Abstract
We previously described a role for adrenergic signaling in the hippocampus to promote contextual and spatial memory retrieval. A subsequent study performing expression analysis of the immediate-early gene (IEG) Arc suggested that activation of CA1 but not CA3 pyramidal neurons during memory retrieval is impaired in the absence of NE. The current study sought to confirm and extend those observations by performing expression analysis of a second IEG product, Fos, following a much greater variety of testing conditions. In mutant mice lacking NE, induction of Fos was normal in all regions of the hippocampus and amygdala shortly after fear conditioning. In contrast, when testing contextual fear one day after training, induction of Fos in CA1 and the central nucleus of the amygdala (CeA), but not CA3, the dentate gyrus or other amygdaloid nuclei, was impaired in the mutant mice. This pattern corresponded to the memory retrieval deficit exhibited by these mice. On the other hand, induction was normal in CA1 and CeA when testing cued fear one day after training, or contextual fear one week or one month after training, conditions in which retrieval is normal in the absence of NE. Acute restoration of NE in the mutant mice before testing but not before training rescued retrieval of contextual fear and restored Fos induction in CA1 and CeA. Because NE facilitates retrieval through the activation of β1-adrenergic receptors, β1 knockout mice were also examined and found to exhibit reduced induction of Fos in CA1 and CeA following retrieval. Based on these and previous results, we hypothesize that adrenergic signaling is critical for the full activation of CA1 pyramidal neurons in response to excitatory input from CA3 pyramidal neurons conveying retrieved contextual information.
Keywords: Fos, CA1, hippocampus, norepinephrine, beta-adrenergic, memory retrieval
The hippocampus is involved in the acquisition, consolidation and retrieval of explicit memories, which can be readily examined in animals using paradigms that depend on contextual (Anagnostaras et al., 2001) or spatial learning (Morris et al., 2003). One goal for understanding memory is to define the neurons activated during storage and retrieval. Induction of immediate early gene (IEG) expression has been used because it offers high sensitivity and cellular resolution that can be analyzed throughout the brain (Clayton, 2000; Kubik et al., 2007). During memory retrieval, the IEGs c-fos and zif268 are induced in the hippocampus following reexposure to the training context after fear conditioning (Milanovic et al., 1998; Hall et al., 2001a, b; Frankland et al., 2004). Changes in IEG expression in the hippocampus are not due to arousal, fear or freezing, for example, because these changes are not observed when testing is performed while freezing is intact and retrieval is independent of the hippocampus (Hall et al., 2001a, b; Frankland et al., 2004). Thus, it is hypothesized that IEG induction in the hippocampus during testing reflects the genomic activation of neurons relevant to the retrieval of contextual memory.
We previously demonstrated a critical role for norepinephrine (NE) in contextual and spatial memory retrieval that is not due to a role in fear, freezing or the performance of spatial navigation (Murchison et al., 2004). Those findings were initiated by the study of mice genetically altered to lack the endogenous ligands for the adrenergic receptors, NE and epinephrine (E), via targeted disruption of the dopamine β-hydroxylase gene (Dbh) (Thomas et al., 1995; Thomas et al., 1998). Intracerebral infusions indicated that β1-adrenergic receptor signaling in the dorsal hippocampus (DH) is necessary and sufficient for contextual memory retrieval mediated by NE.
One goal has been to determine whether there are neurons in the DH whose activation depends on NE during the retrieval of contextual memory. Initial experiments designed to address this employed a within-subjects design analyzing expression of the IEG Arc in control and Dbh−/− mice that was induced following reexposure to the fear conditioning apparatus or a distinct context not associated with shock (Zhang et al., 2005). The results suggested that activation of CA1 but not CA3 pyramidal neurons in the DH is impaired in the absence of NE. The results also indicated that activation in the DH is considerably greater than in the ventral hippocampus. That study was limited by the lack of rapid, biphasic induction of Arc in dentate granule cells, and by the single behavioral condition that was studied (contextual memory retrieval one day after training) due to the intensive analysis required for the within-subjects approach.
