Abstract
The Rule of Five predicts suitability of drug candidates, but was developed primarily using orally administered drugs. Here, we test whether the Rule of Five predicts drugs for delivery via non-oral routes, specifically ophthalmic, inhalation and transdermal. We assessed 111 drugs approved by FDA for those routes of administration and found that >98% of current non-oral drugs have physicochemical properties within the limits of the Rule of Five. However, given the inherent bias in the dataset, this analysis was not able to assess whether drugs with properties outside those limits are poor candidates. Indeed, further analysis indicates that drugs well outside the Rule of Five limits, including hydrophilic macromolecules, can be delivered by inhalation. In contrast, drugs currently administered across skin fall within more stringent limits than predicted by the Rule of Five, but new transdermal delivery technologies may make these constraints obsolete by dramatically increasing skin permeability. The Rule of Five does appear to apply well to ophthalmic delivery. We conclude that although current non-oral drugs mostly have physicochemical properties within the Rule of Five thresholds, the Rule of Five should not be used to predict non-oral drug candidates, especially for inhalation and transdermal routes.
Keywords: absorption, drug design, drug-like properties, inhalation, Lipinski's Rule of Five, ophthalmic, physicochemical properties, predictive drug delivery, pulmonary, tissue permeability, transdermal
INTRODUCTION
Drug candidates are triaged early during drug development based on computer modeling, high-throughput screening and cell-based assays that predict pharmacologic activity (1). It is, however, much more difficult to predict drug absorption, distribution, metabolism and excretion (ADME), which typically require evaluation in vivo. Because in vivo studies are slow and expensive, it is desirable to have simple methods to predict ADME properties of drug candidates.
A widely accepted method to predict ADME properties is the Rule of Five proposed by Lipinski in 1997 (2). To develop this rule, Lipinski carried out retrospective analysis of 2245 drugs at entry to Phase II, most of which were orally active, lipophilic drugs, and identified which physicochemical properties they had in common. The resulting correlation identified four physicochemical parameters: molecular weight (MW), number of H-bond donors (NHD), number of H-bond acceptors (NHA) and octanol-water partition coefficient (log P). The Rule of Five states that poor absorption or permeation is expected when MW>500, NHD>5, NHA>10 or log P>5.
Although oral delivery remains dominant, other routes of administration are widely used and of growing interest for targeted drug delivery and increased patient compliance. For example, most ophthalmic drugs are given via local ocular delivery using eye drops to increase targeting efficiency and reduce systemic side effects (3). Similarly, inhalation delivery is preferred for local treatment of the lung, which avoids systemic side effects; for drugs needing rapid onset enabled by quick absorption in the lung; for drugs affected by first-pass metabolism; and for macromolecule drugs that would otherwise need to be injected (4). Finally, transdermal delivery is preferred for drugs that undergo significant or variable first-pass hepatic metabolism or drugs that would benefit from steady plasma concentrations enabled by a controlled release patch worn for up to one week (5). The global market for non-oral drugs is expected to double from its current value of about $45 billion within 5 years (6-8).
While there exist models and correlations that predict the permeability of, for example, the skin or the cornea (3,5), we are not aware of any integrated approaches that enable straightforward triage of poor drug candidates for non-oral delivery. Previous computational work has considered molecular descriptors that correlate with non-oral drugs, but these analyses have nonetheless focused on oral delivery and lumped various non-oral routes together without individually considering the ophthalmic, inhalation and transdermal routes specifically (9,10). The physicochemical properties of marketed respiratory drugs, both inhaled and intranasal drugs combined, were analyzed in comparison with those of their orally administered counterpart drugs used for the same indications (11).
To address this need, this study tested whether the Rule of Five can be used to predict drugs for delivery via non-oral routes. We performed retrospective analysis on 111 drugs approved by the FDA for ophthalmic, inhalation and transdermal delivery, following the same method that Lipinski employed (2). Our approach is based on the expectation that Lipinski's rule is relevant, because both oral and non-oral routes involve diffusion across lipid epithelial barriers and solubility in aqueous bodily fluids. On the other hand, we recognize that each route of delivery has different barriers and constraints. Thus, our analysis also assesses deviations from the Rule of Five and proposes alternative models.
