequencies of the CYP2A6 *4, *5, *7, *9 and *10 alleles were similar to the ranges reported in previous studies of Chinese people outside of Han Chinese of southern China (4.9–15.1%, 0.5–1.2%, 5.7–9.8%, 15.6–15.7%, and 1.7–4.3%, respectively) (22, 26, 30, 47). However, as seen in Table 4, the frequencies of CYP2A6 alleles vary widely between ancestral groups. In the present study population, reduced activity CYP2A6 alleles were more prevalent than previously observed in European (20, 22, 23, 25, 29), African (27), African-American (22) and Indian ancestry populations (30), and generally comparable for other Asian populations (i.e., Indian, Korean, Malaysian, Thai) (27–30), except for Japanese, for whom the frequency of major reduced-activity alleles combined is about twice as high as those reported among Chinese populations (16, 22, 24, 27, 29).
Table 4.
CYP2A6 allele frequencies from present study and other published reports across multiple ethnic groups
| CYP2A6 allele | Caucasian | Black-African | African-American | Chinese | Southern China† | Japanese | Korean | Thai | Indian | Malays | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| *4 | 0–4.2 | 0.9–1.9 | 1.9 | 4.9–15.1 | 8.5 | 17.0–24.2 | 10.8 | 14.0 | 1.4 | 7.0 | (22–31, 47) |
| *5 | 0–0.3 | 0 | 0 | 0.5–1.2 | 1.2 | 0 | 0.5 | 0.9 | 0.9 | (22, 26, 27, 29–31) | |
| *7 | 0 | 0 | 0 | 5.7–9.8 | 6.5 | 9.8–12.5 | 9.4–9.8 | 5.0 | 0 | 4.3 | (20, 24, 27–29, 47) |
| *9 | 5.2–8.0 | 5.7 | 7.1–8.5 | 15.6–15.7 | 13.8 | 19.0–20.3 | 19.6 | 20.0 | (22–25, 27, 28, 46) | ||
| *10 | 0 | 0 | 0 | 1.7–4.3 | 2.5 | 2.2–3.2 | 1.0–4.1 | 2.0 | 0 | 4.3 | (27–30, 47) |
Note. Numbers in columns represent allele frequency ranges, as percent of total alleles, in published studies and in the present study;
Allele frequencies from the present study.
Limitations
There are several limitations to the present study. The data is retrospective and prone to recall bias. The age of first smoking attempt was also not queried and nor was it possible to determine transition time to nicotine dependence. Non-smokers were not included in the study. However, as CYP2A6 metabolizes nicotine and carcinogens but is not implicated in reward signaling, it is unlikely to play a role in whether or not individuals experiment with smoking and there is no clear justification for including non-smokers as they would mainly be a study population of untested genetic risk. Given our study sample, we would have needed to identify non-smokers who had tried smoking and not become regular smokers so they had a chance for their genetic variability in CYP2A6 to act (on nicotine) and then follow participants prospectively. Given the way the data was collected, this was not possible since there was no distinction between individuals who had never experimented with smoking and those who had smoked less than 100 cigarettes.
While not all possible CYP2A6 alleles were genotyped, the selection of alleles were those previously reported to be present in more than 1% of Asians and so we do not anticipate a high ‘false negative’ rate for reduced activity alleles. If anything, the lack of variant allele testing would weaken the power of the present analyses, biasing results toward the null. Another limitation of the data is the lack of biochemical verification of abstinence in former smokers or biomarkers of nicotine exposure and CYP2A6 metabolic activity. Finally, dietary and medication histories were not available, and so were not able to control the potential effects of diet (68) or drug (69) on induction of CYP2A6 activity or effects of oral contraceptives on nicotine clearance. (12).
Conclusions
In this study of mostly male smokers of Han ethnicity from southern China, CYP2A6 poor metabolizer genotypes were associated with lighter smoking, a later age of initiation and a shorter duration of smoking. In addition, both slow and poor metabolizer genotypes were associated with decreased smoking duration and decreased likelihood of smoking cessation. If replicated these results have important public health implications in order to reduce the harm associated with smoking and to develop more targeted efforts to increase smoking cessation and harm reduction.
Supplementary Material
Acknowledgments
This study was funded by the Guangzhou Health Bureau (2005-Zda-001) and National Institute on Drug Abuse/National Institutes of Health grants K08-014276 and R21-027331.
We acknowledge the financial support from the Centre for Addiction and Mental Health (RFT QZ) and Canadian Institutes for Health Research MOP86471 (RFT). RF Tyndale holds a Canada Research Chair in Pharmacogenetics.