Therefore, the present study set out to test whether a between-subjects design analyzing induced expression of a second IEG, Fos, would permit the examination of dentate granule cells and confirm the original results in DH CA1 and CA3 based on the analysis of Arc expression. Fos immunoreactivity was used as a marker for “genomic” activation of neurons in the CNS for several reasons. First, induction of Fos is the most widely characterized IEG response (Morgan et al., 1987; Sagar et al., 1988). Second, induction of Fos has been assessed under conditions that elicit contextual memory retrieval following brief fear conditioning (Milanovic et al., 1998; Hall et al., 2001a, b; Frankland et al., 2004), which is the focus of the current study. Third, basal numbers of Fos-immunoreactive neurons throughout the CNS is unaffected by the absence of NE/E in Dbh−/− mice (S.-H. J. and S.A.T., unpublished), consistent with a previous report examining c-fos mRNA (Szot et al., 1999), permitting comparison between genotypes under various experimental conditions. Fourth, differences have been reported between the induction of Arc and Fos in the hippocampus. For example, induction of Arc in the DH is maintained with repeated exposure to a context across days (Guzowski et al., 1999), similar to the firing of DH pyramidal neuron place cells (Thompson and Best, 1990), while induction of Fos declines with repeated exposure (Papa et al., 1993; Radulovic et al., 1998), similar to the reduction in behavioral exploration. These differences in induction could be due to differences in transcriptional regulation of the IEGs or expression product analyzed (message versus protein), for example.
Once the results employing Arc in the DH were verified using Fos in the current study, analysis of Fos induction was extended to a variety of behavioral testing conditions to determine whether impaired induction of Fos in the absence of NE/E correlates with the impairment of memory retrieval. These studies focused on the DH because it is here that adrenergic signaling facilitates retrieval (Murchison et al., 2004). In addition to the DH, this study also focused on the amygdala because this region is critical to the expression of fear memory (Pape and Pare, 2010), and because induction of Arc was altered in the amygdala following contextual fear memory retrieval in the absence of NE/E (Zhang et al., 2005). While induction of Arc was also altered in other brain regions following contextual fear memory retrieval in the absence of NE/E, these were not examined the current study because the primary goal was to examine induction in the DH under a multitude of testing conditions.
EXPERIMENTAL PROCEDURES
Subjects
Wild-type, Dbh+/−, Dbh−/− and β1 knockout (KO) mice were on a hybrid 129/Sv × C57BL/6 background, and were derived by mating either heterozygotes or homozygotes (Thomas et al., 1995; Rohrer et al., 1996). Dbh−/− mice were rescued prenatally as described (Ouyang et al., 2004). Gender and parental genotype did not affect the results, so data were combined. Genotype was determined by PCR. Mice were maintained on ad lib food and water and a 12 h light:dark cycle, with lights on beginning at 07:00, and were 3–6 months old when tested. Studies were performed during the light phase, with experiments taking place between 09:00 – 18:00. Studies were in accordance with NIH guidelines and had the approval of the IACUC at the University of Pennsylvania.
Pavlovian fear conditioning
Fear conditioning was performed in context S (for salient or shock; ENV-010MC, Med Associates, St. Albans, VT) that was cleaned with VersaClean (Fisher). Mice were given two 3-min handling sessions over 2 days in the training room. Saline was injected at the end of handling each day. The day after handling, mice were either sacrificed (untrained group = U) or trained by first being placed in context N (for neutral or non-shock) for 3 min in the morning. Context N was a Plexiglas cylinder (21 cm diameter, 24 cm tall) with green wire grid floor and vertical green and white wall stripes 240° around, and was cleaned with lemon-scented Ajax. Three hours later they were placed in context S for 2 min, after which a tone (84 dB, 4.5 kHz) was activated for 30 s. A 2 s, 1 mA footshock co-terminated with the tone, and the mouse was returned to its home cage 30 s later. Mice were tested for contextual fear 1–31 days after training by being placed in context N (group N) or S (group S) for 5 min without tone or shock. Cued fear was tested 2 min after mice were placed in context N by activating the training tone for 3 min (group N+T). For one group (HN+T), mice were habituated to context N by exposure to this context for 15 min per day for 3 days prior to training in context S and testing cued fear in context N the next day. Percent freezing was estimated by scoring the presence or absence of non-respiratory movement every 5 s.