DATA COLLECTION AND ANALYSIS
We first identified all drugs approved by the FDA for delivery by ophthalmic, inhalation and transdermal routes using FDA's Orange Book (last updated March, 2010) (12). The drugs were then categorized specifically for each of the administration routes, and their physicochemical properties were examined based on the Rule of Five, following Lipinski's method (2). Drugs exceeding the limits of each of the four parameters (i.e., MW>500, NHD>5, NHA>10, and log P>5) were identified as violating the Rule of Five, and the drugs exceeding the limits of two or more of the four parameters were classified as “ALERT” (Supplementary Material Tables S1, S2 and S3). The distribution of the drug properties for each parameter was also prepared as histograms. We finally performed bootstrap analysis (13) on the physicochemical parameters of the non-oral drugs to test the hypothesis that the thresholds originally generated by Lipinski also apply to the non-oral drugs examined in this work. Through this method, we generated new thresholds for each of the physicochemical parameters. Detailed analytic methods are described in the Supplementary Material.
ANALYTIC RESULTS
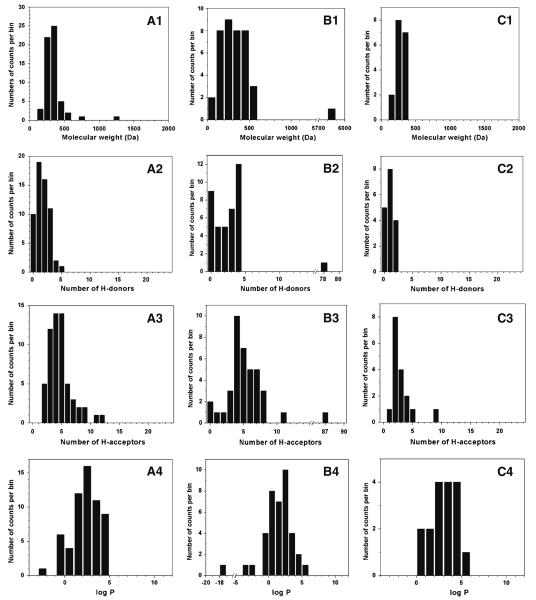
A total of 59 ophthalmic drugs approved by the FDA were evaluated based on the Rule of Five, as shown in Fig. 1A. Almost all drugs adhered to the Rule. The percentages of drugs within the thresholds for each parameter were: MW: 93%; NHD: 100%; NHA: 97%; log P: 100%. There were only five ophthalmic drugs violating the Rule of Five: cromolyn (NHA=11), cyclosporine (MW=1203; NHA= 12), demecarium (MW=717), difluprednate (MW=509) and travoprost (MW=501). Only one drug (cyclosporine) was marked as ALERT (i.e., violation of two combination parameters, MW and NHA) and the other drugs, except for demecarium (MW=717), exhibited MW and NHA very close to the thresholds (Supplementary Material Table S1).
Fig. 1.
Distributions of physicochemical parameters among FDA-approved non-oral drugs administered via ophthalmic (A), inhalation (B) and transdermal (C) routes. For each route, the following physicochemical parameters are evaluated: molecular weight (1), number of H-bond donors (2), number of H-bond acceptors (3) and octanol-water partition coefficient (log P) (4). The dashed line indicates the threshold for Lipinski's Rule of Five.
The Rule of Five also correlated well with the 39 inhalation drugs, as shown in Fig. 1B. The percentages of drugs in the desirable range of the Rule of Five were MW: 89%; NHD: 97%; NHA: 95% and log P: 97%. There are six inhalation drugs violating the Rule of Five: bitolterol (log P=5.80), ciclesonide (MW=541), ergotamine (MW=582), cromolyn (NHA=11), fluticasone (MW=501) and recombinant human insulin (MW=5808, NHD=78, NHA=87). Only one drug (recombinant human insulin) was associated with ALERT. Among the other five violations, the drugs were close to the thresholds of each parameter (MW<583, NHA=11) (Supplementary Material Table S2).
Figure 1C shows the physicochemical distributions of all 17 transdermal drugs, where only one drug (oxybutynin, log P=5.19) was slightly over the threshold of log P. Thus, the percentages of the drugs in the desirable ranges were: MW – 100%; NHD – 100%; NHA – 100% and log P – 94%. No transdermal drugs were associated with “ALERT” (Supplementary Table S3).
Notably, there were only two drugs (cyclosporine: MW= 1203, NHA=12; recombinant human insulin: MW=5808, NHD=78, NHA=87) marked as ALERT among all 111 non-oral drugs tested in this work, indicating a 98% overall match with the Rule of Five for non-oral routes. Among the other nine drugs that each violated the limits of one parameter, only demacarium exhibited a relatively large MW (MW=717), and the other eight drugs were just outside the thresholds. Although the Rule of Five was originally derived based primarily using data on oral drugs, the excellent correlation of the Rule with non-oral drugs can be attributed at least in part to similarity of the epithelial barriers and biological fluids present in both oral and non-oral routes and the fact that many drugs administered by non-oral routes were originally developed for oral delivery but were later switched to a non-oral route.