Footnotes
Conflict of interest declaration
Dr. RF Tyndale holds shares in Nicogen, a company focused on the development of novel smoking cessation treatments. No support was provided by Nicogen for this study and the manuscript was not reviewed by other members of the company. Dr RF Tyndale has also consulted for Novartis on tobacco cessation. All other authors have no conflicts of interests to declare in relation to this report.
References
- 1.Liu BQ, Peto R, Chen ZM, Boreham J, Wu YP, Li JY, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. Bmj. 1998;317:1411–22. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 National Prevalence Survey. Jama. 1999;282:1247–53. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 3.Niu SR, Yang GH, Chen ZM, Wang JL, Wang GH, He XZ, et al. Emerging tobacco hazards in China: 2. Early mortality results from a prospective study. Bmj. 1998;317:1423–4. doi: 10.1136/bmj.317.7170.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization. W. H. Towards a tobacco-free China. Geneva. Switzerland: World Health Organization; 2007. [accessed 15 Aug 2007]. http://www.wpro.who.int/china/sites/tfi/ [Google Scholar]
- 5.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Ma J, Chen A, Zhang Y, Samet JM, Taylor CE, et al. Smoking cessation in China: findings from the 1996 national prevalence survey. Tob Control. 2001;10:170–4. doi: 10.1136/tc.10.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 8.Li MD, Ma JZ, Beuten J. Progress in searching for susceptibility loci and genes for smoking-related behaviour. Clin Genet. 2004;66:382–92. doi: 10.1111/j.1399-0004.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 9.Han S, Gelernter J, Luo X, Yang BZ. Meta-analysis of 15 genome-wide linkage scans of smoking behavior. Biol Psychiatry. 67:12–9. doi: 10.1016/j.biopsych.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 35:702–19. doi: 10.1038/npp.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(TAG) TaGC. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–7. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–15. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 14.Sellers EM, Kaplan HL, Tyndale RF. Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clin Pharmacol Ther. 2000;68:35–43. doi: 10.1067/mcp.2000.107651. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima M, Yokoi T. Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab Pharmacokinet. 2005;20:227–35. doi: 10.2133/dmpk.20.227. [DOI] [PubMed] [Google Scholar]
- 16.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 17.Oscarson M, McLellan RA, Gullsten H, Yue QY, Lang MA, Bernal ML, et al. Characterisation and PCR-based detection of a CYP2A6 gene deletion found at a high frequency in a Chinese population. FEBS Lett. 1999;448:105–10. doi: 10.1016/s0014-5793(99)00359-2. [DOI] [PubMed] [Google Scholar]
- 18.Nunoya KI, Yokoi T, Kimura K, Kainuma T, Satoh K, Kinoshita M, et al. A new CYP2A6 gene deletion responsible for the in vivo polymorphic metabolism of (+)-cis-3,5-dimethyl-2-(3-pyridyl)thiazolidin-4-one hydrochloride in humans. J Pharmacol Exp Ther. 1999;289:437–42. [PubMed] [Google Scholar]
- 19.Yoshida R, Nakajima M, Nishimura K, Tokudome S, Kwon JT, Yokoi T. Effects of polymorphism in promoter region of human CYP2A6 gene (CYP2A6*9) on expression level of messenger ribonucleic acid and enzymatic activity in vivo and in vitro. Clin Pharmacol Ther. 2003;74:69–76. doi: 10.1016/S0009-9236(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 20.Mwenifumbo JC, Myers MG, Wall TL, Lin SK, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6*7, CYP2A6*8 and CYP2A6*10 as assessed with a novel haplotyping method. Pharmacogenet Genomics. 2005;15:189–92. doi: 10.1097/01213011-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, Krasnow RE, Swan GE, Benowitz NL, et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin Pharmacol Ther. 2008;83:115–21. doi: 10.1038/sj.clpt.6100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–26. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–74. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 24.Minematsu N, Nakamura H, Furuuchi M, Nakajima T, Takahashi S, Tateno H, et al. Limitation of cigarette consumption by CYP2A6*4, *7 and *9 polymorphisms. Eur Respir J. 2006;27:289–92. doi: 10.1183/09031936.06.00056305. [DOI] [PubMed] [Google Scholar]