Drug administration
The NE precursor L-threo-3,4-dihydroxyphenylserine (L-DOPS, 1 g/kg, Dainippon Sumitomo Pharma, Osaka, Japan) and the peripheral aromatic L-amino acid decarboxylase inhibitor benserazide (50 mg/kg, Sigma, St. Louis, MO) were administered together subcutaneously 5 h before training or testing as described (Murchison et al., 2004).
Fos immunohistochemistry
Mice were anesthetized 2 h after conditioning or 1 h after memory testing and perfused with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. These time points were chosen because pilot studies indicated that peak induction of Fos occurred around these times, and because contextual memory retrieval requires NE at these times (however, no retrieval testing was performed 2 h after conditioning). Brains were fixed overnight (4°C), placed in 30% sucrose (4°C) for 2–3 days and stored (−80°C). Coronal sections (30 µm) were cut by cryostat (HM505E, Microm, Waldorf, Germany). Sections were incubated (all at 22°C in PBS) sequentially in 1.0% H2O2 (25 min), in 8% normal goat serum with 0.3% Triton X-100 (50 min) and in Fos polyclonal antibody (1:15,000; Santa Cruz Biotechnology, Santa Cruz, CA) with 2% normal goat serum and Triton (16 h). Sections were then incubated in biotinylated anti-rabbit IgG goat antibody (1:200, 1 h, Santa Cruz) and in horseradish peroxidase – streptavidin (1:500, 1 h, Vector) and developed using diaminobenzidine with nickel (Vector). PBS washes were performed following each step. Sections were dehydrated, cleared and coverslipped with Permount (Fisher, Pittsburgh, PA). Immunoreactive nuclei were counted in non-adjacent sections separated by 30 µm. Images were captured using a color CCD camera (CoolSNAP-ProCf, Media Cybernetics, Silver Spring, MD) at 100× using an Eclipse E600W microscope (Nikon, Tokyo, Japan). ImagePro Plus software (Media Cybernetics) was used for counting. Fos-immunoreactive nuclei were normalized to area (0.1 mm2).
Statistics
Four to six mice of each genotype were used for each condition. Data were analyzed with Statistica 9.0 (StatSoft, Tulsa, OK) using factorial two-way ANOVA of genotype and condition. Condition was “context” for behavioral data and either “brain region” or “treatment group” for Fos labeling. One-way ANOVA using treatment group was performed for Fos labeling and behavior for the L-DOPS study. Post-hoc comparisons were made using Duncan’s range test. Data are presented as mean ± standard error, and levels of significance are: *, P < 0.05; ^, P < 0.01; #, P < 0.001.
RESULTS
Induction of Fos following fear conditioning
To examine neuronal activation in the presence and absence of NE/E, Dbh+/− and Dbh−/− mice were used, respectively. Dbh+/− mice were used as controls for Dbh−/− mice because the former have normal tissue levels of NE/E and are phenotypically indistinguishable from Dbh+/+ mice (Thomas et al., 1998). Prior to examining induction of Fos following memory retrieval, induction following fear conditioning was assessed. In this way it could be determined whether any differences between genotypes observed for induction following memory retrieval might be due to alterations in induction during training. Fos labeling was quantified in the DH and amygdala 2 h after conditioning. The three major subfields of the DH (DG = dentate gyrus, CA3 and CA1) and four nuclei of the amygdala (lateral – LA, basolateral – BLA, basomedial – BMA and central – CeA) were analyzed (Fig. 1). Fos labeling was 5- to 10-fold higher in all regions 2 h after training when compared to labeling in untrained mice, and there was no difference in Fos labeling between genotypes (Fig. 2).
Fig. 1.