While Rule of Five predictions correlate with current non-oral drugs, it may not be optimal. Indeed, bootstrap statistical analysis (13) shows that most of the Lipinski thresholds are not optimal for the non-oral drugs except for the MW threshold of ophthalmic drugs, the MW and NHA thresholds of inhalation drugs and the log P threshold of transdermal drugs (Supplementary Material Table S4). Therefore, we statistically developed new rules for each of the three non-oral routes considered in this study, which displayed stricter thresholds of the physicochemical parameters (see Supplementary Material). The new thresholds for current ophthalmic drugs are NHD≤3, NHA≤8 and log P <4.2; those for current inhalation drugs are NHD≤4 and log P<3.4; and those for current transdermal drugs are MW<335, NHD≤2 and NHA≤5 (Table 1).
Table 1.
Modified Rule of Five for FDA-Approved Non-Oral Drugs Generated by the Bootstrap Method
| Administration route |
MW (Da) |
# of H donors | # of H acceptors | log P |
|---|---|---|---|---|
| Ophthalmic | 500 a | 3 | 8 | 4.2 |
| Inhalation | 500 a | 4 | 10 a | 3.4 |
| Transdermal | 335 | 2 | 5 | 5.0 a |
The threshold values for the physicochemical parameters where the Rule of Five statistically proves to be inapplicable were generated by identifying the median values from the bootstrap confidence interval of each of the thresholds below which 90% of the non-oral drugs would fall (N = 10000) (see Supplementary Material).
Bootstrap analysis demonstrated that the original Rule of Five values provided appropriate threshold values and did not require changing.
RELEVANCE OF THE RULE OF FIVE FOR NON-ORAL ROUTES OF DELIVERY
This analysis shows that the Rule of Five predictions correlate with current drugs delivered by non-oral routes. Our analysis also shows that drugs approved for delivery by non-oral routes adhere to more stringent rules compared to oral drugs. This suggests that candidates for non-oral drugs may be more limited compared to oral delivery. However, this statement is perhaps misleading. First of all, the thresholds for ophthalmic and inhalation drugs were close to those of the Rule of Five, and only the transdermal thresholds were dramatically more limiting. In addition, drugs administered orally not only need to have physicochemical properties in agreement with the Rule of Five, but must also overcome the significant enzymatic barriers in the gastrointestinal tract and first pass of the liver. Such enzymatic issues are much less important when drugs are administered by non-oral routes. Moreover, local treatment of indications in the lung, eye or skin requires much lower doses than systemic delivery by mouth, which further expands the list of drug candidates for non-oral routes. On the other hand, systemic delivery of drugs via non-oral routes may be limited by the maximum dose that can be absorbed across their smaller surface area, especially for ocular and transdermal delivery, compared to the large surface area available via the oral route.
Another significant consideration when evaluating this study is the selection bias inherent to our analysis. Lipinski found that most drugs at entry to Phase II adhere to the Rule of Five and concluded that drugs violating the Rule of Five are unlikely to be good candidates (2). This conclusion is valid because a large number of drug candidates have been evaluated over the years—including compounds with a broad range of physicochemical parameters that exceed the ranges of the Rule of Five—but very few that violate the Rule have become successful drugs. This is especially true with oral drugs because the majority of drugs analyzed in Lipinski's work were orally active compounds.
Our analysis with non-oral drugs is different because the drug candidates considered for these routes of delivery over the years have been much more limited. Indeed, many of the drug candidates evaluated for non-oral delivery were originally developed as oral drugs and later adapted for non-oral delivery for improved patient compliance or new indications. Thus, there is an inherent selection bias, such that many fewer drugs violating the Rule of Five have been evaluated for non-oral delivery, and, therefore, it should not be surprising that current non-oral drugs also adhere to the Rule.