- 25.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–9. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 26.Oscarson M, McLellan RA, Gullsten H, Agundez JA, Benitez J, Rautio A, et al. Identification and characterisation of novel polymorphisms in the CYP2A locus: implications for nicotine metabolism. FEBS Lett. 1999;460:321–7. doi: 10.1016/s0014-5793(99)01364-2. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80:282–97. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Peamkrasatam S, Sriwatanakul K, Kiyotani K, Fujieda M, Yamazaki H, Kamataki T, et al. In vivo evaluation of coumarin and nicotine as probe drugs to predict the metabolic capacity of CYP2A6 due to genetic polymorphism in Thais. Drug Metab Pharmacokinet. 2006;21:475–84. doi: 10.2133/dmpk.21.475. [DOI] [PubMed] [Google Scholar]
- 29.Gyamfi MA, Fujieda M, Kiyotani K, Yamazaki H, Kamataki T. High prevalence of cytochrome P450 2A6*1A alleles in a black African population of Ghana. Eur J Clin Pharmacol. 2005;60:855–7. doi: 10.1007/s00228-004-0854-9. [DOI] [PubMed] [Google Scholar]
- 30.Nurfadhlina M, Foong K, Teh LK, Tan SC, Mohd Zaki S, Ismail R. CYP2A6 polymorphisms in Malays, Chinese and Indians. Xenobiotica. 2006;36:684–92. doi: 10.1080/00498250600715932. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Cook DG, Hinks LJ, Chen XH, Ye S, Gilg JA, et al. CYP2A6, MAOA, DBH, DRD4, and 5HT2A genotypes, smoking behaviour and cotinine levels in 1518 UK adolescents. Pharmacogenet Genomics. 2005;15:839–50. doi: 10.1097/01213011-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 32.O’Loughlin J, Paradis G, Kim W, DiFranza J, Meshefedjian G, McMillan-Davey E, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–8. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu DF, Hinks LJ, Morton NE, Day IN. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet. 2000;64:383–90. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- 34.Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, et al. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–55. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- 35.Minematsu N, Nakamura H, Iwata M, Tateno H, Nakajima T, Takahashi S, et al. Association of CYP2A6 deletion polymorphism with smoking habit and development of pulmonary emphysema. Thorax. 2003;58:623–8. doi: 10.1136/thorax.58.7.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujieda M, Yamazaki H, Saito T, Kiyotani K, Gyamfi MA, Sakurai M, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25:2451–8. doi: 10.1093/carcin/bgh258. [DOI] [PubMed] [Google Scholar]
- 37.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loriot MA, Rebuissou S, Oscarson M, Cenee S, Miyamoto M, Ariyoshi N, et al. Genetic polymorphisms of cytochrome P450 2A6 in a case-control study on lung cancer in a French population. Pharmacogenetics. 2001;11:39–44. doi: 10.1097/00008571-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Amemo K, Ameno S, Iwahashi K, Kinoshita H, Kubota T, et al. Lack of association between smoking and CYP2A6 gene polymorphisms in A Japanese population. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2001;36:486–90. [PubMed] [Google Scholar]
- 40.Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87:553–7. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–43. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–5. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 43.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malaiyandi V, Goodz SD, Sellers EM, Tyndale RF. CYP2A6 genotype, phenotype, and the use of nicotine metabolites as biomarkers during ad libitum smoking. Cancer Epidemiol Biomarkers Prev. 2006;15:1812–9. doi: 10.1158/1055-9965.EPI-05-0723. [DOI] [PubMed] [Google Scholar]
- 46.Pitarque M, von Richter O, Oke B, Berkkan H, Oscarson M, Ingelman-Sundberg M. Identification of a single nucleotide polymorphism in the TATA box of the CYP2A6 gene: impairment of its promoter activity. Biochem Biophys Res Commun. 2001;284:455–60. doi: 10.1006/bbrc.2001.4990. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Rao YS, Xu B, Hoffmann E, Jones J, Sellers EM, et al. An in vivo pilot study characterizing the new CYP2A6*7, *8, and *10 alleles. Biochem Biophys Res Commun. 2002;290:318–24. doi: 10.1006/bbrc.2001.6209. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z, Lee L, Chen J, Collins R, Wu F, Guo Y, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–9. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Qiu Q, Tan LL, Liu T, Deng XQ, Chen YM, et al. Prevalence and determinants of diabetes and impaired fasting glucose among urban community-dwelling adults in Guangzhou, China. Diabetes Metab. 2009;35:378–84. doi: 10.1016/j.diabet.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukami T, Nakajima M, Sakai H, McLeod HL, Yokoi T. CYP2A7 polymorphic alleles confound the genotyping of CYP2A6*4A allele. Pharmacogenomics J. 2006;6:401–12. doi: 10.1038/sj.tpj.6500390. [DOI] [PubMed] [Google Scholar]