Coronal mouse brain diagram indicating regions where fos induction was quantified. For hippocampus: dDG, dCA3 and dCA1 are dorsal dentate gyrus, CA3 and CA1; and for amygdala: LA, BLA, BMA and CeA are lateral, basolateral, basomedial and central nuclei. The number in parentheses is the distance posterior to Bregma in mm.
Fig. 2.
Fos immunolabeling in DH and amygdala shortly after fear conditioning. Mice were sacrificed either unconditioned (U) or 2 h after conditioning (2). For all 7 brain regions (see Fig. 1), Fos labeling is significantly greater 2 h after training relative to no training (P < 0.001 for each main effect of conditioning). To examine the potential contribution of adrenergic signaling, mice with (Dbh+/−) and without (Dbh−/−) NE/E were studied. For all 7 brain regions, no significant differences between genotype were present (P > 0.4 for each main effect of genotype). For all figures, Fos-immunoreactive (Fos-IR) nuclei are normalized to 0.1 mm2 and n = 4–6 per group per genotype.
Induction of Fos following contextual memory retrieval
To examine induction of Fos following contextual memory retrieval, reexposure to the training context (S = salient or shock) was performed one day after fear conditioning. To gauge induction in a salient versus neutral context, other mice were reexposed to the non-shock context (N) that they were also exposed to on the day of training. Mice were sacrificed 1 h after context exposure to examine Fos expression. There was a significant increase in Fos labeling in context S versus N in each subfield of the DH and nucleus in the amygdala (Fig. 3). There were no significant differences in Fos induction between genotypes in the DG or CA3. In contrast, there was a significantly greater induction of Fos in CA1 in Dbh+/− versus Dbh−/− mice (Figs. 3 and 4). In the amygdala, the only significant difference in Fos induction between genotypes was in CeA, where induction was impaired in Dbh−/− mice. The reductions in Fos labeling in CA1 and CeA in the Dbh−/− mice corresponded with the reduction in freezing by these mice due to impaired contextual memory retrieval (Fig. 3A). Because the impairment in induction was specific to CA1 and CeA, subsequent analyses focused on these regions.
Fig. 3.
Fos immunolabeling in DH and amygdala following context reexposure one day after conditioning. (A) During reexposure to the conditioning apparatus, NE/E-deficient Dbh−/− mice froze significantly less than control Dbh+/− mice. (B) and (C) Mice were exposed to a neutral (non-shock) context (N) and the salient (shock) context (S) on training day. The next day, the mice were reexposed to one or the other context during testing and sacrificed 1 h later. Results were compared to those from unconditioned mice (U). For all 7 regions, Fos labeling is significantly greater in context S versus N, as indicated by the symbols with lines above the bars (P < 0.01 to 0.001 for each main effect of conditioning, as indicated). Significant differences in Fos labeling by genotype were present only in context S in CA1 and CeA. For CA1 and for CeA, P < 0.05 for the main effect of genotype and the interaction of condition and genotype. For all figures, significance is indicated as: *, P < 0.05; ^, P < 0.01; #, P < 0.001.
Fig. 4.
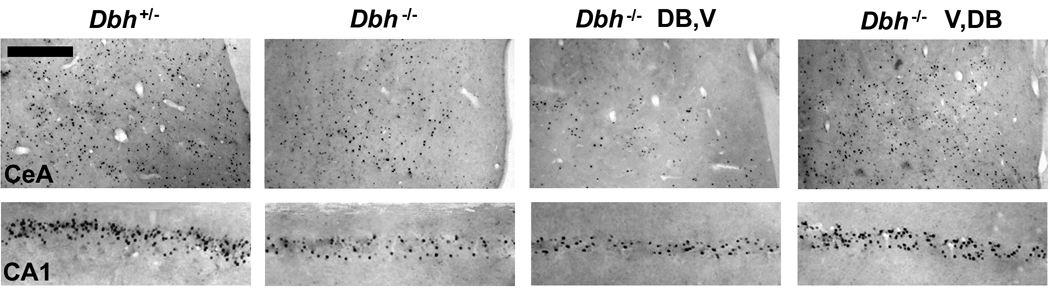
Representative Fos-immunolabeled sections from Dbh+/− and Dbh−/− mice following retrieval testing. The two panels on the left are from Dbh+/− and Dbh−/− mice that did not receive injections. Analogous results were obtained with Dbh+/− and Dbh−/− mice that received vehicle injections (V,V) 5 h prior to training and testing (Fig. 5). The two panels on the right are from Dbh−/− mice that received injections of L-DOPS with benserazide 5 h before training and vehicle 5 h before testing (DB,V), or vehicle 5 h before training and L-DOPS with benserazide 5 h before testing (V,DB). Scale bar is 50 µm.