Ophthalmic Drugs
Similar to orally active drugs, the ophthalmic drugs tested in this study require crossing an epithelial barrier to reach their intraocular targets. However, the permeability barrier of the cornea is significantly greater than the intestine, given the seven layers of corneal epithelial cells compared to the monolayer of intestinal epithelium (3,14). Moreover, residence time of drugs applied as topical eye drops is just minutes, due to tear fluid drainage and elimination by subconjunctival clearance, compared to a residence time of hours in the gastrointestinal tract after oral delivery. In addition, corneal surface area is orders of magnitude smaller than that of the small intestine. Altogether, these differences would suggest that corneal absorption is much less efficient than intestinal absorption, and indeed it is, as shown by ocular bioavailabilities typically well below 5% (3). However, drugs given to the eye are for local treatment and therefore require orders of magnitude lower doses than for systemic delivery by mouth. That is, the reduction in absorption in the eye (due to lower corneal permeability and lower residence time) turns out to often be about equal to the reduction in dose needed in the eye, and, as such, the same drugs that are effective for systemic therapy in the intestine are also found to be effective for local therapy in the eye. In this way, drugs suitable to cross the intestinal epithelium, as indicated by the Rule of Five, are also generally suitable to cross the corneal epithelium, albeit less efficiently.
Inhaled Drugs
In the lung, drugs crossing the pulmonary epithelium involve just one monolayer of cells like the oral route, but without the large presence of enzymes. Assuming that a drug reaches the deep lung, alveolar transport becomes dominant, where permeability is extremely high, surface area is large, enzymatic degradation is low, and residence times can be relatively long (4). Therefore, drug delivery via the lung is limited to a large extent by transport to the alveolar surface, which depends mostly on the delivery system and minimally on drug physicochemical properties. This suggests that drug candidates for pulmonary delivery should be at least as plentiful compared to oral delivery, yet almost all current inhalation drugs adhere to the Rule of Five. We believe that this outcome is a result of selection bias, in which relatively few drugs not adhering to the Rule of Five have been evaluated for pulmonary delivery. For this reason, there is overlap between the properties of current oral and inhaled drugs. In recent years, however, a number of macromolecular drugs that flagrantly violate the Rule of Five have been successfully administered via the lung, such as insulin, due to the high permeability of alveolar epithelium. We therefore conclude that although current inhalation drugs mostly adhere to the Rule of Five, this Rule is overly restrictive and should not be used as the basis for removing pulmonary drug candidates from consideration.
Transdermal Drugs
Despite the relatively large surface area, relatively small enzymatic degradation, and extremely long application times permitted for transdermal delivery, the permeability barrier of skin's outer layer of stratum corneum is enormous (5). Although skin and intestinal barrier properties are both based on lipid bilayers, the monolayer of intestinal epithelial cells is orders of magnitude more permeable compared to a cross-section through the stratum corneum, which contains on the order of 100 multilamellar lipid bilayers filling its extracellular spaces. For this reason, successful transdermal drugs have been limited by parameter thresholds even more restrictive than the Rule of Five. Moreover, drugs delivered passively across the skin also have a more stringent lower limit on log P (i.e., log P≥0), probably further reflecting the highly selective stratum corneum barrier. Although Lipinski's Rule of Five does not have a lower limit on log P, oral and other routes of delivery are also limited by drugs that are too polar (2). Although our analysis was limited to the 17 FDA-approved transdermal drugs, a reading of the skin permeability literature and knowledge of the extensively studied properties of stratum corneum suggest that the conclusions derived from these 17 drugs are consistent with the broader literature (5). Therefore, the more restrictive variation on the Rule of Five proposed in this analysis seems appropriate to predict drug candidates for passive transdermal delivery. These rules, however, do not apply to newer, active methods of transdermal delivery discussed below.
OVERCOMING LIMITATIONS OF NON-ORAL TISSUE BARRIERS
Our analysis so far has focused on delivery across normal, unaltered tissue. Other than perhaps pulmonary epithelium, these natural tissue barriers significantly limit suitable drug candidates. Historically, drugs violating the Rule of Five have largely been administered via hypodermic injection, which utilizes a needle to make tissue barrier properties irrelevant. However, advances in drug delivery offer other alternatives that permit delivery of many more drugs across epithelial barriers without the use of a needle.
Advances in pulmonary delivery systems are limited because inhaled dosage forms typically must dissolve or biodegrade in the lung, since they cannot easily be removed after use. Thus, advances in inhaled delivery systems have focused mostly on increasing the efficiency of drugs reaching the epithelial surface of alveolar sacs and, to a lesser extent, increasing duration of delivery through controlled release (4). Because the alveolar epithelium is so permeable and the Rule of Five does not appear to be rate limiting, getting drug efficiently to the deep lung may be the most important advance for pulmonary delivery. Patient safety and acceptance of inhalation therapy are also important.