- 52.Ho MK, Mwenifumbo JC, Zhao B, Gillam EM, Tyndale RF. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black-African descent. Pharmacogenet Genomics. 2008;18:67–75. doi: 10.1097/FPC.0b013e3282f3606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ariyoshi N, Sawamura Y, Kamataki T. A novel single nucleotide polymorphism altering stability and activity of CYP2a6. Biochem Biophys Res Commun. 2001;281:810–4. doi: 10.1006/bbrc.2001.4422. [DOI] [PubMed] [Google Scholar]
- 54.Oscarson M, Gullsten H, Rautio A, Bernal ML, Sinues B, Dahl ML, et al. Genotyping of human cytochrome P450 2A6 (CYP2A6), a nicotine C-oxidase. FEBS Lett. 1998;438:201–5. doi: 10.1016/s0014-5793(98)01297-6. [DOI] [PubMed] [Google Scholar]
- 55.Hadidi HF, Cholerton S, Monkman SC, Armstrong M, Irshaid YM, Rawashdeh NM, et al. Debrisoquine 4-hydroxylation (CYP2D6) polymorphism in Jordanians. Pharmacogenetics. 1994;4:159–61. doi: 10.1097/00008571-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Ariyoshi N, Takahashi Y, Miyamoto M, Umetsu Y, Daigo S, Tateishi T, et al. Structural characterization of a new variant of the CYP2A6 gene (CYP2A6*1B) apparently diagnosed as heterozygotes of CYP2A6*1A and CYP2A6*4C. Pharmacogenetics. 2000;10:687–93. doi: 10.1097/00008571-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Kitagawa K, Kunugita N, Kitagawa M, Kawamoto T. CYP2A6*6, a novel polymorphism in cytochrome p450 2A6, has a single amino acid substitution (R128Q) that inactivates enzymatic activity. J Biol Chem. 2001;276:17830–5. doi: 10.1074/jbc.M009432200. [DOI] [PubMed] [Google Scholar]
- 58.Nunoya K, Yokoi T, Kimura K, Inoue K, Kodama T, Funayama M, et al. A new deleted allele in the human cytochrome P450 2A6 (CYP2A6) gene found in individuals showing poor metabolic capacity to coumarin and (+)-cis-3,5-dimethyl-2-(3-pyridyl)thiazolidin-4-one hydrochloride (SM-12502) Pharmacogenetics. 1998;8:239–49. doi: 10.1097/00008571-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Al Koudsi N, Hoffmann EB, Assadzadeh A, Tyndale RF. Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. Eur J Clin Pharmacol. 66:239–51. doi: 10.1007/s00228-009-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Bmj. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doll R. Smoking and disease: prospects for control. R Soc Health J. 1977;97:167–76. doi: 10.1177/146642407709700409. [DOI] [PubMed] [Google Scholar]
- 62.Doll SR. Smoking and lung cancer. Am J Respir Crit Care Med. 2000;162:4–6. doi: 10.1164/ajrccm.162.1.16221. [DOI] [PubMed] [Google Scholar]
- 63.Kohrman M. New steps for tobacco control in and outside of China. Asia Pac J Public Health. 22:189S–196S. doi: 10.1177/1010539510373012. [DOI] [PubMed] [Google Scholar]
- 64.Hyland A, Higbee C, Borland R, Travers M, Hastings G, Fong GT, et al. Attitudes and beliefs about secondhand smoke and smoke-free policies in four countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2009;11:642–9. doi: 10.1093/ntr/ntp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sussman S, Pokhrel P, Black D, Kohrman M, Hamann S, Vateesatokit P, et al. Tobacco control in developing countries: Tanzania, Nepal, China, and Thailand as examples. Nicotine Tob Res. 2007;9(Suppl 3):S447–57. doi: 10.1080/14622200701587078. [DOI] [PubMed] [Google Scholar]
- 66.Kohrman M. Smoking among doctors: governmentality, embodiment, and the diversion of blame in contemporary China. Med Anthropol. 2008;27:9–42. doi: 10.1080/01459740701831401. [DOI] [PubMed] [Google Scholar]
- 67.Tyndale RF, Sellers EM. Variable CYP2A6-mediated nicotine metabolism alters smoking behavior and risk. Drug Metab Dispos. 2001;29:548–52. [PubMed] [Google Scholar]
- 68.Runkel M, Bourian M, Tegtmeier M, Legrum W. The character of inhibition of the metabolism of 1,2-benzopyrone (coumarin) by grapefruit juice in human. Eur J Clin Pharmacol. 1997;53:265–9. doi: 10.1007/s002280050374. [DOI] [PubMed] [Google Scholar]
- 69.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003;31:421–31. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