One of several observations that implicates NE in contextual memory retrieval, rather than in acquisition or consolidation, is the ability to instate normal contextual fear in Dbh−/− mice when NE is restored prior to testing but not prior to training (Murchison et al., 2004). To determine whether this would also be the case for Fos induction, mice were treated with the NE precursor L-DOPS. Analogous to the rescue in freezing, NE restoration resulted in normal induction of Fos in CA1 and CeA when restoration was performed before testing but not before training (Figs. 4 and 5).
Fig. 5.
Instatement of normal freezing and Fos labeling in Dbh−/− mice in response to the training context following restoration of NE before testing. Mice were injected 5 h before training and again 5 h before testing 2 days later with either vehicle (V) or L-DOPS plus benserazide (DB). Benserazide is a peripheral decarboxylase inhibitor that acts to restore NE selectively to the CNS in Dbh−/− mice. Only Dbh−/− mice treated with L-DOPS plus benserazide before testing (V,DB) exhibited freezing (A) and Fos labeling (B and C) that was comparable to that for Dbh+/− mice, consistent with the requirement for NE during contextual memory retrieval. For behavior, CA1 and CeA, P < 0.01 for the main effect of group.
The role for NE in contextual memory retrieval is time-limited, apparent by 2 h after training and lasting for ~4 days. That observation offers an opportunity to examine Fos induction in Dbh−/− mice that lack NE/E but have intact contextual memory retrieval. For this purpose, mice were tested for contextual fear 4, 7 or 31 days after training and results were compared to those for testing one day after training. As expected, freezing was significantly lower in the Dbh−/− mice 1 and 4 days after training but not 7 or 31 days after training (Fig. 6). Similarly, Fos levels were significantly lower in CA1 and CeA of the Dbh−/− mice 1 and 4 days after training but not 7 or 31 days after training. Of note, induction of Fos in CA1 at 31 days is not significantly different from that observed in a neutral context, a result that dissociates CA1 Fos induction from the expression of fear and freezing, as others have observed (Frankland et al., 2004).
Fig. 6.
Freezing and Fos labeling in Dbh−/− mice is normal following reexposure to the training context 7 or 31 days after fear conditioning. Mice were reexposed to the training context 1, 4, 7 or 31 days after training. Induction of Fos was significantly reduced in Dbh−/− mice at 1 and 4 days after training. In controls, there was a significant reduction in Fos labeling following reexposure to the training context at 31 days relative to earlier times in CA1 (P < 0.001), and at 7 and 31 days relative to earlier times in CeA (P < 0.05 for 7 days and P < 0.01 for 31 days). For behavior, P < 0.001 for the main effects of genotype and time, as well as the interaction of genotype and time. For CA1, P < 0.001 for the main effect of time, and P < 0.05 for the main effect of genotype and the interaction of genotype and time. For CeA, P < 0.001 for the main effect of time, P < 0.01 for the main effect of genotype, and P < 0.05 for the interaction of genotype and time.