Transdermal drug delivery, in contrast, has a highly restrictive permeability barrier, but fortunately offers a number of opportunities to overcome this limitation (5). This is because skin is an easily accessible external organ, and the stratum corneum barrier is a non-living tissue that can withstand minor disruptions in structure. Transdermal patches can be worn for as long as one week, which increases bioavailability and reduces dosing frequency. Iontophoretic and other driving forces have also been applied to increase transdermal delivery especially of charged compounds that typically violate the modified Rule of Five (15). Most significantly, stratum corneum permeability can be increased by orders of magnitude using nanometer-scale disruptions created, for example, by chemical enhancers and ultrasound, and using micron-scale disruptions created, for example, by microneedles and thermal poration, which enable delivery of large hydro-philic molecules (5). These advanced delivery technologies make Rule of Five permeability limitations obsolete.
Finally, although the eye is externally accessible, its anatomy, function and sensitivity limit the aggressiveness of drug delivery systems (3,14). Most advances have concerned increasing the duration of delivery using controlled release methods. Limited work has applied an iontophoretic driving force to increase drug transport (15), but other, more powerful methods used on the skin may not be suitable for the eye.
CONCLUSION
The cost, duration and complexity of drug development make early triage of drug candidates a priority. Lipinski's Rule of Five has provided a simple method to identify suitable compounds mostly for oral administration based on ADME considerations using easy-to-calculate physicochemical properties. In this work, we tested whether the Rule of Five can also predict drugs for delivery via non-oral routes and found that this Rule correlated with FDA-approved drugs for ophthalmic, inhalation and transdermal delivery with up to 98% accuracy.
More specifically, we conclude that drug candidates violating the Rule of Five are unlikely to be suitable for ophthalmic delivery. Although currently approved drugs for pulmonary delivery mostly adhere to the Rule of Five, a broader reading of the literature suggests that drug candidates in violation of the Rule of Five, including macromolecules, can be administered by inhalation if suitable delivery systems are used to efficiently deposit drugs in the deep lung. Passive transdermal delivery drug candidates are limited by a rule even more restrictive than the Rule of Five, given the extreme barrier properties of skin's stratum corneum. However, a number of different advanced delivery technologies can increase skin permeability to enable delivery of compounds well beyond the limitations of the Rule of Five, including macromolecules.
Supplementary Material
ACKNOWLEDGMENTS
We thank Prof. Harvinder Gill for helpful discussions and research assistance and Prof. Branislav Vidakovic for helping us conduct statistical analysis. This work was carried out at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at Georgia Tech and was supported in part by the National Institutes of Health.
Footnotes
Electronic Supplementary Material The online version of this article (doi:10.1007/s11095-010-0292-6) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Bleicher KH, Bohm HJ, Muller K, Alanine AI. Hit and lead generation: beyond high-throughput screening. Nat Rev Drug Discov. 2003;2:369–78. doi: 10.1038/nrd1086. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 3.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–87. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 4.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 5.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Ophthalmic Pharmaceutical Drugs Market. New York, NY: Apr, 2008. ReporterLinker.com. [Google Scholar]
- 7.Drug Delivery Markets . Vol.3: Pulmonary Delivery Systems, Kalorama Information. New York, NY: Mar, 2007. [Google Scholar]
- 8.Transdermal Drug Delivery - Technologies, Markets, and Companies, Jain PharmaBiotech. Basel, Switzerland: May, 2005. [Google Scholar]
- 9.Vieth M, Siegel MG, Higgs RE, Watson IA, Robertson DH, Savin KA, Durst GL, Hipskind PA. Characteristic physical properties and structural fragments of marketed oral drugs. J Med Chem. 2004;47:224–232. doi: 10.1021/jm030267j. [DOI] [PubMed] [Google Scholar]
- 10.Wenlock MC, Austin RP, Barton P, Davis AM, Lesson PD. A comparison of physiochemical property profiles of development and marketed oral drugs. J Med Chem. 2003;46:1250–1256. doi: 10.1021/jm021053p. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie TJ, Luscombe CN, Macdonald SJ. Analysis of the Calculated Physicochemical Properties of Respiratory Drugs: Can We Design for Inhaled Drugs Yet? J Chem Inf Model. 2009;49:1025–1032. doi: 10.1021/ci800429e. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration (FDA) Approved Drug Products with Therapeutic Equivalence Evaluations (FDA Orange Book) FDA web site (online), accessed on March 10, 2010 < http://www.fda.gov/cder/ob/>. [Google Scholar]
- 13.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall/CRC; Boca Raton: 1994. [Google Scholar]
- 14.Fatt I. Physiology of the Eye: An Introduction to the Vegetative Functions. Butterworth-Heinemann; Boston: 1992. [Google Scholar]
- 15.Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006;110:479–89. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.