Induction of Fos following cued memory retrieval
Unlike contextual fear memory, the retrieval of cued fear memory is intact one day after training in Dbh−/− mice (Murchison et al., 2004). To determine whether there is a general refractoriness to Fos induction in CA1 and CeA in the absence of NE/E one day after training, Fos was examined following reexposure to the training tone in context N. In contrast to the results for contextual fear testing, induction of Fos was normal in CA1 and CeA from Dbh−/− mice tested for cued fear following the normal conditioning protocol (Fig. 7). Induction was also normal in CeA from Dbh−/− mice tested for cued fear following a modified protocol where habituation to context N was performed prior to conditioning. Induction of Fos in CA1 did not occur under this condition, again dissociating Fos induction from fear and freezing behavior, as others have shown (Hall et al., 2001a, b).
Fig. 7.
Responses following cued fear testing one day after training. Mice were subjected to one of three conditions: reexposure to context N in the absence of the training tone (N); reexposure to context N and the training tone (N+T); reexposure to context N and the training tone following habituation to context N prior to conditioning (HN+T). No significant difference by genotype was present (P > 0.7 for each main effect of genotype). Note the dissociation between freezing and induction of Fos in CA1 but not CeA in the habituated (HN+T) mice. For behavior, CA1 and CeA, P < 0.001 for the main effect of condition.
Induction of Fos and β1-adrenergic signaling
Finally, NE promotes contextual memory retrieval through stimulation of β1-adrenergic receptors in the DH (Murchison et al., 2004). Thus, it was of interest to determine how specific loss of β1 signaling rather than loss of all adrenergic signaling (in Dbh−/− mice) would affect induction of Fos during contextual memory retrieval. Toward this goal, Fos induction was examined in wild-type and β1-adrenergic receptor knockout (β1 KO) mice tested for contextual fear one day after training. Similar to the results from Dbh−/− mice, significant reductions in freezing and Fos induction in CA1 and CeA were apparent (Fig. 8).
Fig. 8.
Freezing and Fos induction are significantly reduced in β1 KO mice. Mice were reexposed to the training context one day after conditioning.
DISCUSSION
Adrenergic signaling and IEG expression
Critical to our analyses, we found that the number of Fos-positive cells under basal conditions and 2 h after fear conditioning was unaffected by the absence of NE/E (we cannot exclude the possibility that Fos induction may differ between genotypes 1 h after conditioning). Similar observations have been made when examining basal mRNA expression for the IEGs c-fos and Arc (Szot et al., 1999; Zhang et al., 2005). Normal basal expression of Fos and Arc in the Dbh−/− mice is perhaps surprising. Lesions of the locus coeruleus, which supplies much of the adrenergic innervation to the forebrain, cause reductions in the expression of these IEGs (Cirelli et al., 1996; Cirelli and Tononi, 2000). However, those experiments measured IEG levels following prolonged wakefulness, whereas our experiments examined basal expression and short-term induction of Fos in response to context exposure or fear conditioning. Another difference is that lesions incapacitate adrenergic terminals, which are intact in Dbh−/− mice (Jin et al., 2004).
Contextual memory retrieval, adrenergic signaling and the hippocampus
Stimulation of β1-adrenergic signaling in the DH is necessary and sufficient for the actions of NE in promoting contextual memory retrieval (Murchison et al., 2004). However, it is unknown how β1 signaling acts in the DH to promote contextual memory retrieval. To address this, we asked whether genomic activation of hippocampal neurons would be altered following retrieval in the absence of NE/E. Our results indicate that activation of DG and CA3 is normal. In contrast, activation of CA1 is reduced in the absence of NE/E. A similar observation was made when examining induction of Arc in the DH following contextual fear testing – induction was normal in CA3 but impaired in CA1 in the absence of NE/E (Zhang et al., 2005). Induction of Arc in the DG that was specific to the training context could not be examined in that study due to the unique properties in Arc expression in those neurons. This was an important advantage to analyzing Fos in the current study.
Impaired induction of Fos in CA1 in the Dbh−/− mice correlated with reduced freezing under all conditions tested. Induction of Fos in CA1 during testing is thought reflect contextual memory retrieval for two reasons. First, fear and freezing elicited by the training tone one day after conditioning do not induce Fos in CA1 when mice have been habituated to the testing context prior to training (Fig. 7B), as was shown by others (Hall et al., 2001a, b). Second, induction of Fos in CA1 by reexposure to the training context is time-limited (Fig. 6B), similar to the time-limited role of the DH in contextual memory retrieval (Frankland et al., 2004).
One might have predicted that an absence of NE/E would lead to a general reduction in fear, given the hypothesized role of adrenergic signaling in fear and anxiety (Morilak et al., 2005). However, no such effect of NE/E deficiency was found when testing the Dbh−/− mice for cued fear one day after training or contextual fear one or more weeks after training. The same pattern of results was found for induction of Fos in CA1 in the absence of NE/E, i.e. no diminution in Fos induction for cued fear one day after training or contextual fear one or more weeks after training. Thus, the blunted induction of Fos in CA1 in the absence of NE/E is not due to a general impairment in fear that is independent of the role for adrenergic signaling in memory retrieval. The induction of Fos in CA1 following cued fear testing may be due to the animal being exposed to a fearful conditioned stimulus (the training tone) in a context not associated with shock, which could initiate second order fear conditioning to context N (Talk et al., 2002). That this induction of Fos in CA1 does not depend on NE/E is consistent with our observation that induction following initial fear conditioning does not depend on NE/E.
Our results are consistent with a model whereby DG/CA3 are important for contextual memory storage and retrieval, and CA1 is important for comparing memory of context coming from DG/CA3 to information about the current context coming from the perforant path (Treves and Rolls, 1994; Lisman, 1999). Adopting such a model, we hypothesize that perforant path activity is normal in the absence of NE/E, allowing normal contextual memory storage in DG/CA3 and normal online contextual information processing in CA1. This is consistent with the normal activation of all subfields 2 h after conditioning, and of DG/CA3 following reexposure to the training context in the Dbh−/− mice. Importantly, activation of CA1 was normal in Dbh−/− mice following reexposure to the training context one week after conditioning. In general, deficits in activation of CA1 neurons in the absence of NE/E only occurred when NE was required for retrieval.
The model and results suggest that activation of DG/CA3 during contextual memory retrieval may fail to be properly received by CA1, or that CA1 fails to properly transmit DG/CA3 input as CA1 output. This could be for several reasons. β1-adrenergic receptors are expressed by CA1 pyramidal neurons and dentate granule cells (Booze et al., 1993; Nicholas et al., 1993). It is possible that β1 receptors expressed in CA1 play a key role in gating the strength of the Schäffer collateral input from CA3 or in determining the responsiveness of CA1 pyramidal neurons to excitatory input from CA3. Alternatively or in addition, β1-adrenergic receptors expressed by DG cells could indirectly gate the firing of CA3 pyramidal neurons via the mossy fiber pathway. The latter possibility seems less likely given the normal induction of Arc and Fos in CA3 in the Dbh−/− mice during retrieval. However, because the relationship between induction of IEGs and action potential generation is complex (Clayton, 2000; Fields et al., 2001), it is possible that the synaptic activation that leads to normal IEG induction in CA3 does not lead to normal action potential generation in CA3 in the absence of NE.
Conditioned responding and the amygdala
We predicted that when contextual memory retrieval was impaired, induction of Fos in the amygdala would also be altered. This is because impaired retrieval leads to reduced fear expression. The amygdala is essential for fear conditioning (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Goosens and Maren, 2001), and there are prominent reciprocal connections between the ventral hippocampus and amygdala (Pitkanen et al., 2000). It is not clear how information about context might be relayed from the DH to the amygdala, however. This could occur via longitudinal connections within the hippocampus from dorsal to ventral (Witter, 1993), or via cortical association areas that receive indirect input from the DH and project to the amygdala (Pitkanen et al., 2000).
Learned associations of conditioned and unconditioned stimuli reside within the amygdala, and their activation results in the modulation of behavior (Fanselow and LeDoux, 1999). The lateral and basal nuclei are important for associative fear acquisition and expression, while the central nucleus is thought to be a critical relay for mediating behavioral manifestations of fear (Muller et al., 1997; Goosens and Maren, 2001). Because cued fear memory does not depend on NE/E, it is our hypothesis that elements (cues) within a fearful context result in the activation of the locus coeruleus, inducing release of NE in the DH to facilitate retrieval of contextual memory that further supports learned behavior (in this case fear). During contextual fear testing, induction of Fos was most prominent in CeA, an important output nucleus of the amygdala. Further, induction of Fos in the amygdala was impaired only in CeA in the absence of NE/E. These results are consistent with the hypothesis that context-specific activation of the CeA depends on hippocampal output. This could be tested directly in future studies by examining IEG expression following loss of β1 signaling selectively in the hippocampus using a Cre-loxP deletion strategy, for example.
Finally, the decrease in Fos induction in CeA in the absence of NE/E is consistent with results obtained by analyzing induction of Arc (Zhang et al., 2005). Differences between outcomes in the two studies occurred for other nuclei in the amygdala, however. We observed diminished induction of Arc in the absence of NE/E in the LA, BLA and BMA, which was not the case when analyzing Fos. The reason for these differences is not clear. It is possible that these two IEGs have different roles in the reconsolidation of fear memory that likely follows from retrieval testing. For example, Arc but not Fos could play a role in fear memory reconsolidation in the LA, BLA and BMA. If contextual fear memory retrieval is reduced and reconsolidation processes are not invoked, then Arc expression might be selectively diminished in these nuclei in the absence of NE/E.
Acknowledgments
We thank H. Luo for technical assistance and Dainippon Sumitomo Pharma (Osaka, Japan) for their generous gift of L-DOPS. This work was supported by NIH grant 5R01MH063352 to S.A.T.
Abbreviations
- BLA
basolateral nucleus of the amygdala
- BMA
basomedial nucleus of the amygdala
- CeA
central nucleus of the amygdala
- DG
dentate gyrus
- E
epinephrine
- IEG
immediate-early gene
- KO
knockout
- L-DOPS
L-threo-3,4-dihydroxyphenylserine
- LA
lateral nucleus of the amygdala
- NE
norepinephrine
- PBS
phosphate-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Booze RM, Crisostomo EA, Davis JN. Beta-adrenergic receptors in the hippocampal and retrohippocampal regions of rats and guinea pigs: autoradiographic and immunohistochemical studies. Synapse. 1993;13:206–214. doi: 10.1002/syn.890130303. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Dudek S, Ozsarac N, Stevens B. Regulation of gene expression by action potentials: dependence on complexity in cellular information processing; Novartis Found Symp; 2001. pp. 160–172. discussion 172-166, 234-140. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001a;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci. 2001b;13:1453–1458. doi: 10.1046/j.0953-816x.2001.01531.x. [DOI] [PubMed] [Google Scholar]
- Jin S-H, Kim HJT, Harris DC, Thomas SA. Postnatal development of the cerebellum and adrenergic system is independent of norepinephrine and epinephrine. J Comp Neurol. 2004;477:300–309. doi: 10.1002/cne.20263. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang X-Y, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hokfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience. 1993;56:1023–1039. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- Papa M, Pellicano MP, Welzl H, Sadile AG. Distributed changes in c-Fos and c-Jun immunoreactivity in the rat brain associated with arousal and habituation to novelty. Brain Res Bull. 1993;32:509–515. doi: 10.1016/0361-9230(93)90299-q. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci U S A. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, Palmiter RD. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci. 1999;19:10985–10992. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talk AC, Gandhi CC, Matzel LD. Hippocampal function during behaviorally silent associative learning: dissociation of memory storage and expression. Hippocampus. 2002;12:648–656. doi: 10.1002/hipo.10098. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509:299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3 Spec No:33–44. [PubMed] [Google Scholar]
- Zhang WP, Guzowski JF, Thomas SA. Mapping neuronal activation and the influence of adrenergic signaling during contextual memory retrieval. Learn Mem. 2005;12:239–247. doi: 10.1101/lm.90005. [DOI] [PMC free article] [PubMed] [Google Scholar]